Legal Status
This site displays a prototype of a “Web 2.0” version of the daily Federal Register. It is not an official legal edition of the Federal Register, and does not replace the official print version or the official electronic version on GPO’s govinfo.gov.
The documents posted on this site are XML renditions of published Federal Register documents. Each document posted on the site includes a link to the corresponding official PDF file on govinfo.gov. This prototype edition of the daily Federal Register on FederalRegister.gov will remain an unofficial informational resource until the Administrative Committee of the Federal Register (ACFR) issues a regulation granting it official legal status. For complete information about, and access to, our official publications and services, go to About the Federal Register on NARA's archives.gov.
The OFR/GPO partnership is committed to presenting accurate and reliable regulatory information on FederalRegister.gov with the objective of establishing the XML-based Federal Register as an ACFR-sanctioned publication in the future. While every effort has been made to ensure that the material on FederalRegister.gov is accurately displayed, consistent with the official SGML-based PDF version on govinfo.gov, those relying on it for legal research should verify their results against an official edition of the Federal Register. Until the ACFR grants it official status, the XML rendition of the daily Federal Register on FederalRegister.gov does not provide legal notice to the public or judicial notice to the courts.
Legal Status
Rule
Medicare Program: Changes to the Hospital Outpatient Prospective Payment System and CY 2010 Payment Rates; Changes to the Ambulatory Surgical Center Payment System and CY 2010 Payment Rates
A Rule by the Centers for Medicare & Medicaid Services on 11/20/2009
Document Details
Information about this document as published in the Federal Register.
- Printed version:
- Publication Date:
- 11/20/2009
- Agencies:
- Department of Health and Human Services
- Centers for Medicare & Medicaid Services
- Dates:
- Effective Date: The provisions of this rule are effective January 1, 2010.
- Effective Date:
- 01/01/2010
- Document Type:
- Rule
- Document Citation:
- 74 FR 60316
- Page:
- 60316-60983 (668 pages)
- CFR:
- 42 CFR 410
- 42 CFR 416
- 42 CFR 419
- Agency/Docket Number:
- CMS-1414-FC
- RIN:
- 0938-AP41
- Document Number:
- E9-26499
Document Details
Document Statistics
- Page views:
- 4,778
- as of 04/18/2024 at 10:15 pm EDT
Document Statistics
Published Document
This document has been published in the Federal Register. Use the PDF linked in the document sidebar for the official electronic format.
Published Document
-
Enhanced Content - Table of Contents
This table of contents is a navigational tool, processed from the headings within the legal text of Federal Register documents. This repetition of headings to form internal navigation links has no substantive legal effect.
- AGENCY:
- ACTION:
- SUMMARY:
- DATES:
- ADDRESSES:
- FOR FURTHER INFORMATION CONTACT:
- SUPPLEMENTARY INFORMATION:
- Electronic Access
- Alphabetical List of Acronyms Appearing in This Final Rule
- Table of Contents
- Regulation Text
- Addenda
- I. Background and Summary of the CY 2010 OPPS/ASC Final Rule With Comment Period
- A. Legislative and Regulatory Authority for the Hospital Outpatient Prospective Payment System
- B. Excluded OPPS Services and Hospitals
- C. Prior Rulemaking
- D. Advisory Panel on Ambulatory Payment Classification (APC) Groups
- 1. Authority of the APC Panel
- 2. Establishment of the APC Panel
- 3. APC Panel Meetings and Organizational Structure
- E. Background and Summary of the CY 2010 OPPS/ASC Proposed Rule
- 1. Updates Affecting OPPS Payments
- 2. OPPS Ambulatory Payment Classification (APC) Group Policies
- 3. OPPS Payment for Devices
- 4. OPPS Payment Changes for Drugs, Biologicals, and Radiopharmaceuticals
- 5. Estimate of OPPS Transitional Pass-Through Spending for Drugs, Biologicals, Radiopharmaceuticals, and Devices
- 6. OPPS Payment for Brachytherapy Sources
- 7. OPPS Payment for Drug Administration Services
- 8. OPPS Payment for Hospital Outpatient Visits
- 9. Payment for Partial Hospitalization Services
- 10. Procedures That Will Be Paid Only as Inpatient Procedures
- 11. OPPS Nonrecurring Technical and Policy Changes and Clarifications
- 12. OPPS Payment Status and Comment Indicators
- 13. OPPS Policy and Payment Recommendations
- 14. Updates to the Ambulatory Surgical Center (ASC) Payment System
- 15. Reporting Quality Data for Annual Payment Rate Updates
- 16. Healthcare-Associated Conditions
- 17. Regulatory Impact Analysis
- F. Public Comments Received in Response to the CY 2010 OPPS/ASC Proposed Rule
- G. Public Comments Received in Response to the November 18, 2008 OPPS/ASC Final Rule With Comment Period
- II. Updates Affecting OPPS Payments
- A. Recalibration of APC Relative Weights
- 1. Database Construction
- a. Database Source and Methodology
- b. Use of Single and Multiple Procedure Claims
- c. Calculation of CCRs
- (1) Development of the CCRs
- (2) Charge Compression
- 2. Data Development Process and Calculation of Median Costs
- a. Claims Preparation
- b. Splitting Claims and Creation of “Pseudo” Single Claims
- (1) Splitting Claims
- (2) Creation of “Pseudo” Single Claims
- c. Completion of Claim Records and Median Cost Calculations
- d. Calculation of Single Procedure APC Criteria-Based Median Costs
- (1) Device-Dependent APCs
- (2) Blood and Blood Products
- (3) Single Allergy Tests
- (4) Echocardiography Services
- (5) Nuclear Medicine Services
- (6) Hyperbaric Oxygen Therapy
- e. Calculation of Composite APC Criteria-Based Median Costs
- (1) Extended Assessment and Management Composite APCs (APCs 8002 and 8003)
- (2) Low Dose Rate (LDR) Prostate Brachytherapy Composite APC (APC 8001)
- (3) Cardiac Electrophysiologic Evaluation and Ablation Composite APC (APC 8000)
- (4) Mental Health Services Composite APC (APC 0034)
- (5) Multiple Imaging Composite APCs (APCs 8004, 8005, 8006, 8007, and 8008)
- 3. Calculation of OPPS Scaled Payment Weights
- 4. Changes to Packaged Services
- a. Background
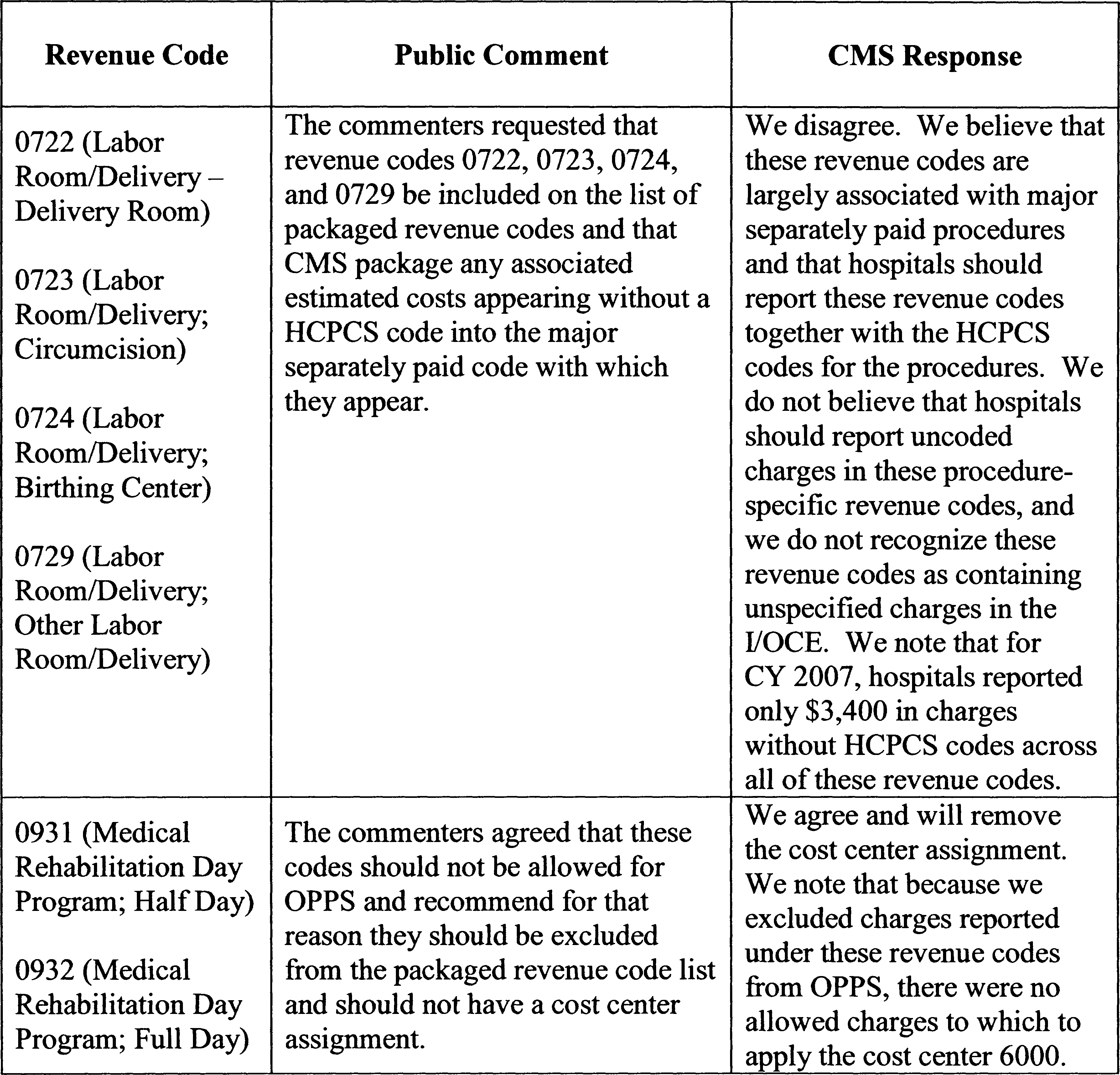
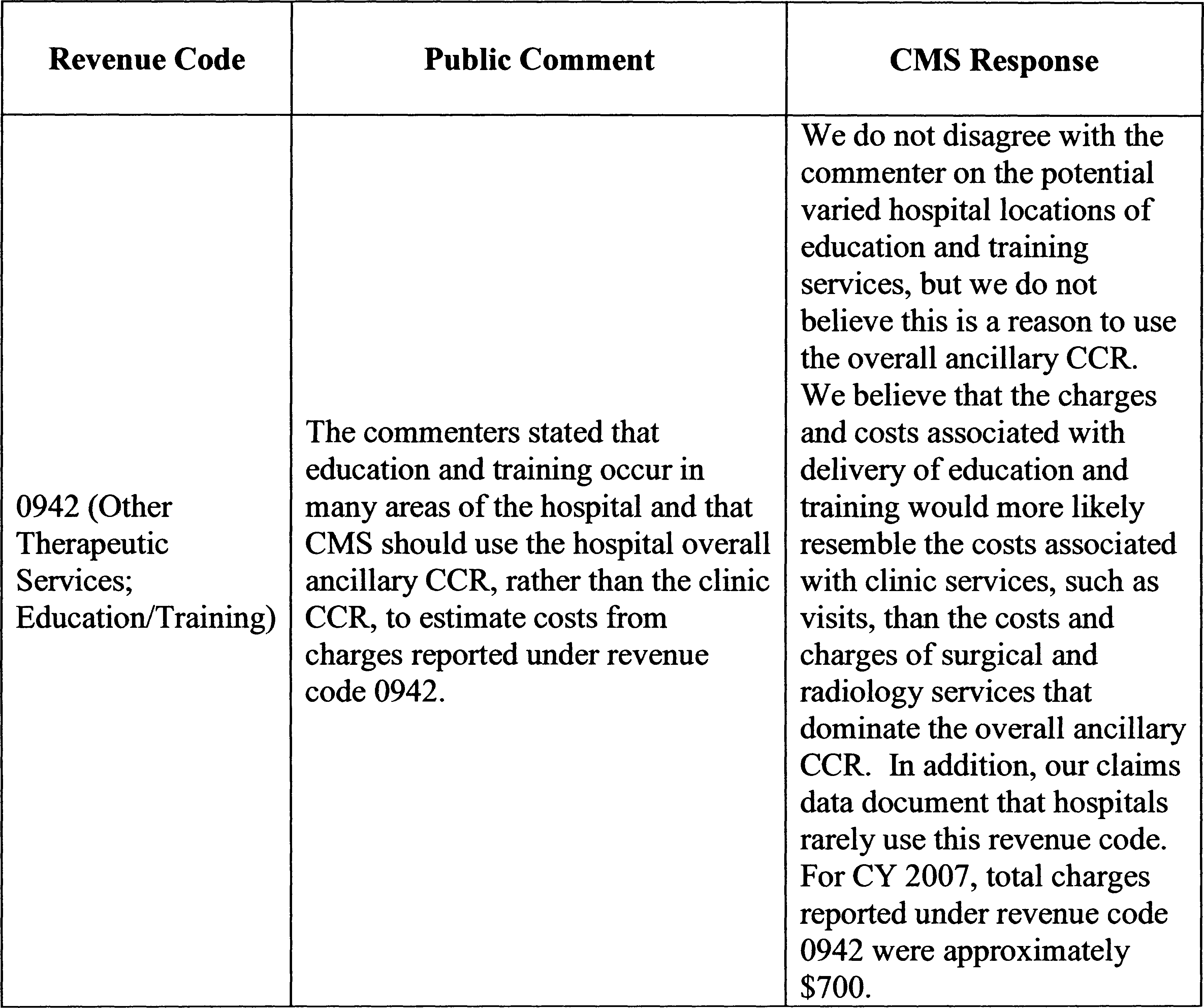
- b. Packaging Issues
- (1) Packaged Services Addressed by the February 2009 APC Panel Recommendations
- Recommendation 1
- Recommendation 2
- Recommendation 3
- Recommendation 4
- Recommendation 5
- (2) Packaged Services Addressed by the August 2009 APC Panel Recommendations
- (3) Other Service-Specific Packaging Issues
- B. Conversion Factor Update
- C. Wage Index Changes
- D. Statewide Average Default CCRs
- E. OPPS Payment to Certain Rural and Other Hospitals
- 1. Hold Harmless Transitional Payment Changes Made by Public Law 110–275 (MIPPA)
- 2. Adjustment for Rural SCHs Implemented in CY 2006 Related to Public Law 108–173 (MMA)
- F. Hospital Outpatient Outlier Payments
- 1. Background
- 2. Outlier Calculation
- 3. Final Outlier Calculation
- 4. Outlier Reconciliation
- G. Calculation of an Adjusted Medicare Payment From the National Unadjusted Medicare Payment
- H. Beneficiary Copayments
- 1. Background
- 2. Copayment Policy
- 3. Calculation of an Adjusted Copayment Amount for an APC Group
- III. OPPS Ambulatory Payment Classification (APC) Group Policies
- A. OPPS Treatment of New CPT and Level II HCPCS Codes
- 1. Treatment of New Level II HCPCS Codes and Category I CPT Vaccine Codes and Category III CPT Codes
- 2. Process for New Level II HCPCS Codes and Category I and Category III CPT Codes for Which We Are Soliciting Public Comments on the CY 2010 OPPS/ASC Final Rule With Comment Period
- B. OPPS Changes—Variations Within APCs
- 1. Background
- 2. Application of the 2 Times Rule
- 3. Exceptions to the 2 Times Rule
- C. New Technology APCs
- 1. Background
- 2. Movement of Procedures From New Technology APCs to Clinical APCs
- D. OPPS APC-Specific Policies
- 1. Cardiovascular Services
- a. Cardiovascular Telemetry (APC 0209)
- b. Implantable Loop Recorder Monitoring (APC 0689)
- c. Transluminal Balloon Angioplasty (APC 0279)
- 2. Gastrointestinal Services
- a. Change of Gastrostomy Tube (APC 0676)
- b. Laparoscopic Liver Cryoablation (APC 0131)
- c. Cholangioscopy (APC 0151)
- d. Laparoscopic Hernia Repair (APC 0131)
- 3. Genitourinary Services
- a. Percutaneous Renal Cryoablation (APC 0423)
- b. Hemodialysis (APC 0170)
- c. Radiofrequency Remodeling of Bladder Neck (APC 0165)
- d. Change of Bladder Tube (APC 0121)
- 4. Nervous System Services
- a. Pain-Related Procedures (APCs 0203, 0204, 0206, 0207, 0221, 0224, and 0388)
- b. Magnetoencephalography (APCs 0065 and 0067)
- 5. Ocular Services
- a. Insertion of Anterior Segment Aqueous Drainage Device (APC 0234)
- b. Backbench Preparation of Corneal Allograft
- 6. Orthopedic and Musculoskeletal Services
- a. Arthroscopic Procedures (APCs 0041 and 0042)
- b. Knee Arthroscopy (APCs 0041 and 0042)
- c. Shoulder Arthroscopy (APC 0042)
- d. Fasciotomy Procedures (APC 0049)
- e. Fibula Repair (APC 0062)
- f. Forearm Orthopedic Procedures (APCs 0050, 0051, and 0052)
- g. Low Energy Extracorporeal Shock Wave Therapy (Low Energy ESWT)
- h. Insertion of Posterior Spinous Process Distraction Device (APC 0052)
- 7. Radiation Therapy Services
- a. Proton Beam Therapy (APCs 0664 and 0667)
- b. Stereotactic Radiosurgery (SRS) Treatment Delivery Services (APCs 0065, 0066, 0067, and 0127)
- c. Clinical Brachytherapy (APCs 0312 and 0651)
- 8. Other Services
- a. Low Frequency, Non-Contact, Non-Thermal Ultrasound (APC 0013)
- b. Skin Repair (APCs 0134 and 0135)
- c. Group Psychotherapy (APC 0325)
- d. Portable X-Ray Services
- e. Home Sleep Study Tests (APC 0213)
- IV. OPPS Payment for Devices
- A. Pass-Through Payments for Devices
- 1. Expiration of Transitional Pass-Through Payments for Certain Devices
- 2. Provisions for Reducing Transitional Pass-Through Payments To Offset Costs Packaged Into APC Groups
- a. Background
- b. Final Policy
- B. Adjustment to OPPS Payment for No Cost/Full Credit and Partial Credit Devices
- 1. Background
- 2. APCs and Devices Subject to the Adjustment Policy
- V. OPPS Payment Changes for Drugs, Biologicals, and Radiopharmaceuticals
- A. OPPS Transitional Pass-Through Payment for Additional Costs of Drugs, Biologicals, and Radiopharmaceuticals
- 1. Background
- 2. Drugs and Biologicals With Expiring Pass-Through Status in CY 2009
- 3. Drugs, Biologicals, and Radiopharmaceuticals With New or Continuing Pass-Through Status in CY 2010
- 4. Pass-Through Payment for Implantable Biologicals
- a. Background
- b. Policy for CY 2010
- 5. Definition of Pass-Through Payment Eligibility Period for New Drugs and Biologicals
- 6. Provisions for Reducing Transitional Pass-Through Payments for Diagnostic Radiopharmaceuticals and Contrast Agents To Offset Costs Packaged Into APC Groups
- a. Background
- b. Payment Offset Policy for Diagnostic Radiopharmaceuticals
- c. Payment Offset Policy for Contrast Agents
- B. OPPS Payment for Drugs, Biologicals, and Radiopharmaceuticals Without Pass-Through Status
- 1. Background
- 2. Criteria for Packaging Payment for Drugs, Biologicals, and Radiopharmaceuticals
- a. Background
- b. Cost Threshold for Packaging of Payment for HCPCS Codes that Describe Certain Drugs, Nonimplantable Biologicals, and Therapeutic Radiopharmaceuticals (“Threshold-Packaged Drugs”)
- c. Packaging Determination for HCPCS Codes That Describe the Same Drug or Biological But Different Dosages
- d. Packaging of Payment for Diagnostic Radiopharmaceuticals, Contrast Agents, and Implantable Biologicals (“Policy-Packaged” Drugs and Devices)
- 3. Payment for Drugs and Biologicals Without Pass-Through Status That Are Not Packaged
- a. Payment for Specified Covered Outpatient Drugs (SCODs) and Other Separately Payable and Packaged Drugs and Biologicals
- b. Payment Policy
- 4. Payment for Blood Clotting Factors
- 5. Payment for Therapeutic Radiopharmaceuticals
- a. Background
- b. Payment Policy
- 6. Payment for Nonpass-Through Drugs, Biologicals, and Radiopharmaceuticals With HCPCS Codes, but Without OPPS Hospital Claims Data
- VI. Estimate of OPPS Transitional Pass-Through Spending for Drugs, Biologicals, Radiopharmaceuticals, and Devices
- A. Background
- B. Estimate of Pass-Through Spending
- VII. OPPS Payment for Brachytherapy Sources
- A. Background
- B. OPPS Payment Policy
- VIII. OPPS Payment for Drug Administration Services
- A. Background
- B. Coding and Payment for Drug Administration Services
- IX. OPPS Payment for Hospital Outpatient Visits
- A. Background
- B. Policies for Hospital Outpatient Visits
- 1. Clinic Visits: New and Established Patient Visits
- 2. Emergency Department Visits
- 3. Visit Reporting Guidelines
- X. Payment for Partial Hospitalization Services
- A. Background
- B. PHP APC Update for CY 2010
- C. Separate Threshold for Outlier Payments to CMHCs
- XI. Procedures That Will Be Paid Only as Inpatient Procedures
- A. Background
- B. Changes to the Inpatient List
- XII. OPPS Nonrecurring Technical and Policy Changes and Clarifications
- A. Kidney Disease Education Services
- 1. Background
- 2. Payment for Services Furnished by Providers of Services Located in a Rural Area
- B. Pulmonary Rehabilitation, Cardiac Rehabilitation, and Intensive Cardiac Rehabilitation Services
- 1. Legislative Changes
- 2. Payments for Services Furnished to Hospital Outpatients in a Pulmonary Rehabilitation Program
- 3. Payment for Services Furnished to Hospital Outpatients Under a Cardiac Rehabilitation or an Intensive Cardiac Rehabilitation Program
- 4. Physician Supervision for Pulmonary Rehabilitation, Cardiac Rehabilitation, and Intensive Cardiac Rehabilitation Services
- C. Stem Cell Transplant
- D. Physician Supervision
- 1. Background
- 2. Issues Regarding the Physician Supervision of Hospital Outpatient Services Raised by Hospitals and Other Stakeholders
- 3. Policies for Direct Supervision of Hospital and CAH Outpatient Therapeutic Services
- 4. Policies for Direct Supervision of Hospital and CAH Outpatient Diagnostic Services
- 5. Summary of CY 2010 Physician Supervision Final Policy
- E. Direct Referral for Observation Services
- XIII. OPPS Payment Status and Comment Indicators
- A. OPPS Payment Status Indicator Definitions
- 1. Payment Status Indicators to Designate Services That Are Paid Under the OPPS
- 2. Payment Status Indicators to Designate Services That Are Paid Under a Payment System Other Than the OPPS
- 3. Payment Status Indicators To Designate Services That are Not Recognized Under the OPPS but That May Be Recognized by Other Institutional Providers
- 4. Payment Status Indicators To Designate Services That Are Not Payable by Medicare on Outpatient Claims
- B. Comment Indicator Definitions
- XIV. OPPS Policy and Payment Recommendations
- A. MedPAC Recommendations
- B. APC Panel Recommendations
- C. OIG Recommendations
- XV. Updates to the Ambulatory Surgical Center (ASC) Payment System
- A. Background
- 1. Legislative Authority for the ASC Payment System
- 2. Prior Rulemaking
- 3. Policies Governing Changes to the Lists of Codes and Payment Rates for ASC Covered Surgical Procedures and Covered Ancillary Services
- B. Treatment of New Codes
- 1. Treatment of New Category I and Category III CPT Codes and Level II HCPCS Codes
- 2. Treatment of New Level II HCPCS Codes Implemented in April and July 2009
- C. Update to the Lists of ASC Covered Surgical Procedures and Covered Ancillary Services
- 1. Covered Surgical Procedures
- a. Additions to the List of ASC Covered Surgical Procedures
- b. Covered Surgical Procedures Designated as Office-Based
- (1) Background
- (2) Changes to Covered Surgical Procedures Designated as Office-Based for CY 2010
- c. ASC Covered Surgical Procedures Designated as Device-Intensive
- (1) Background
- (2) Changes to List of Covered Surgical Procedures Designated as Device-Intensive for CY 2010
- d. ASC Treatment of Surgical Procedures Removed From the OPPS Inpatient List for CY 2010
- 2. Covered Ancillary Services
- D. ASC Payment for Covered Surgical Procedures and Covered Ancillary Services
- 1. Payment for Covered Surgical Procedures
- a. Background
- b. Update to ASC Covered Surgical Procedure Payment Rates for CY 2010
- c. Adjustment to ASC Payments for No Cost/Full Credit and Partial Credit Devices
- 2. Payment for Covered Ancillary Services
- a. Background
- b. Payment for Covered Ancillary Services for CY 2010
- E. New Technology Intraocular Lenses (NTIOLs)
- 1. Background
- 2. NTIOL Application Process for Payment Adjustment
- 3. Classes of NTIOLs Approved and New Requests for Payment Adjustment
- a. Background
- b. Request To Establish New NTIOL Class for CY 2010 and Deadline for Public Comment
- 4. Payment Adjustment
- 5. ASC Payment for Insertion of IOLs
- 6. Announcement of CY 2010 Deadline for Submitting Requests for CMS Review of Appropriateness of ASC Payment for Insertion of an NTIOL Following Cataract Surgery
- F. ASC Payment and Comment Indicators
- 1. Background
- 2. ASC Payment and Comment Indicators
- G. ASC Policy and Payment Recommendations
- H. Revision to Terms of Agreements for Hospital-Operated ASCs
- 1. Background
- 2. Change to the Terms of Agreements for ASCs Operated by Hospitals
- I. Calculation of the ASC Conversion Factor and ASC Payment Rates
- 1. Background
- 2. Calculation of the ASC Payment Rates
- a. Updating the ASC Relative Payment Weights for CY 2010 and Future Years
- b. Updating the ASC Conversion Factor
- 3. Display of ASC Payment Rates
- XVI. Reporting Quality Data for Annual Payment Rate Updates
- A. Background
- 1. Overview
- 2. Hospital Outpatient Quality Data Reporting Under Section 109(a) of Pub. L. 109–432
- 3. Reporting ASC Quality Data for Annual Payment Update
- 4. HOP QDRP Quality Measures for the CY 2009 Payment Determination
- 5. HOP QDRP Quality Measures for the CY 2010 Payment Determination
- a. Background
- b. Maintenance of Technical Specifications for Quality Measures
- c. Publication of HOP QDRP Data
- B. Quality Measures for the CY 2011 Payment Determination
- 1. Considerations in Expanding and Updating Quality Measures Under the HOP QDRP
- 2. Retirement of HOP QDRP Quality Measures
- 3. HOP QDRP Quality Measures for the CY 2011 Payment Determination
- • OP–4: Aspirin at Arrival & OP–5: Median Time to ECG
- • Imaging Efficiency Measures Generally
- • OP–8: MRI Lumbar Spine for Low Back Pain
- • OP–9: Mammography Follow-up Rates
- • OP–10: Abdomen CT—Use of Contrast Material
- • OP–11: Thorax CT—Use of Contrast Material
- C. Possible Quality Measures Under Consideration for CY 2012 and Subsequent Years
- • Cancer (Potential Measures 1, 2, and 3)
- • Emergency Department Throughput (Potential Measure 4)
- • Diabetes (Potential Measures 5, 6, 7, and 8)
- • Medication Reconciliation (Potential Measure 9)
- • Immunization (Potential Measures 10 and 11)
- • Imaging Efficiency—SPECT MPI and Stress Echocardiography (Potential Measures 12 and 13)
- • Imaging Efficiency—Computed Tomography (Potential Measures 14 and 15)
- • Surgery (Potential Measure 16)
- • Other Suggested Measures or Measurement Areas
- D. Payment Reduction for Hospitals That Fail To Meet the HOP QDRP Requirements for the CY 2010 Payment Update
- 1. Background
- 2. Reporting Ratio Application and Associated Adjustment Policy for CY 2010
- E. Requirements for HOPD Quality Data Reporting for CY 2011 and Subsequent Years
- 1. Administrative Requirements
- 2. Data Collection and Submission Requirements
- a. General Data Collection and Submission Requirements
- b. Extraordinary Circumstance Extension or Waiver for Reporting Quality Data
- 3. HOP QDRP Validation Requirements
- b. Data Validation Approach for CY 2012 and Subsequent Years
- c. Additional Data Validation Conditions Under Consideration for CY 2012 and Subsequent Years
- F. 2010 Publication of HOP QDRP Data
- G. HOP QDRP Reconsideration and Appeals Procedures
- H. Reporting of ASC Quality Data
- I. Electronic Health Records
- XVII. Healthcare-Associated Conditions
- A. Background
- 1. Preventable Medical Errors and Hospital-Acquired Conditions (HACs) Under the IPPS
- 2. Expanding the Principles of the IPPS HACs Payment Provision to the OPPS
- 3. Discussion in the CY 2009 OPPS/ASC Final Rule With Comment Period
- B. Public Comments and Recommendations on Issues Regarding Healthcare-Associated Conditions From the Joint IPPS/OPPS Listening Session
- C. CY 2010 Approach to Healthcare-Associated Conditions Under the OPPS
- XVIII. Files Available to the Public Via the Internet
- A. Information in Addenda Related to the CY 2010 Hospital OPPS
- B. Information in Addenda Related to the CY 2010 ASC Payment System
- XIX. Collection of Information Requirements
- A. Legislative Requirement for Solicitation of Comments
- B. Associated Information Collections Not Specified in Regulatory Text
- 1. Hospital Outpatient Quality Data Reporting Program (HOP QDRP)
- 2. HOP QDRP Quality Measures for the CY 2010 and CY 2011 Payment Determinations
- 3. HOP QDRP Validation Requirements
- 4. HOP QDRP Reconsideration and Appeals Procedures
- 5. Additional Topics
- XX. Response to Comments
- XXI. Regulatory Impact Analysis
- A. Overall Impact
- 1. Executive Order 12866
- 2. Regulatory Flexibility Act (RFA)
- 3. Small Rural Hospitals
- 4. Unfunded Mandates
- 5. Federalism
- B. Effects of OPPS Changes in This Final Rule With Comment Period
- 1. Alternatives Considered
- a. Alternatives Considered for Pass-Through Payment for Implantable Biologicals
- b. Alternatives Considered for Payment of the Acquisition and Pharmacy Overhead Costs of Drugs and Biologicals That Do Not Have Pass-Through Status
- c. Alternatives Considered for the Physician Supervision of Hospital Outpatient Services
- 2. Limitations of Our Analysis
- 3. Estimated Effects of This Final Rule With Comment Period on Hospitals
- Column 1: Total Number of Hospitals
- Column 2: APC Changes Due to Reassignment and Recalibration
- Column 3: New Wage Indices and the Effect of the Rural Adjustment
- Column 4: All Budget Neutrality Changes and Market Basket Update
- Column 5: All Changes for CY 2010
- 4. Estimated Effects of This Final Rule With Comment Period on CMHCs
- 5. Estimated Effect of This Final Rule With Comment Period Rule on Beneficiaries
- 6. Conclusion
- 7. Accounting Statement
- C. Effects of ASC Payment System Changes in This Final Rule With Comment Period Rule
- 1. Alternatives Considered
- a. Alternatives Considered for Office-Based Procedures
- b. Alternatives Considered for Covered Surgical Procedures
- 2. Limitations of Our Analysis
- 3. Estimated Effects of This Final Rule With Comment Period on Payments to ASCs
- 4. Estimated Effects of This Final Rule With Comment Period on Beneficiaries
- 5. Conclusion
- 6. Accounting Statement
- D. Effects of Requirements for Hospital Reporting of Quality Data for Annual Hospital Payment Update
- E. Executive Order 12866
- List of Subjects
- 42 CFR Part 410
- 42 CFR Part 416
- 42 CFR Part 419
- PART 410—SUPPLEMENTARY MEDICAL INSURANCE (SMI) BENEFITS
- PART 416—AMBULATORY SURGICAL SERVICES
- PART 419—PROSPECTIVE PAYMENT SYSTEM FOR HOSPITAL OUTPATIENT DEPARTMENT SERVICES
- Footnotes
Enhanced Content - Table of Contents
-
Enhanced Content - Submit Public Comment
- This feature is not available for this document.
Enhanced Content - Submit Public Comment
-
Enhanced Content - Read Public Comments
- This feature is not available for this document.
Enhanced Content - Read Public Comments
-
Enhanced Content - Sharing
- Shorter Document URL
- https://www.federalregister.gov/d/E9-26499
Enhanced Content - Sharing
-
Enhanced Content - Document Tools
These tools are designed to help you understand the official document better and aid in comparing the online edition to the print edition.
-
These markup elements allow the user to see how the document follows the Document Drafting Handbook that agencies use to create their documents. These can be useful for better understanding how a document is structured but are not part of the published document itself.
Display Non-Printed Markup Elements
Enhanced Content - Document Tools
-
-
Enhanced Content - Developer Tools
This document is available in the following developer friendly formats:
- JSON: Normalized attributes and metadata
- XML: Original full text XML
- MODS: Government Publishing Office metadata
More information and documentation can be found in our developer tools pages.
Enhanced Content - Developer Tools
Published Document
This document has been published in the Federal Register. Use the PDF linked in the document sidebar for the official electronic format.
AGENCY:
Centers for Medicare & Medicaid Services (CMS), HHS.
ACTION:
Final rule with comment period.
SUMMARY:
This final rule with comment period revises the Medicare hospital outpatient prospective payment system (OPPS) to implement applicable statutory requirements and changes arising from our continuing experience with this system. In this final rule with comment period, we describe the changes to the amounts and factors used to determine the payment rates for Medicare hospital outpatient services paid under the prospective payment system. These changes are applicable to services furnished on or after January 1, 2010.
In addition, this final rule with comment period updates the revised Medicare ambulatory surgical center (ASC) payment system to implement applicable statutory requirements and changes arising from our continuing experience with this system. In this final rule with comment period, we set forth the applicable relative payment weights and amounts for services furnished in ASCs, specific HCPCS codes to which these changes will apply, and other pertinent ratesetting information for the CY 2010 ASC payment system. These changes are applicable to services furnished on or after January 1, 2010.
DATES:
Effective Date: The provisions of this rule are effective January 1, 2010.
Comment Period: We will consider comments on the subject areas listed in the SUPPLEMENTARY INFORMATION section of this rule that are received at one of the addresses provided in the ADDRESSES section of this rule no later than 5 p.m. EST on December 29, 2009.
Application Deadline for New Class of New Technology Intraocular Lenses: Request for review of applications for a new class of new technology intraocular lenses must be received by 5 p.m. EST on March 8, 2010.
ADDRESSES:
In commenting, please refer to file code CMS–1414–FC. Because of staff and resource limitations, we cannot accept comments by facsimile (FAX) transmission.
You may submit comments in one of four ways (no duplicates, please):
1. Electronically. You may submit electronic comments on this regulation to http://www.regulations.gov. Follow the instructions for “Comment or Submission” and enter the file code to find the document accepting comments.
2. By regular mail. You may mail written comments (one original and two copies) to the following address ONLY: Centers for Medicare & Medicaid Services, Department of Health and Human Services, Attention: CMS–1414–FC, P.O. Box 8013, Baltimore, MD 21244–1850.
Please allow sufficient time for mailed comments to be received before the close of the comment period.
3. By express or overnight mail. You may send written comments (one original and two copies) to the following address ONLY: Centers for Medicare & Medicaid Services, Department of Health and Human Services, Attention: CMS–1414–FC, Mail Stop C4–26–05, 7500 Security Boulevard, Baltimore, MD 21244–1850.
4. By hand or courier. If you prefer, you may deliver (by hand or courier) your written comments (one original and two copies) before the close of the comment period to one of the following addresses:
a. Room 445–G, Hubert H. Humphrey Building, 200 Independence Avenue, SW., Washington, DC 20201.
(Because access to the interior of the HHH Building is not readily available to persons without Federal Government identification, commenters are encouraged to leave their comments in the CMS drop slots located in the main lobby of the building. A stamp-in clock is available for persons wishing to retain a proof of filing by stamping in and retaining an extra copy of the comments being filed.)
b. 7500 Security Boulevard, Baltimore, MD 21244–1850.
If you intend to deliver your comments to the Baltimore address, please call the telephone number (410) 786–9994 in advance to schedule your arrival with one of our staff members.
Comments mailed to the addresses indicated as appropriate for hand or courier delivery may be delayed and received after the comment period.
For information on viewing public comments, see the beginning of the SUPPLEMENTARY INFORMATION section.
Applications for a new class of new technology intraocular lenses: Requests for review of applications for a new class of new technology intraocular lenses must be sent by regular mail to ASC/NTOL, Division of Outpatient Care, Mailstop C4–05–17, Centers for Medicare & Medicaid Services, 7500 Security Boulevard, Baltimore, MD 21244–1850.
Start Further InfoFOR FURTHER INFORMATION CONTACT:
Alberta Dwivedi, (410) 786–0378, Hospital outpatient prospective payment issues.
Dana Burley, (410) 786–0378, Ambulatory surgical center issues.
Michele Franklin, (410) 786–4533, and Jana Lindquist, (410) 786–4533, Partial hospitalization and community mental health center issues.
James Poyer, (410) 786–2261, Reporting of quality data issues.
End Further Info End Preamble Start Supplemental InformationSUPPLEMENTARY INFORMATION:
Comment Subject Areas: We will consider comments on the following subject areas discussed in this final rule with comment period that are received by the date and time indicated in the DATES section of this final rule with comment period:
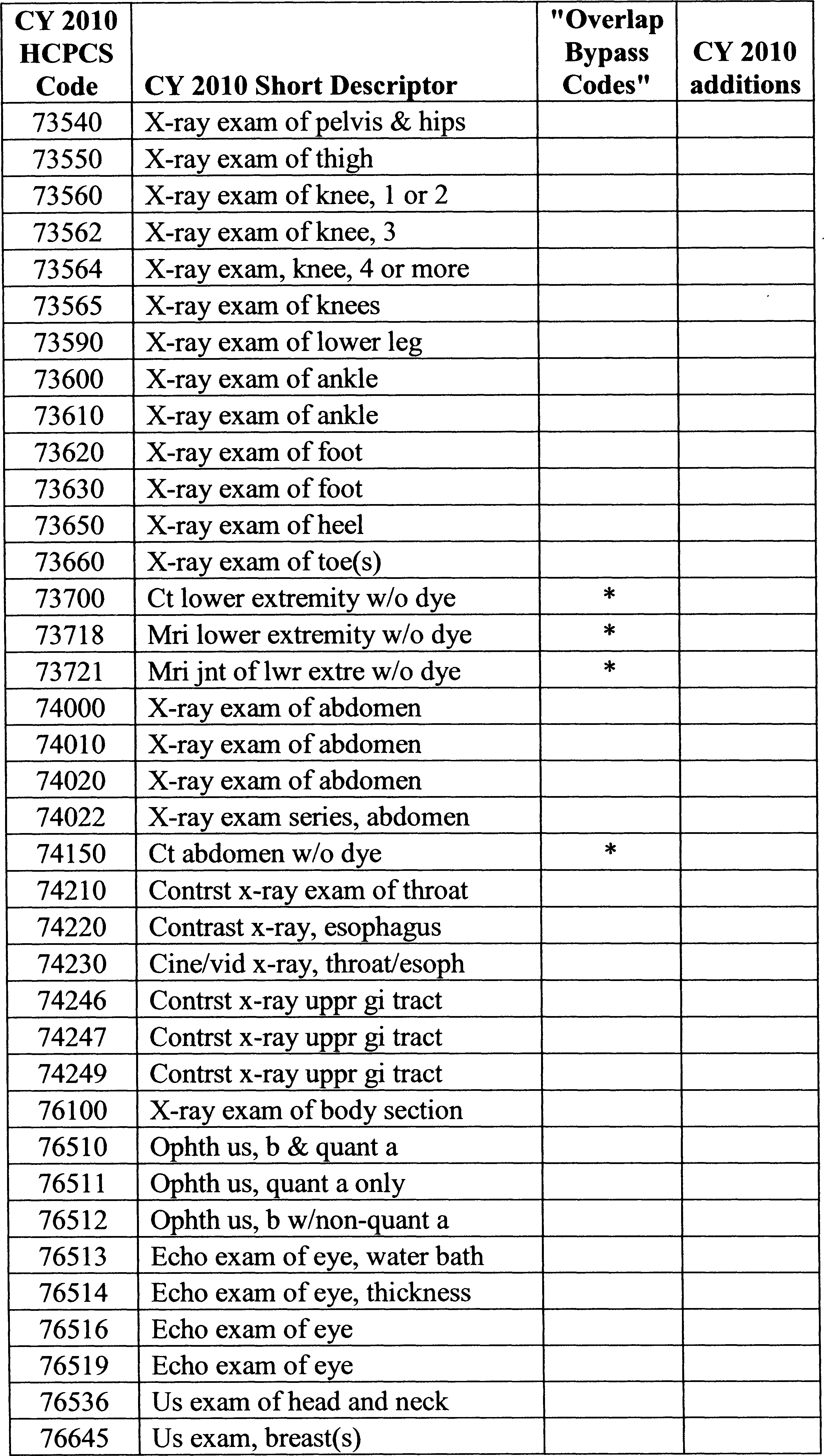
(1) The payment classifications assigned to HCPCS codes identified in Addenda B, AA, and BB to this final rule with comment period with the “NI” comment indicator;
(2) Recognition of plasma protein fraction as a blood product or a biological for OPPS payment, as discussed in section II.A.1.d.(2) of this final rule with comment period;
(3) Potential alternative coding schemes for reporting hospital clinic visits for new and established patients, as discussed in section IX.B.1. of this final rule with comment period;
(4) The possibility of extending the direct supervision requirements for hospital-based partial hospitalization program services to those same services in community mental health centers, as discussed in section XII.D.3. of this final rule with comment period; and
(5) The possibility of establishing direct physician supervision requirements for ASC services, as discussed in section XV.A.3. of this final rule with comment period.
Inspection of Public Comments: All comments received before the close of the comment period are available for viewing by the public, including any personally identifiable or confidential business information that is included in a comment. We post all comments received before the close of the comment period on the following Web site as soon as possible after they have been received: http://www.regulations.gov. Follow the search Start Printed Page 60317 instructions on that Web site to view public comments.
Comments received timely will also be available for public inspection as they are received, generally beginning approximately 3 weeks after publication of a document, at the headquarters of the Centers for Medicare & Medicaid Services, 7500 Security Boulevard, Baltimore, MD 21244, on Monday through Friday of each week from 8:30 a.m. to 4 p.m. EST. To schedule an appointment to view public comments, phone 1–800–743–3951.
Electronic Access
This Federal Register document is also available from the Federal Register online database through GPO Access, a service of the U.S. Government Printing Office. Free public access is available on a Wide Area Information Server (WAIS) through the Internet and via asynchronous dial-in. Internet users can access the database by using the World Wide Web; the Superintendent of Documents' home page address is http://www.gpoaccess.gov/index.html, by using local WAIS client software, or by telnet to swais.access.gpo.gov, then login as guest (no password required). Dial-in users should use communications software and modem to call (202) 512–1661; type swais, then login as guest (no password required).
Alphabetical List of Acronyms Appearing in This Final Rule
ACEP American College of Emergency Physicians
AHA American Hospital Association
AHIMA American Health Information Management Association
AMA American Medical Association
AMP Average manufacturer price
AOA American Osteopathic Association
APC Ambulatory payment classification
ASC Ambulatory Surgical Center
ASP Average sales price
AWP Average wholesale price
BBA Balanced Budget Act of 1997, Public Law 105–33
BBRA Medicare, Medicaid, and SCHIP [State Children's Health Insurance Program] Balanced Budget Refinement Act of 1999, Public Law 106–113
BCA Blue Cross Association
BCBSA Blue Cross and Blue Shield Association
BIPA Medicare, Medicaid, and SCHIP Benefits Improvement and Protection Act of 2000, Public Law 106–554
CAH Critical access hospital
CAP Competitive Acquisition Program
CBSA Core-Based Statistical Area
CCR Cost-to-charge ratio
CERT Comprehensive Error Rate Testing
CKD Chronic kidney disease
CMHC Community mental health center
CMS Centers for Medicare & Medicaid Services
CORF Comprehensive outpatient rehabilitation facility
CPT [Physicians'] Current Procedural Terminology, Fourth Edition, 2009, copyrighted by the American Medical Association
CR Cardiac rehabilitation
CRNA Certified registered nurse anesthetist
CY Calendar year
DMEPOS Durable medical equipment, prosthetics, orthotics, and supplies
DMERC Durable medical equipment regional carrier
DRA Deficit Reduction Act of 2005, Public Law 109–171
DSH Disproportionate share hospital
EACH Essential Access Community Hospital
E/M Evaluation and management
EPO Erythropoietin
ESRD End-stage renal disease
FACA Federal Advisory Committee Act, Public Law 92–463
FAR Federal Acquisition Regulations
FDA Food and Drug Administration
FFS Fee-for-service
FSS Federal Supply Schedule
FTE Full-time equivalent
FY Federal fiscal year
GAO Government Accountability Office
GME Graduate medical education
HCPCS Healthcare Common Procedure Coding System
HCRIS Hospital Cost Report Information System
HHA Home health agency
HIPAA Health Insurance Portability and Accountability Act of 1996, Public Law 104–191
HOPD Hospital outpatient department
HOPQDRP Hospital Outpatient Quality Data Reporting Program
ICD–9–CM International Classification of Diseases, Ninth Edition, Clinical Modification
ICR Intensive cardiac rehabilitation
IDE Investigational device exemption
IME Indirect medical education
I/OCE Integrated Outpatient Code Editor
IOL Intraocular lens
IPPS [Hospital] Inpatient prospective payment system
IVIG Intravenous immune globulin
KDE Kidney disease education
MAC Medicare Administrative Contractor
MedPAC Medicare Payment Advisory Commission
MDH Medicare-dependent, small rural hospital
MIEA–TRHCA Medicare Improvements and Extension Act Under Division B, Title I of the Tax Relief Health Care Act of 2006, Public Law 109–432
MIPPA Medicare Improvements for Patients and Providers Act of 2008, Public Law 110–275
MMA Medicare Prescription Drug, Improvement, and Modernization Act of 2003, Public Law 108–173
MMSEA Medicare, Medicaid, and SCHIP Extension Act of 2007, Public Law 110–173
MPFS Medicare Physician Fee Schedule
MSA Metropolitan Statistical Area
NCCI National Correct Coding Initiative
NCD National Coverage Determination
NTIOL New technology intraocular lens
OIG [HHS] Office of the Inspector General
OMB Office of Management and Budget
OPD [Hospital] Outpatient department
OPPS [Hospital] Outpatient prospective payment system
PBD Provider-based department
PHP Partial hospitalization program
PM Program memorandum
PPI Producer Price Index
PPS Prospective payment system
PR Pulmonary rehabilitation
PRA Paperwork Reduction Act
QAPI Quality Assessment and Performance Improvement
QIO Quality Improvement Organization
RAC Recovery Audit Contractor
RFA Regulatory Flexibility Act
RHQDAPU Reporting Hospital Quality Data for Annual Payment Update [Program]
RHHI Regional home health intermediary
SBA Small Business Administration
SCH Sole community hospital
SDP Single Drug Pricer
SI Status indicator
TEFRA Tax Equity and Fiscal Responsibility Act of 1982, Public Law 97–248
TOPS Transitional outpatient payments
USPDI United States Pharmacopoeia Drug Information
WAC Wholesale acquisition cost
In this document, we address two payment systems under the Medicare program: the hospital outpatient prospective payment system (OPPS) and the revised ambulatory surgical center (ASC) payment system. The provisions relating to the OPPS are included in sections I. through XIV., and XVI. through XXI. of this final rule with comment period and in Addenda A, B, C (Addendum C is available on the Internet only; we refer readers to section XVIII.A. of this final rule with comment period), D1, D2, E, L, and M to this final rule with comment period. The provisions related to the revised ASC payment system are included in sections XV., XVI., and XVIII. through XXI. of this final rule with comment period and in Addenda AA, BB, DD1, DD2, and EE to this final rule with comment period. (Addendum EE is available on the Internet only; we refer readers to section XVIII.B. of this final rule with comment period.)
Table of Contents
I. Background and Summary of the CY 2010 OPPS/ASC Final Rule With Comment Period
A. Legislative and Regulatory Authority for the Hospital Outpatient Prospective Payment System
B. Excluded OPPS Services and Hospitals
C. Prior Rulemaking
D. Advisory Panel on Ambulatory Payment Classification (APC) Groups
1. Authority of the APC Panel
2. Establishment of the APC Panel
3. APC Panel Meetings and Organizational Structure
E. Background and Summary of the CY 2010 OPPS/ASC Proposed Rule
1. Updates Affecting OPPS Payments Start Printed Page 60318
2. OPPS Ambulatory Payment Classification (APC) Group Policies
3. OPPS Payment for Devices
4. OPPS Payment Changes for Drugs, Biologicals, and Radiopharmaceuticals
5. Estimate of OPPS Transitional Pass-Through Spending for Drugs, Biologicals, Radiopharmaceuticals, and Devices
6. OPPS Payment for Brachytherapy Sources
7. OPPS Payment for Drug Administration Services
8. OPPS Payment for Hospital Outpatient Visits
9. Payment for Partial Hospitalization Services
10. Procedures That Will Be Paid Only as Inpatient Services
11. OPPS Nonrecurring Technical and Policy Changes and Clarifications
12. OPPS Payment Status and Comment Indicators
13. OPPS Policy and Payment Recommendations
14. Updates to the Ambulatory Surgical Center (ASC) Payment System
15. Reporting Quality Data for Annual Payment Rate Updates
16. Healthcare-Associated Conditions
17. Regulatory Impact Analysis
F. Public Comments Received in Response to the CY 2010 OPPS/ASC Proposed Rule
G. Public Comments Received in Response to the November 18, 2008 OPPS/ASC Final Rule With Comment Period
II. Updates Affecting OPPS Payments
A. Recalibration of APC Relative Weights
1. Database Construction
a. Database Source and Methodology
b. Use of Single and Multiple Procedure Claims
c. Calculation of CCRs
(1) Development of the CCRs
(2) Charge Compression
2. Data Development Process and Calculation of Median Costs
a. Claims Preparation
b. Splitting Claims and Creation of “Pseudo” Single Claims
(1) Splitting Claims
(2) Creation of “Pseudo” Single Claims
c. Completion of Claim Records and Median Cost Calculations
d. Calculation of Single Procedure APC Criteria-Based Median Costs
(1) Device-Dependent APCs
(2) Blood and Blood Products
(3) Single Allergy Tests
(4) Echocardiography Services
(5) Nuclear Medicine Services
(6) Hyperbaric Oxygen Therapy
(7) Payment for Ancillary Outpatient Services When Patient Expires (CA Modifier)
e. Calculation of Composite APC Criteria-Based Median Costs
(1) Extended Assessment and Management Composite APCs (APCs 8002 and 8003)
(2) Low Dose Rate (LDR) Prostate Brachytherapy Composite APC (APC 8001)
(3) Cardiac Electrophysiologic Evaluation and Ablation Composite APC (APC 8000)
(4) Mental Health Services Composite APC (APC 0034)
(5) Multiple Imaging Composite APCs (APCs 8004, 8005, 8006, 8007, and 8008)
3. Calculation of OPPS Scaled Payment Weights
4. Changes to Packaged Services
a. Background
b. Packaging Issues
(1) Packaged Services Addressed by the February 2009 APC Panel Recommendations
(2) Packaged Services Addressed by the August 2009 APC Panel Recommendations
(3) Other Service-Specific Packaging Issues
B. Conversion Factor Update
C. Wage Index Changes
D. Statewide Average Default CCRs
E. OPPS Payment to Certain Rural and Other Hospitals
1. Hold Harmless Transitional Payment Changes Made by Public Law 110–275 (MIPPA)
2. Adjustment for Rural SCHs Implemented in CY 2006 Related to Pub. L. 108–173 (MMA)
F. Hospital Outpatient Outlier Payments
1. Background
2. Outlier Calculation
3. Final Outlier Calculation
4. Outlier Reconciliation
G. Calculation of an Adjusted Medicare Payment From the National Unadjusted Medicare Payment
H. Beneficiary Copayments
1. Background
2. Copayment Policy
3. Calculation of an Adjusted Copayment Amount for an APC Group
III. OPPS Ambulatory Payment Classification (APC) Group Policies
A. OPPS Treatment of New CPT and Level II HCPCS Codes
1. Treatment of New Level II HCPCS Codes and Category I CPT Vaccine Codes and Category III CPT Codes
2. Process for New Level II HCPCS Codes and Category I and Category III CPT Codes for Which We Are Soliciting Public Comments on the CY 2010 OPPS/ASC Final Rule With Comment Period
B. OPPS Changes—Variations Within APCs
1. Background
2. Application of the 2 Times Rule
3. Exceptions to the 2 Times Rule
C. New Technology APCs
1. Background
2. Movement of Procedures From New Technology APCs to Clinical APCs
D. OPPS APC–Specific Policies
1. Cardiovascular Services
a. Cardiovascular Telemetry (APC 0209)
b. Implantable Loop Recorder Monitoring (APC 0689)
c. Transluminal Balloon Angioplasty (APC 0279)
2. Gastrointestinal Services
a. Change of Gastrostomy Tube (APC 0676)
b. Laparoscopic Liver Cryoablation (APC 0131)
c. Cholangioscopy (APC 0151)
d. Laparoscopic Hernia Repair (APC 0131)
3. Genitourinary Services
a. Percutaneous Renal Cryoablation (APC 0423)
b. Hemodialysis (APC 0170)
c. Radiofrequency Remodeling of Bladder Neck (APC 0165)
d. Change of Bladder Tube (APC 0121)
4. Nervous System Services
a. Pain-Related Procedures (APCs 0203, 0204, 0206, 0207, 0221, 0224, and 0388)
b. Magnetoencephalography (APCs 0065 and 0067)
5. Ocular Services
a. Insertion of Anterior Segment Aqueous Drainage Device (APC 0234)
b. Backbench Preparation of Corneal Allograft
6. Orthopedic and Musculoskeletal Services
a. Arthroscopic Procedures (APCs 0041 and 0042)
b. Knee Arthroscopy (APCs 0041 and 0042)
c. Shoulder Arthroscopy (APC 0042)
d. Fasciotomy Procedures (APC 0049)
e. Fibula Repair (APC 0062)
f. Forearm Orthopedic Procedures (APCs 0050, 0051, and 0052)
g. Low Energy Extracorporeal Shock Wave Therapy (Low Energy ESWT)
h. Insertion of Posterior Spinous Process Distraction Device (APC 0052)
7. Radiation Therapy Services
a. Proton Beam Therapy (APCs 0664 and 0667)
b. Stereotactic Radiosurgery (SRS) Treatment Delivery Services (APCs 0065, 0066, 0067, and 0127)
c. Clinical Brachytherapy (APCs 0312 and 0651)
8. Other Services
a. Low Frequency, Non-Contact, Non-Thermal Ultrasound (APC 0013)
b. Skin Repair (APCs 0134 and 0135)
c. Group Psychotherapy (APC 0325)
d. Portable X–Ray Services
e. Home Sleep Study Tests (APC 0213)
IV. OPPS Payment for Devices
A. Pass-Through Payments for Devices
1. Expiration of Transitional Pass-Through Payments for Certain Devices
2. Provisions for Reducing Transitional Pass-Through Payments To Offset Costs Packaged Into APC Groups
a. Background
b. Final Policy
B. Adjustment to OPPS Payment for No Cost/Full Credit and Partial Credit Devices
1. Background
2. APCs and Devices Subject to the Adjustment Policy
V. OPPS Payment Changes for Drugs, Biologicals, and Radiopharmaceuticals
A. OPPS Transitional Pass-Through Payment for Additional Costs of Drugs, Biologicals, and Radiopharmaceuticals
1. Background
2. Drugs and Biologicals With Expiring Pass-Through Status in CY 2009
3. Drugs, Biologicals, and Radiopharmaceuticals With New or Continuing Pass-Through Status in CY 2010
4. Pass-Through Payments for Implantable Biologicals
a. Background
b. Policy for CY 2010
5. Definition of Pass-Through Payment Eligibility Period for New Drugs and Biologicals
6. Provision for Reducing Transitional Pass-Through Payments for Diagnostic Start Printed Page 60319 Radiopharmaceuticals and Contrast Agents To Offset Costs Packaged Into APC Groups
a. Background
b. Payment Offset Policy for Diagnostic Radiopharmaceuticals
c. Payment Offset Policy for Contrast Agents
B. OPPS Payment for Drugs, Biologicals, and Radiopharmaceuticals Without Pass-Through Status
1. Background
2. Criteria for Packaging Payment for Drugs, Biologicals, and Radiopharmaceuticals
a. Background
b. Cost Threshold for Packaging of Payment for HCPCS Codes That Describe Certain Drugs, Nonimplantable Biologicals, and Therapeutic Radiopharmaceuticals (“Threshold-Packaged Drugs”)
c. Packaging Determination for HCPCS Codes That Describe the Same Drug or Biological But Different Dosages
d. Packaging of Payment for Diagnostic Radiopharmaceuticals, Contrast Agents, and Implantable Biologicals (“Policy-Packaged” Drugs and Devices)
3. Payment for Drugs and Biologicals Without Pass-Through Status That Are Not Packaged
a. Payment for Specified Covered Outpatient Drugs (SCODs) and Other Separately Payable and Packaged Drugs and Biologicals
b. Payment Policy
4. Payment for Blood Clotting Factors
5. Payment for Therapeutic Radiopharmaceuticals
a. Background
b. Payment Policy
6. Payment for Nonpass-Through Drugs, Biologicals, and Radiopharmaceuticals With HCPCS Codes, But Without OPPS Hospital Claims Data
VI. Estimate of OPPS Transitional Pass-Through Spending for Drugs, Biologicals, Radiopharmaceuticals, and Devices
A. Background
B. Estimate of Pass-Through Spending
VII. OPPS Payment for Brachytherapy Sources
A. Background
B. OPPS Payment Policy
VIII. OPPS Payment for Drug Administration Services
A. Background
B. Coding and Payment for Drug Administration Services
IX. OPPS Payment for Hospital Outpatient Visits
A. Background
B. Policies for Hospital Outpatient Visits
1. Clinic Visits: New and Established Patient Visits
2. Emergency Department Visits
3. Visit Reporting Guidelines
X. Payment for Partial Hospitalization Services
A. Background
B. PHP APC Update for CY 2010
C. Separate Threshold for Outlier Payments to CMHCs
XI. Procedures That Will Be Paid Only as Inpatient Procedures
A. Background
B. Changes to the Inpatient List
XII. OPPS Nonrecurring Technical and Policy Changes and Clarifications
A. Kidney Disease Education Services
1. Background
2. Payment for Services Furnished by Providers of Services Located in a Rural Area
B. Pulmonary Rehabilitation, Cardiac Rehabilitation, and Intensive Cardiac Rehabilitation Services
1. Legislative Changes
2. Payment for Services Furnished to Hospital Outpatients in a Pulmonary Rehabilitation Program
3. Payment for Services Furnished to Hospital Outpatients Under a Cardiac Rehabilitation or an Intensive Cardiac Rehabilitation Program
4. Physician Supervision for Pulmonary Rehabilitation, Cardiac Rehabilitation, and Intensive Cardiac Rehabilitation Services
C. Stem Cell Transplants
D. Physician Supervision
1. Background
2. Issues Regarding the Physician Supervision of Hospital Outpatient Services Raised by Hospitals and Other Stakeholders
3. Policies for Direct Supervision of Hospital and CAH Outpatient Therapeutic Services
4. Policies for Direct Supervision of Hospital and CAH Outpatient Diagnostic Services
5. Summary of CY 2010 Physician Supervision Final Policies
E. Direct Referral for Observation Services
XIII. OPPS Payment Status and Comment Indicators
A. OPPS Payment Status Indicator Definitions
1. Payment Status Indicators To Designate Services That Are Paid Under the OPPS
2. Payment Status Indicators To Designate Services That Are Paid Under a Payment System Other Than the OPPS
3. Payment Status Indicators To Designate Services That Are Not Recognized Under the OPPS But That May Be Recognized by Other Institutional Providers
4. Payment Status Indicators To Designate Services That Are Not Payable by Medicare on Outpatient Claims
B. Comment Indicator Definitions
XIV. OPPS Policy and Payment Recommendations
A. MedPAC Recommendations
B. APC Panel Recommendations
C. OIG Recommendations
XV. Updates to the Ambulatory Surgical Center (ASC) Payment System
A. Background
1. Legislative Authority for the ASC Payment System
2. Prior Rulemaking
3. Policies Governing Changes to the Lists of Codes and Payment Rates for ASC Covered Surgical Procedures and Covered Ancillary Services
B. Treatment of New Codes
1. Treatment of New Category I and III CPT Codes and Level II HCPCS Codes
2. Treatment of New Level II HCPCS Codes Implemented in April and July 2009
C. Update to the List of ASC Covered Surgical Procedures and Covered Ancillary Services
1. Covered Surgical Procedures
a. Additions to the List of ASC Covered Surgical Procedures
b. Covered Surgical Procedures Designated as Office-Based
(1) Background
(2) Changes to Covered Surgical Procedures Designated as Office-Based for CY 2010
c. ASC Covered Surgical Procedures Designated as Device-Intensive
(1) Background
(2) Changes to List of Covered Surgical Procedures Designated as Device-Intensive for CY 2010
d. ASC Treatment of Surgical Procedures Removed From the OPPS Inpatient List for CY 2010
2. Covered Ancillary Services
D. ASC Payment for Covered Surgical Procedures and Covered Ancillary Services
1. Payment for Covered Surgical Procedures
a. Background
b. Update to ASC Covered Surgical Procedure Payment Rates for CY 2010
c. Adjustment to ASC Payments for No Cost/Full Credit and Partial Credit Devices
2. Payment for Covered Ancillary Services
a. Background
b. Payment for Covered Ancillary Services for CY 2010
E. New Technology Intraocular Lenses (NTIOLs)
1. Background
2. NTIOL Application Process for Payment Adjustment
3. Classes of NTIOLs Approved and New Requests for Payment Adjustment
a. Background
b. Request To Establish New NTIOL Class for CY 2010 and Deadline for Public Comment
4. Payment Adjustment
5. ASC Payment for Insertion of IOLs
6. Announcement of CY 2010 Deadline for Submitting Requests for CMS Review of Appropriateness of ASC Payment for Insertion of an NTIOL Following Cataract Surgery
F. ASC Payment and Comment Indicators
1. Background
2. ASC Payment and Comment Indicators
G. ASC Policy and Payment Recommendations
H. Revision to Terms of Agreements for Hospital-Operated ASCs
1. Background
2. Changes to the Terms of Agreements for ASCs Operated by Hospitals
I. Calculation of the ASC Conversion Factor and ASC Payment Rates
1. Background
2. Calculation of the ASC Payment Rates
a. Updating the ASC Relative Payment Weights for CY 2010 and Future Years
b. Updating the ASC Conversion Factor
3. Display of ASC Payment Rates
XVI. Reporting Quality Data for Annual Payment Rate Updates
A. Background
1. Overview Start Printed Page 60320
2. Hospital Outpatient Quality Data Reporting Under Section 109(a) of Public Law 109–432
3. Reporting ASC Quality Data for Annual Payment Update
4. HOPQDRP Quality Measures for the CY 2009 Payment Determination
5. HOP QDRP Quality Measures for the CY 2010 Payment Determination
a. Background
b. Maintenance of Technical Specifications for Quality Measures
c. Publication of HOP QDRP Data
B. Quality Measures for the CY 2011 Payment Determination
1. Considerations in Expanding and Updating Quality Measures Under the HOP QDRP Program
2. Retirement of HOP QDRP Quality Measures
3. HOP QDRP Quality Measures for the CY 2011 Payment Determination
C. Possible Quality Measures Under Consideration for CY 2012 and Subsequent Years
D. Payment Reduction for Hospitals That Fail To Meet the HOP QDRP Requirements for the CY 2010 Payment Update
1. Background
2. Reporting Ratio Application and Associated Adjustment Policy for CY 2010
E. Requirements for HOPD Quality Data Reporting for CY 2011 and Subsequent Years
1. Administrative Requirements
2. Data Collection and Submission Requirements
a. General Data Collection and Submission Requirements
b. Extraordinary Circumstance Extension or Waiver for Reporting Quality Data
3. HOP QDRP Validation Requirements
a. Data Validation Requirements for CY 2011
b. Data Validation Approach for CY 2012 and Subsequent Years
c. Additional Data Validation Conditions Under Consideration for CY 2012 and Subsequent Years
F. 2010 Publication of HOP QDRP Data
G. HOP QDRP Reconsideration and Appeals Procedures
H. Reporting of ASC Quality Data
I. Electronic Health Records
XVII. Healthcare-Associated Conditions
A. Background
1. Preventable Medical Errors and Hospital-Acquired Conditions (HACs) Under the IPPS
2. Expanding the Principles of the IPPS HACs Payment Provision to the OPPS
3. Discussion in the CY 2009 OPPS/ASC Final Rule With Comment Period
B. Public Comments and Recommendations on Issues Regarding Healthcare-Associated Conditions From the Joint IPPS/OPPS Listening Session
C. CY 2010 Approach to Healthcare-Associated Conditions Under the OPPS
XVIII. Files Available to the Public via the Internet
A. Information in Addenda Related to the CY 2010 Hospital OPPS
B. Information in Addenda Related to the CY 2010 ASC Payment System
XIX. Collection of Information Requirements
A. Legislative Requirements for Solicitation of Comments
B. Associated Information Collections Not Specified in Regulatory Text
1. Hospital Outpatient Quality Data Reporting Program (HOP QDRP)
2. HOP QDRP Quality Measures for the CY 2010 and CY 2011 Payment Determinations
3. HOP QDRP Validation Requirements
4. HOP QDRP Reconsideration and Appeals Procedures
5. Additional Topics
XX. Response to Comments
XXI. Regulatory Impact Analysis
A. Overall Impact
1. Executive Order 12866
2. Regulatory Flexibility Act (RFA)
3. Small Rural Hospitals
4. Unfunded Mandates
5. Federalism
B. Effects of OPPS Changes in This Final Rule With Comment Period
1. Alternatives Considered
2. Limitations of Our Analysis
3. Estimated Effects of This Final Rule With Comment Period on Hospitals
4. Estimated Effects of This Final Rule With Comment Period on CMHCs
5. Estimated Effects of This Final Rule With Comment Period on Beneficiaries
6. Conclusion
7. Accounting Statement
C. Effects of ASC Payment System Changes in This Final Rule With Comment Period
1. Alternatives Considered
2. Limitations of Our Analysis
3. Estimated Effects of This Final Rule With Comment Period on Payments to ASCs
4. Estimated Effects of This Final Rule With Comment Period on Beneficiaries
5. Conclusion
6. Accounting Statement
D. Effects of Requirements for Reporting of Quality Data for Annual Hospital Payment Update
E. Executive Order 12866
Regulation Text
Addenda
Addendum A—Final OPPS APCs for CY 2010
Addendum AA—Final ASC Covered Surgical Procedures for CY 2010 (Including Surgical Procedures for Which Payment Is Packaged)
Addendum B—Final OPPS Payment by HCPCS Code for CY 2010
Addendum BB—Final ASC Covered Ancillary Services Integral to Covered Surgical Procedures for CY 2010 (Including Ancillary Services for Which Payment Is Packaged)
Addendum D1—Final OPPS Payment Status Indicators for CY 2010
Addendum DD1—Final ASC Payment Indicators for CY 2010
Addendum D2—Final OPPS Comment Indicators for CY 2010
Addendum DD2—Final ASC Comment Indicators for CY 2010
Addendum E— HCPCS Codes That Are Paid as Inpatient Procedures for CY 2010
Addendum L–CY 2010 OPPS Out-Migration Adjustment
Addendum M—HCPCS Codes for Assignment to Composite APCs for CY 2010
I. Background and Summary of the CY 2010 OPPS/ASC Final Rule With Comment Period
A. Legislative and Regulatory Authority for the Hospital Outpatient Prospective Payment System
When Title XVIII of the Social Security Act (the Act) was enacted, Medicare payment for hospital outpatient services was based on hospital-specific costs. In an effort to ensure that Medicare and its beneficiaries pay appropriately for services and to encourage more efficient delivery of care, the Congress mandated replacement of the reasonable cost-based payment methodology with a prospective payment system (PPS). The Balanced Budget Act (BBA) of 1997 (Pub. L. 105–33) added section 1833(t) to the Act authorizing implementation of a PPS for hospital outpatient services. The OPPS was first implemented for services furnished on or after August 1, 2000. Implementing regulations for the OPPS are located at 42 CFR part 419.
The Medicare, Medicaid, and SCHIP Balanced Budget Refinement Act (BBRA) of 1999 (Pub. L. 106–113) made major changes in the hospital outpatient prospective payment system (OPPS). The following Acts made additional changes to the OPPS: the Medicare, Medicaid, and SCHIP Benefits Improvement and Protection Act (BIPA) of 2000 (Pub. L. 106–554); the Medicare Prescription Drug, Improvement, and Modernization Act (MMA) of 2003 (Pub. L. 108–173); the Deficit Reduction Act (DRA) of 2005 (Pub. L. 109–171), enacted on February 8, 2006; the Medicare Improvements and Extension Act under Division B of Title I of the Tax Relief and Health Care Act (MIEA–TRHCA) of 2006 (Pub. L. 109–432), enacted on December 20, 2006; the Medicare, Medicaid, and SCHIP Extension Act (MMSEA) of 2007 (Pub. L. 110–173), enacted on December 29, 2007; and the Medicare Improvements for Patients and Providers Act (MIPPA) of 2008 (Pub. L. 110–275), enacted on July 15, 2008.
Under the OPPS, we pay for hospital outpatient services on a rate-per-service basis that varies according to the ambulatory payment classification (APC) group to which the service is assigned. We use the Healthcare Common Procedure Coding System (HCPCS) codes (which include certain Current Procedural Terminology (CPT) codes) and descriptors to identify and Start Printed Page 60321 group the services within each APC group. The OPPS includes payment for most hospital outpatient services, except those identified in section I.B. of this final rule with comment period. Section 1833(t)(1)(B)(ii) of the Act provides for payment under the OPPS for hospital outpatient services designated by the Secretary (which includes partial hospitalization services furnished by community mental health centers (CMHCs)) and hospital outpatient services that are furnished to inpatients who have exhausted their Part A benefits, or who are otherwise not in a covered Part A stay. Section 611 of Public Law 108–173 added provisions for Medicare coverage for an initial preventive physical examination, subject to the applicable deductible and coinsurance, as an outpatient department service, payable under the OPPS.
The OPPS rate is an unadjusted national payment amount that includes the Medicare payment and the beneficiary copayment. This rate is divided into a labor-related amount and a nonlabor-related amount. The labor-related amount is adjusted for area wage differences using the hospital inpatient wage index value for the locality in which the hospital or CMHC is located.
All services and items within an APC group are comparable clinically and with respect to resource use (section 1833(t)(2)(B) of the Act). In accordance with section 1833(t)(2) of the Act, subject to certain exceptions, services and items within an APC group cannot be considered comparable with respect to the use of resources if the highest median (or mean cost, if elected by the Secretary) for an item or service in the APC group is more than 2 times greater than the lowest median cost for an item or service within the same APC group (referred to as the “2 times rule”). In implementing this provision, we generally use the median cost of the item or service assigned to an APC group.
For new technology items and services, special payments under the OPPS may be made in one of two ways. Section 1833(t)(6) of the Act provides for temporary additional payments, which we refer to as “transitional pass-through payments,” for at least 2 but not more than 3 years for certain drugs, biological agents, brachytherapy devices used for the treatment of cancer, and categories of other medical devices. For new technology services that are not eligible for transitional pass-through payments, and for which we lack sufficient data to appropriately assign them to a clinical APC group, we have established special APC groups based on costs, which we refer to as New Technology APCs. These New Technology APCs are designated by cost bands which allow us to provide appropriate and consistent payment for designated new procedures that are not yet reflected in our claims data. Similar to pass-through payments, an assignment to a New Technology APC is temporary; that is, we retain a service within a New Technology APC until we acquire sufficient data to assign it to a clinically appropriate APC group.
B. Excluded OPPS Services and Hospitals
Section 1833(t)(1)(B)(i) of the Act authorizes the Secretary to designate the hospital outpatient services that are paid under the OPPS. While most hospital outpatient services are payable under the OPPS, section 1833(t)(1)(B)(iv) of the Act excludes payment for ambulance, physical and occupational therapy, and speech-language pathology services, for which payment is made under a fee schedule. Section 614 of Public Law 108–173 amended section 1833(t)(1)(B)(iv) of the Act to exclude payment for screening and diagnostic mammography services from the OPPS. The Secretary exercised the authority granted under the statute to also exclude from the OPPS those services that are paid under fee schedules or other payment systems. Such excluded services include, for example, the professional services of physicians and nonphysician practitioners paid under the Medicare Physician Fee Schedule (MPFS); laboratory services paid under the clinical diagnostic laboratory fee schedule (CLFS); services for beneficiaries with end-stage renal disease (ESRD) that are paid under the ESRD composite rate; and services and procedures that require an inpatient stay that are paid under the hospital inpatient prospective payment system (IPPS). We set forth the services that are excluded from payment under the OPPS in § 419.22 of the regulations.
Under § 419.20(b) of the regulations, we specify the types of hospitals and entities that are excluded from payment under the OPPS. These excluded entities include: Maryland hospitals, but only for services that are paid under a cost containment waiver in accordance with section 1814(b)(3) of the Act; critical access hospitals (CAHs); hospitals located outside of the 50 States, the District of Columbia, and Puerto Rico; and Indian Health Service hospitals.
C. Prior Rulemaking
On April 7, 2000, we published in the Federal Register a final rule with comment period (65 FR 18434) to implement a prospective payment system for hospital outpatient services. The hospital OPPS was first implemented for services furnished on or after August 1, 2000. Section 1833(t)(9) of the Act requires the Secretary to review certain components of the OPPS, not less often than annually, and to revise the groups, relative payment weights, and other adjustments that take into account changes in medical practices, changes in technologies, and the addition of new services, new cost data, and other relevant information and factors.
Since initially implementing the OPPS, we have published final rules in the Federal Register annually to implement statutory requirements and changes arising from our continuing experience with this system. These rules can be viewed on the CMS Web site at: http://www.cms.hhs.gov/HospitalOutpatientPPS/. We published in the Federal Register on November 18, 2008 the CY 2009 OPPS/ASC final rule with comment period (73 FR 68502). In that final rule with comment period, we revised the OPPS to update the payment weights and conversion factor for services payable under the CY 2009 OPPS on the basis of claims data from January 1, 2007, through December 31, 2007, and to implement certain provisions of Public Law 110–173 and Public Law 110–275. In addition, we responded to public comments received on the provisions of the November 27, 2007 final rule with comment period (72 FR 66580) pertaining to the APC assignment of HCPCS codes identified in Addendum B to that rule with the new interim (“NI”) comment indicator, and public comments received on the July 18, 2008 OPPS/ASC proposed rule for CY 2009 (73 FR 41416).
Subsequent to publication of the CY 2009 OPPS/ASC final rule with comment period, we published in the Federal Register on January 26, 2009, a correction notice (74 FR 4343 through 4344) to correct certain technical errors in the CY 2009 OPPS/ASC final rule with comment period.
On July 20, 2009, we issued in the Federal Register (74 FR 35232) a proposed rule for the CY 2010 OPPS/ASC payment system to implement statutory requirements and changes arising from our continuing experience with both systems. Start Printed Page 60322
D. Advisory Panel on Ambulatory Payment Classification (APC) Groups
1. Authority of the APC Panel
Section 1833(t)(9)(A) of the Act, as amended by section 201(h) of Public Law 106–113, and redesignated by section 202(a)(2) of Public Law 106–113, requires that we consult with an outside panel of experts to review the clinical integrity of the payment groups and their weights under the OPPS. The Act further specifies that the panel will act in an advisory capacity. The Advisory Panel on Ambulatory Payment Classification (APC) Groups (the APC Panel), discussed under section I.D.2. of this final rule with comment period, fulfills these requirements. The APC Panel is not restricted to using data compiled by CMS, and it may use data collected or developed by organizations outside the Department in conducting its review.
2. Establishment of the APC Panel
On November 21, 2000, the Secretary signed the initial charter establishing the APC Panel. This expert panel, which may be composed of up to 15 representatives of providers (currently employed full-time, not as consultants, in their respective areas of expertise) subject to the OPPS, reviews clinical data and advises CMS about the clinical integrity of the APC groups and their payment weights. The APC Panel is technical in nature, and it is governed by the provisions of the Federal Advisory Committee Act (FACA). Since its initial chartering, the Secretary has renewed the APC Panel's charter four times: on November 1, 2002; on November 1, 2004; on November 21, 2006; and on November 2, 2008. The current charter specifies, among other requirements, that: the APC Panel continues to be technical in nature; is governed by the provisions of the FACA; may convene up to three meetings per year; has a Designated Federal Officer (DFO); and is chaired by a Federal official designated by the Secretary.
The current APC Panel membership and other information pertaining to the APC Panel, including its charter, Federal Register notices, membership, meeting dates, agenda topics, and meeting reports, can be viewed on the CMS Web site at: http://www.cms.hhs.gov/FACA/05_AdvisoryPanelonAmbulatoryPaymentClassificationGroups.asp#TopOfPage.
3. APC Panel Meetings and Organizational Structure
The APC Panel first met on February 27 through March 1, 2001. Since the initial meeting, the APC Panel has held 16 meetings, with the last meeting taking place on August 5 and 6, 2009. Prior to each meeting, we publish a notice in the Federal Register to announce the meeting and, when necessary, to solicit nominations for APC Panel membership and to announce new members.
The APC Panel has established an operational structure that, in part, includes the use of three subcommittees to facilitate its required APC review process. The three current subcommittees are the Data Subcommittee, the Visits and Observation Subcommittee, and the Packaging Subcommittee. The Data Subcommittee is responsible for studying the data issues confronting the APC Panel and for recommending options for resolving them. The Visits and Observation Subcommittee reviews and makes recommendations to the APC Panel on all technical issues pertaining to observation services and hospital outpatient visits paid under the OPPS (for example, APC configurations and APC payment weights). The Packaging Subcommittee studies and makes recommendations on issues pertaining to services that are not separately payable under the OPPS, but whose payments are bundled or packaged into APC payments. Each of these subcommittees was established by a majority vote from the full APC Panel during a scheduled APC Panel meeting, and their continuation as subcommittees was last approved at the August 2009 APC Panel meeting. At that meeting, the APC Panel recommended that the work of these three subcommittees continue, and we accept those recommendations of the APC Panel. All subcommittee recommendations are discussed and voted upon by the full APC Panel.
Discussions of the other recommendations made by the APC Panel at the August 2009 meeting are included in the sections of this final rule with comment period that are specific to each recommendation. For discussions of earlier APC Panel meetings and recommendations, we refer readers to previously published hospital OPPS/ASC proposed and final rules, the CMS Web site mentioned earlier in this section, and the FACA database at: http://fido.gov/facadatabase/public.asp.
Comment: Several commenters requested that CMS include ASC representation on the APC Panel. Because the revised ASC payment system is based upon the same APC groups and relative payment weights as the OPPS, the commenters believed that ASC representation on the APC Panel would ensure input from representatives of all care settings that provide surgical services whose payment groups and payment weights are affected by the OPPS. Further, the commenters urged CMS to revise the APC Panel's charter to reflect the current alignment of the OPPS and the revised ASC payment system by including representation from the ASC industry on the APC Panel, as the commenters believed is permitted by the statute.
Response: We acknowledge that the revised ASC payment system provides Medicare payments to ASCs for surgical procedures that are based, in most cases, on the relative payment weights of the OPPS. However, CMS is statutorily required to have an appropriate selection of representatives of “providers” as members of the APC Panel. The current APC Panel charter requires that “Each Panel member must be employed full-time by a hospital, hospital system, or other Medicare provider subject to payment under the OPPS,” which does not include ASCs because ASCs are not providers. We refer readers to section 1833(t)(9)(A) of the Act and § 400.202 of our regulations for specific requirements and definitions. ASCs are suppliers, not providers. The charter must comply with the statute, which does not include representatives of suppliers on the APC Panel. Therefore, although we understand the concerns of the commenters regarding ASC input on the APC Panel now that the ASC payment system is based on the OPPS relative payment weights, we cannot revise the charter to include ASC representation.
E. Background and Summary of the CY 2010 OPPS/ASC Proposed Rule
A proposed rule appeared in the July 20, 2009 Federal Register (74 FR 35232) that set forth proposed changes to the Medicare hospital OPPS for CY 2010 to implement statutory requirements and changes arising from our continuing experience with the system. In addition, we set forth proposed changes to the revised Medicare ASC payment system for CY 2010, including updated payment weights, covered surgical procedures, and covered ancillary items and services based on the proposed OPPS update. Finally, we set forth proposed quality measures for the Hospital Outpatient Quality Data Reporting Program (HOP QDRP) for reporting quality data for annual payment rate updates for CY 2011 and subsequent calendar years, the requirements for data collection and submission for the annual payment Start Printed Page 60323 update, and a proposed reduction in the OPPS payment for hospitals that fail to meet the HOP QDRP requirements for the CY 2010 payment update, in accordance with the statutory requirement. The following is a summary of the major proposed changes included in the CY 2010 OPPS/ASC proposed rule:
1. Updates Affecting OPPS Payments
In section II. of the proposed rule, we set forth—
- The methodology used to recalibrate the APC relative payment weights.
- The proposed changes to packaged services.
- The proposed update to the conversion factor used to determine payment rates under the OPPS. In this section, we set forth proposed changes in the amounts and factors for calculating the full annual update increase to the conversion factor.
- The proposed retention of our current policy to use the IPPS wage indices to adjust, for geographic wage differences, the portion of the OPPS payment rate and the copayment standardized amount attributable to labor-related cost.
- The proposed update of statewide average default CCRs.
- The proposed application of hold harmless transitional outpatient payments (TOPs) for certain small rural hospitals.
- The proposed payment adjustment for rural SCHs.
- The proposed calculation of the hospital outpatient outlier payment.
- The calculation of the proposed national unadjusted Medicare OPPS payment.
- The proposed beneficiary copayments for OPPS services.
2. OPPS Ambulatory Payment Classification (APC) Group Policies
In section III. of the proposed rule, we discussed—
- The proposed additions of new HCPCS codes to APCs.
- The proposed establishment of a number of new APCs.
- Our analyses of Medicare claims data and certain recommendations of the APC Panel.
- The application of the 2 times rule and proposed exceptions to it.
- The proposed changes to specific APCs.
- The proposed movement of procedures from New Technology APCs to clinical APCs.
3. OPPS Payment for Devices
In section IV. of the proposed rule, we discussed the proposed pass-through payment for specific categories of devices and the proposed adjustment for devices furnished at no cost or with partial or full credit.
4. OPPS Payment Changes for Drugs, Biologicals, and Radiopharmaceuticals
In section V. of the proposed rule, we discussed the proposed CY 2010 OPPS payment for drugs, biologicals, and radiopharmaceuticals, including the proposed payment for drugs, biologicals, and radiopharmaceuticals with and without pass-through status.
5. Estimate of OPPS Transitional Pass-Through Spending for Drugs, Biologicals, Radiopharmaceuticals, and Devices
In section VI. of the proposed rule, we discussed the estimate of CY 2010 OPPS transitional pass-through spending for drugs, biologicals, and devices.
6. OPPS Payment for Brachytherapy Sources
In section VII. of the proposed rule, we discussed payment for brachytherapy sources.
7. OPPS Payment for Drug Administration Services
In section VIII. of the proposed rule, we set forth our proposed policy concerning coding and payment for drug administration services.
8. OPPS Payment for Hospital Outpatient Visits
In section IX. of the proposed rule, we set forth our proposed policies for the payment of clinic and emergency department visits and critical care services based on claims data.
9. Payment for Partial Hospitalization Services
In section X. of the proposed rule, we set forth the proposed payment for partial hospitalization services, including the proposed separate threshold for outlier payments for CMHCs.
10. Procedures That Will Be Paid Only as Inpatient Procedures
In section XI. of the proposed rule, we discussed the procedures that we proposed to remove from the inpatient list and assign to APCs for payment under the OPPS.
11. OPPS Nonrecurring Technical and Policy Changes and Clarifications
In section XII. of the proposed rule, we discussed nonrecurring technical issues, proposed policy changes, and provided policy clarifications.
12. OPPS Payment Status and Comment Indicators
In section XIII. of the proposed rule, we discussed our proposed changes to the definitions of status indicators assigned to APCs and presented our proposed comment indicators for the final rule with comment period.
13. OPPS Policy and Payment Recommendations
In section XIV. of the proposed rule, we addressed recommendations made by the Medicare Payment Advisory Commission (MedPAC) in its March 2009 report to Congress, by the Office of Inspector General (OIG), and by the APC Panel regarding the OPPS for CY 2010.
14. Updates to the Ambulatory Surgical Center (ASC) Payment System
In section XV. of the proposed rule, we discussed the proposed updates of the revised ASC payment system and payment rates for CY 2010.
15. Reporting Quality Data for Annual Payment Rate Updates
In section XVI. of the proposed rule, we discussed the proposed quality measures for reporting hospital outpatient (HOP) quality data for the annual payment update factor for CY 2011 and subsequent calendar years; set forth the requirements for data collection and submission for the annual payment update; and discussed the reduction in the OPPS payment for hospitals that fail to meet the HOP Quality Data Reporting Program (QDRP) requirements for CY 2010.
16. Healthcare-Associated Conditions
In section XVII. of the proposed rule, we discussed public responses to a December 2008 CMS public listening session addressing the potential extension of the principle of Medicare not paying more under the IPPS for the care of preventable hospital-acquired conditions experienced by a Medicare beneficiary during a hospital inpatient stay to medical care in other settings that are paid under other Medicare payment systems, including the OPPS, for those healthcare-associated conditions that occur or result from care in those other settings.
17. Regulatory Impact Analysis
In section XXI. of the proposed rule, we set forth an analysis of the impact the proposed changes would have on affected entities and beneficiaries. Start Printed Page 60324
F. Public Comments Received in Response to the CY 2010 OPPS/ASC Proposed Rule
We received approximately 1,527 timely pieces of correspondence containing multiple comments on the CY 2010 OPPS/ASC proposed rule. We note that we received some public comments that were outside of the scope of the CY 2010 OPPS/ASC proposed rule. These out-of-scope public comments are not addressed in this final rule with comment period.
New (and substantially revised) CY 2010 HCPCS codes are designated with comment indicator “NI” in Addenda B, AA, and BB of this final rule with comment period to signify that their CY 2010 interim OPPS and/or ASC treatment are open to public comment on this final rule with comment period. Summaries of the public comments that are within the scope of the CY 2010 proposals and our responses to those comments are set forth in the various sections of this final rule with comment period under the appropriate headings.
G. Public Comments Received in Response to the November 18, 2008 OPPS/ASC Final Rule With Comment Period
We received approximately 41 timely pieces of correspondence on the CY 2009 OPPS/ASC final rule with comment period, some of which contained multiple comments on the interim APC assignments and/or status indicators of HCPCS codes identified with comment indicator “NI” in Addendum B of that final rule with comment period. Summaries of those public comments on topics open to comment in the CY 2009 OPPS/ASC final rule with comment period and our responses to them are set forth in the various sections of this final rule with comment period under the appropriate headings.
II. Updates Affecting OPPS Payments
A. Recalibration of APC Relative Weights
1. Database Construction
a. Database Source and Methodology
Section 1833(t)(9)(A) of the Act requires that the Secretary review and revise the relative payment weights for APCs at least annually. In the April 7, 2000 OPPS final rule with comment period (65 FR 18482), we explained in detail how we calculated the relative payment weights that were implemented on August 1, 2000 for each APC group.
For CY 2010, we proposed to use the same basic methodology that we described in the April 7, 2000 OPPS final rule with comment period to recalibrate the APC relative payment weights for services furnished on or after January 1, 2010, and before January 1, 2011 (CY 2010). That is, we proposed to recalibrate the relative payment weights for each APC based on claims and cost report data for hospital outpatient department (HOPD) services. We proposed to use the most recent available data to construct the database for calculating APC group weights. Therefore, for the purpose of recalibrating the APC relative payment weights for CY 2010, we used approximately 141 million final action claims for hospital outpatient department services furnished on or after January 1, 2008, and before January 1, 2009. (For exact counts of claims used, we refer readers to the claims accounting narrative under supporting documentation for this final rule with comment period on the CMS Web site at: http://www.cms.hhs.gov/HospitalOutpatientPPS/HORD/.)
Of the 141 million final action claims for services provided in hospital outpatient settings used to calculate the CY 2010 OPPS payment rates for this final rule with comment period, approximately 107 million claims were the type of bill potentially appropriate for use in setting rates for OPPS services (but did not necessarily contain services payable under the OPPS). Of the 107 million claims, approximately 50 million claims were not for services paid under the OPPS or were excluded as not appropriate for use (for example, erroneous cost-to-charge ratios (CCRs) or no HCPCS codes reported on the claim). From the remaining 58 million claims, we created approximately 99 million single records, of which approximately 68 million were “pseudo” single or “single session” claims (created from 26 million multiple procedure claims using the process we discuss later in this section). Approximately 657,000 claims were trimmed out on cost or units in excess of +/−3 standard deviations from the geometric mean, yielding approximately 99 million single bills for median setting. As described in section II.A.2. of this final rule with comment period, our data development process is designed with the goal of using appropriate cost information in setting the APC relative weights. The bypass process is described in section II.A.1.b. of this final rule with comment period. This section discusses how we develop “pseudo” single claims, with the intention of using more appropriate data from the available claims. In some cases, the bypass process allows us to use some portion of the submitted claim for cost estimation purposes, while the remaining information on the claim continues to be unusable. Consistent with the goal of using appropriate information in our data development process, we only use claims (or portions of each claim) that are appropriate for ratesetting purposes. Ultimately, we were able to use for CY 2010 ratesetting some portion of 95 percent of the CY 2008 claims containing services payable under the OPPS.
As proposed, the APC relative weights and payments for CY 2010 in Addenda A and B to this final rule with comment period were calculated using claims from CY 2008 that were processed before January 1, 2009 and continue to be based on the median hospital costs for services in the APC groups. We selected claims for services paid under the OPPS and matched these claims to the most recent cost report filed by the individual hospitals represented in our claims data. We continue to believe that it is appropriate to use the most current full calendar year claims data and the most recently submitted cost reports to calculate the median costs underpinning the APC relative payment weights and the CY 2010 payment rates.
We did not receive any public comments on our proposal to base the CY 2010 APC relative weights on the most currently available cost reports and on claims for services furnished in CY 2008. Therefore, for the reasons noted above in this section, we are finalizing our data source for the recalibration of the CY 2010 APC relative payment weights as proposed, without modification, as described in this section of this final rule with comment period.
b. Use of Single and Multiple Procedure Claims
For CY 2010, in general, we proposed to continue to use single procedure claims to set the medians on which the APC relative payment weights would be based, with some exceptions as discussed below in this section. We generally use single procedure claims to set the median costs for APCs because we believe that the OPPS relative weights on which payment rates are based should be derived from the costs of furnishing one procedure and because, in many circumstances, we are unable to ensure that packaged costs can be appropriately allocated across multiple procedures performed on the same date of service.
We agree that, optimally, it is desirable to use the data from as many claims as possible to recalibrate the APC relative payment weights, including Start Printed Page 60325 those claims for multiple procedures. As we have for several years, we continued to use date of service stratification and a list of codes to be bypassed to convert multiple procedure claims to “pseudo” single procedure claims. Through bypassing specified codes that we believe do not have significant packaged costs, we are able to use more data from multiple procedure claims. In many cases, this enables us to create multiple “pseudo” single claims from claims that were submitted as multiple procedure claims spanning multiple dates of service, or claims that contained numerous separately paid procedures reported on the same date on one claim. We refer to these newly created single procedure claims as “pseudo” single claims. The history of our use of a bypass list to generate “pseudo” single claims is well documented, most recently in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68512 through 68519). In addition, for CY 2008, we increased packaging and created the first composite APCs. This also increased the number of bills that we were able to use for median calculation by enabling us to use claims that contained multiple major procedures that previously would not have been usable. Further, for CY 2009, we expanded the composite APC model to one additional clinical area, multiple imaging services (73 FR 68559 through 68569), which also increased the number of bills we were able to use to calculate APC median costs. We refer readers to section II.A.2.e. of this final rule with comment period for discussion of the use of claims to establish median costs for composite APCs.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35239 through 35241), we proposed to continue to apply these processes to enable us to use as much claims data as possible for ratesetting for the CY 2010 OPPS. This process enabled us to create, for this final rule with comment period, approximately 68 million “pseudo” single claims, including multiple imaging composite “single session” bills (we refer readers to section II.A.2.e.(5) of this final rule with comment period for further discussion), to add to the approximately 32 million “natural” single bills. For this final rule with comment period, “pseudo” single and “single session” procedure bills represent 68 percent of all single bills used to calculate median costs.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35239 through 35241), we proposed to bypass 438 HCPCS codes for CY 2010. Since the inception of the bypass list, we have calculated the percent of “natural” single bills that contained packaging for each HCPCS code and the amount of packaging on each “natural” single bill for each code. Each year, we generally retain the codes on the previous year's bypass list and use the update year's data (for CY 2010, data available for the February 2009 APC Panel meeting from CY 2008 claims processed through September 30, 2008 and CY 2007 claims data processed through June 30, 2008 used to model the final payment rates for CY 2009) to determine whether it would be appropriate to propose to add additional codes to the previous year's bypass list. For CY 2010, we proposed to continue to bypass all of the HCPCS codes on the CY 2009 OPPS bypass list. We also proposed to add to the bypass list for CY 2010 all HCPCS codes not on the CY 2009 bypass list that, using both CY 2009 final rule and February 2009 APC Panel data, met the same previously established empirical criteria for the bypass list that are summarized below. Because we must make some assumptions about packaging in the multiple procedure claims in order to assess a HCPCS code for addition to the bypass list, we assume that the representation of packaging on “natural” single claims for any given code is comparable to packaging for that code in the multiple claims. The proposed criteria for the bypass list were:
- There are 100 or more “natural” single claims for the code. This number of single claims ensures that observed outcomes are sufficiently representative of packaging that might occur in the multiple claims.
- Five percent or fewer of the “natural” single claims for the code have packaged costs on that single claim for the code. This criterion results in limiting the amount of packaging being redistributed to the separately payable procedures remaining on the claim after the bypass code is removed and ensures that the costs associated with the bypass code represent the cost of the bypassed service.
- The median cost of packaging observed in the “natural” single claims is equal to or less than $50. This criterion also limits the amount of error in redistributed costs. Throughout the bypass process, we do not know the dollar value of the packaged cost that should be appropriately attributed to the other procedures on the claim. Ensuring that redistributed costs associated with a bypass code are small in amount and volume protects the validity of cost estimates for low cost services billed with the bypassed service.
- The code is not a code for an unlisted service.
In addition, we proposed to continue to include on the bypass list HCPCS codes that CMS medical advisors believe have minimal associated packaging based on their clinical assessment of the complete CY 2010 OPPS proposal. Some of these codes were identified by CMS medical advisors and some were identified in prior years by commenters with specialized knowledge of the packaging associated with specific services, especially on a multiple procedure claim. We also proposed to continue to include on the bypass list certain HCPCS codes in order to purposefully direct the assignment of packaged costs to a companion code where services always appear together and where there would otherwise be few single claims available for ratesetting. For example, we have previously discussed our reasoning for adding HCPCS code G0390 (Trauma response team associated with hospital critical care service) and the CPT codes for additional hours of drug administration to the bypass list (73 FR 68513 and 71 FR 68117 through 68118).
As a result of the multiple imaging composite APCs that we established in CY 2009, we note that the program logic for creating “pseudo” singles from bypassed codes that are also members of multiple imaging composite APCs changed. When creating the set of “pseudo” single claims, claims that contain “overlap bypass codes,” that is, those HCPCS codes that are both on the bypass list and are members of the multiple imaging composite APCs, were identified first. These HCPCS codes were then processed to create multiple imaging composite “single session” bills, that is, claims containing HCPCS codes from only one imaging family, thus suppressing the initial use of these codes as bypass codes. However, these “overlap bypass codes” were retained on the bypass list because, at the end of the “pseudo” single processing logic, we reassessed the claims without suppression of the “overlap bypass codes” under our longstanding “pseudo” single process to determine whether we could convert additional claims to “pseudo” single claims. (We refer readers to section II.A.2.b. of this final rule with comment period for further discussion of the treatment of “overlap bypass codes.”) This process also created multiple imaging composite “single session” bills that could be used for calculating composite APC median costs. “Overlap bypass codes” that would be members of the proposed multiple imaging composite APCs were Start Printed Page 60326 identified by asterisks (*) in Table 1 of the CY 2010 OPPS/ASC proposed rule (74 FR 35242 through 35252).
At the February 2009 APC Panel Meeting, the APC Panel recommended that CMS place CPT code 76098 (Radiological examination, surgical specimen) on the bypass list and reassign the code to APC 0260 (Level I Plain Film Except Teeth) in response to a public presentation requesting that CMS makes these changes. Although CPT code 76098 would not be eligible for addition to the bypass list because the frequency and magnitude of packaged costs in its “natural” single claims exceed the empirical criteria, the presenter suggested that the “natural” single claims represented aberrant billing with inappropriate packaged services and pointed out that the packaged services support the surgical procedures that commonly are also reported on claims for CPT code 76098. The presenter suggested that bypassing CPT code 76098 would properly allocate packaged costs to surgical procedures on these claims, and would increase the number of single claims available for ratesetting for both CPT code 76098 and the associated surgical breast procedures. The APC Panel indicated that the issues raised by the presenter appeared to be consistent with clinical practice and subsequently made the recommendation to bypass CPT code 76098 and reassign the code to APC 0260 based on the code's revised cost.
Based on the APC Panel's specific recommendation for CPT code 76098, we studied the billing patterns for the code in the “natural” single and multiple major claims in the CY 2008 claims data available for the February 2009 APC Panel. The presenter asserted that CPT code 76098 is commonly billed with surgical breast procedures and our claims data from the multiple procedure claims confirm this observation. However, as noted above, there are also a significant number of “natural” single bills in those data (1,303), and these “natural” single claims include costly packaged services, such as CPT code 19290 (Preoperative placement of needle localization wire, breast) and CPT 77032 code (Mammographic guidance for needle placement, breast (eg, for wire localization or for injection), each lesion, radiological supervision and interpretation). We have received anecdotal information indicating that hospitals may place guidance wires prior to surgery in the hospital's radiology department and then examine the surgical specimen in the radiology department after its surgical removal. This information, along with the number of observed “natural” single claims, suggests that the packaged costs might appropriately be associated with the radiological examination of the breast specimen. Although bypassing CPT code 76098 would allow for the creation of more “pseudo” single claims for ratesetting, it would also require the assumption that all packaging on the claim would be correctly assigned to the remaining major procedure where it exists and that on “natural” single bills no packaging would be appropriately associated with CPT code 76098. Given the number of “natural” single bills for CPT code 76098 and the significant packaged costs on these claims, we are not confident that placement of this code on the bypass list is appropriate.
While we did not propose to place CPT code 76098 on the bypass list, we wanted to continue to provide separate payment for this procedure when appropriate. We believe that CPT code 76098 is generally ancillary and supportive to surgical breast procedures. In CY 2008 we established a group of conditionally packaged codes, called “T-packaged codes,” whose payment is packaged when one or more separately paid surgical procedures with status indicator “T” are provided during a hospital encounter. In order to provide separate payment for CPT code 76098 when not provided with a separately payable surgical procedure and also to recognize its ancillary and supportive nature when it accompanies separately payable procedures, we proposed to conditionally package CPT code 76098 as a “T-packaged code” for CY 2010, identified with status indicator “Q2” in Addendum B to the CY 2010 OPPS/ASC proposed rule. As a “T-packaged code,” CPT code 76098 would receive separate payment except where it appears with a surgical procedure, in which case its payment would be packaged. Designating CPT 76098 in this way allows the separate payment to appropriately account for the packaged costs that appear on the code's “natural” single bills, while also allowing us to use more multiple procedure claims that include both a surgical procedure and CPT code 76098 to set the payment rates for the related surgical procedures. The CPT code-specific median cost of CPT code 76098 in the CY 2008 claims data available for the February 2009 APC Panel meeting was approximately $346, consistent with its CY 2009 assignment to APC 0317 (Level II Miscellaneous Radiology Procedures), which had an observed APC median cost in those data of approximately $339. In contrast, the median cost of APC 0260, the APC reassignment recommended by the APC Panel, was much lower in the APC Panel data, approximately $46. Therefore, we did not accept the APC Panel's recommendation to reassign CPT code 76098. Instead, we proposed to continue its assignment to APC 0317 for CY 2010 in those cases where CPT code 76098 is separately paid.
Comment: Several commenters requested that CMS add CPT code 76098 to the bypass list and reassign it to APC 0260. The commenters believed that CPT 76098 is similar with respect to resource use to the other codes assigned to APC 0260. The commenters also claimed that including CPT code 76098 on the bypass list would appropriately make more claims available for ratesetting purposes for the CPT code itself and the surgical breast procedures that appear with CPT code 76098 in the multiple major procedure claims. Another commenter supported the proposal to not include CPT code 76098 on the CY 2010 bypass list.
Response: The hospital claims data show that there is significant packaging associated with CPT code 76098. Therefore, we believe CPT code 76098 is not appropriate for inclusion on the bypass list.
In examining the billing patterns for CPT 76098, we noted its failure to meet the empirical criteria for inclusion on the bypass list. The significant number of “natural” single claims suggests that these claims are an accurate representation of hospital billing practices in certain clinical situations. Further, we believe the packaging on these claims is properly associated with the code. Anecdotal information on the placement of wires prior to surgery suggests that the packaging on the “natural” single claims reflects appropriate billing in some clinical scenarios, such as when hospitals place guidance wires prior to surgery in the hospital's radiology department and then examine the surgical specimen in the radiology department after its surgical removal. This example illustrates appropriate billing on “natural” single claims for CPT code 76098 because the hospital has accurately reported all services that the hospital provided to the patient on the claim. In this case, the hospital did not provide the associated surgical breast procedure; therefore, all packaging would be appropriately associated with CPT code 76098, which is the separately payable service that the hospital provided to the patient. This scenario contradicts the commenter's belief that the significant packaging on the “natural” single claims for CPT code Start Printed Page 60327 76098 would represent aberrant hospital billing. As a result, for the CY 2010 proposed rule, we did not propose to add CPT code 76098 to the bypass list. However, based on our examination of the claims data for the proposed rule, we agreed that CPT 76098 is generally ancillary and supportive to surgical breast procedures. In order to provide appropriate separate payment for CPT code 76098 when the service is not furnished with a separately payable surgical procedure, and also to recognize its ancillary and supportive nature when it accompanies separately payable procedures, we proposed to conditionally package CPT code 76098 as a “T-packaged code” for CY 2010, identified with status indicator “Q2” in Addendum B to the proposed rule. Designating CPT code 76098 as a “T-packaged code” allows the separate payment to appropriately account for the packaged costs that appear on the code's “natural” single bills, while also allowing us to use more multiple procedure claims that include a surgical procedure and CPT code 76098 to set the payment rates for the related surgical procedures. In turn, we are able to use more data from the multiple procedure claims with CPT code 76098 to set payment rates for the surgical breast procedures on those claims. We continue to believe that classifying CPT code 76098 as a conditionally packaged code with status indicator “Q2” is the proper policy to both provide appropriate payment when the service is billed by itself and appropriate payment for the associated surgical breast procedures that it supports.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to assign status indicator “Q2” to CPT code 76098. When the service is furnished with a separately payable surgical procedure with status indicator “T” on the same day, payment for CPT code 76098 is packaged. Otherwise, payment for CPT code 76098 is made separately through APC 0317, which has a final APC median cost of approximately $374. We are not adding CPT code 76098 to the bypass list for CY 2010.
Comment: Many commenters supported the current methodology of bypassing HCPCS codes and the goal of using more data from the multiple major claims. A few commenters noted that some of the HCPCS codes on the proposed CY 2010 bypass list do not meet the empirical criteria described above and observed that many codes that meet the empirical criteria were not included on the proposed bypass list. The commenters highlighted findings from supporting data analysis to illustrate their points. Several commenters also raised concerns about the transparency of the bypass process. The commenters suggested that the empirical criteria were not explained clearly and were applied inconsistently. Other commenters believed that there is a lack of transparency regarding the addition of codes to the bypass list and the bypass process in general.
The commenters requested detailed explanations about which codes are included on the bypass list, asking that CMS identify any codes on the bypass list that do not meet the empirical criteria and the reason for their inclusion. Several commenters believed that modifying the specific empirical criteria that the median packaged cost be less than $50 on less than 5 percent of “natural” single bills would increase the number of potential bypass codes and “pseudo” single claims. Some commenters suggested adopting a different threshold of some low percentage of total packaged costs on the code's single claims as a percent of total costs on all single claims. They believed that a percentage approach could provide more stability in the ratesetting process. One commenter also suggested that more generous empirical thresholds could be appropriate for a select set of HCPCS codes by subtracting the average packaged cost of the bypass code from other costs on the date of service where the code appears and is used as a bypass code, specifically to increase the number of claims available for setting payment rates for APCs for low dose rate brachytherapy services. A few commenters recommended that the median packaged cost threshold of $50 on less than 5 percent of “natural” single bills be updated as CMS has not updated the threshold since its introduction, and one commenter claimed the packaged cost threshold was arbitrary. Several commenters also indicated that the HCPCS codes CMS proposed to add to the CY 2010 OPPS bypass list were not actually incorporated into CMS' ratesetting process.
Response: As discussed above in this section, we only apply the empirical criteria to the “natural” single claims. The bypass list is intended to consist of services that have minimal or no associated packaging, and in recent years, also includes codes for services that we wish to explicitly treat as not having packaged costs for purposes of OPPS payment. We refer readers to our previous discussions regarding the inclusion of additional hours of drug administration services (73 FR 68513) and HCPCS G0390 (71 FR 68117 through 68118) on the bypass list for further detail. Extracting “pseudo” single bills or unique estimates of a single service's total resource cost from claims containing multiple procedures requires making some assumptions about the amount of packaging associated with every service. As reflected in the CY 2005 proposed rule (69 FR 50474 through 50475), our empirical criteria of 100 “natural” single claims, 5 percent or fewer “natural” single claims with packaging, and median packaged cost less than $50 are intended to be conservative, that is, to limit the amount and impact of redistributed packaging from expanding the bypass list. These criteria ensure that the packaged costs associated with bypass codes are limited, based on the best information that we have in the “natural” single procedure claims. Bypassing codes with significant associated packaging would inappropriately redistribute these packaged costs to major procedures billed with the bypass codes in the multiple procedure claims, when the individual line-items for the bypass codes are removed to create “pseudo” single claims. Because we recognize that the “natural” single claims are not always good representations of the code when it is reported on multiple major claims, for example, a service with only 20 “natural” single claims, we also judiciously include procedures on the bypass list that both CMS' medical advisors and public commenters identify as not including significant packaging and for which our own data analyses do not suggest that inclusion on the bypass list would result in an inappropriate redistribution of packaged costs. Finally, our general policy each year has been to retain codes from the previous year's bypass list without reevaluation of these codes in the context of the empirical criteria based upon updated data. We listed and discussed these empirical criteria most recently in the CY 2010 OPPS/ASC proposed rule (74 FR 35240 through 35241). The empirical criteria have remained unchanged since first implemented because it has been our experience that they effectively limit the inappropriate redistribution of packaged costs when we create “pseudo” single procedure claims.
In examining the empirical data provided by commenters supporting their requests for additions to the bypass list, we believe that the research supporting these public comments applied the empirical criteria to all single claims rather than only to the “natural” single claims. We note that Start Printed Page 60328 this application of the empirical criteria is inconsistent with our methodology of generalizing about packaging in the multiple procedure claims from the “natural” single procedure claims. We do not believe that it would be appropriate to expand the bypass list by assuming that our packaging redistribution after application of the current bypass list should be used to identify additional candidates for the bypass list. Clearly comparing all single bills, not just “natural” single bills, would lead to the conclusion that many more codes are eligible for inclusion on the bypass list but could also compound any inappropriate cost redistribution created by the current “pseudo” single claim development process. The OPPS pays for individual items and services and some APCs do not contain many services and some of these services are low cost. Further, some payment rates are based on a small sample of single procedure claims. Because redistributing even a small amount of packaging could have a potentially large impact on median costs for small volume or low cost APCs, we believe our current empirical criteria and reliance on “natural” single procedure claims provide the most appropriate bypass policy.
Some commenters indicated that a packaged cost threshold based on a percentage of low packaged costs out of total costs for all single bills would be more appropriate. We believe that using a percentage could allow some significant packaged costs to be redistributed. Specifically, implementing this change to the empirical criteria could redistribute a low percentage of packaged cost out of total cost for all single bills to a very inexpensive service, leading to potential distortions in the APC relative weights. This would be contrary to one primary purpose of the empirical criteria, which is to limit the inappropriate redistribution of packaged costs in the bypass process. We also do not understand how adopting this policy would introduce greater stability. If the policy increased the size of the bypass list, it could introduce greater instability by inappropriately redistributing more variable packaged costs from year to year. With regard to the suggestion that we subtract an average packaged cost for the bypass code from each multiple procedure claim, we believe that this would inappropriately remove cost information from the claims used for ratesetting and assume that the removal of that average cost is appropriate in most cases.
While we are not adopting the commenters' suggested revisions to the empirical criteria for the CY 2010 OPPS bypass list, we acknowledge that the $50 median packaged cost threshold has not been updated for several years and that the real value of this packaged cost threshold criterion has declined due to inflation. Consequently, we will consider whether it would be appropriate to update the $50 dollar packaged cost threshold for inflation when identifying potential bypass codes in future rulemaking.
The bypass list we used to calculate payment rates for this final rule with comment period omits 11 of the 14 HCPCS codes that we newly proposed to add to the bypass list for the CY 2010 OPPS. Although these 14 proposed codes met the empirical criteria for inclusion on the bypass list for CY 2010 and although we listed them in Table 1 of the proposed rule (74 FR 35242 through 35352), we inadvertently omitted them from the bypass list that we used to calculate the median costs and payment rates that we proposed for CY 2010. To ensure consistency between the proposed rule and the final rule with comment period, we began our modeling for this final rule with comment period using the same list of bypass codes that we used to create the median costs and payment rates that we proposed for CY 2010. Three proposed radiation oncology code additions are an exception to this approach. In this final rule with comment period, we are including these three proposed bypass codes both because they meet the empirical criteria and because commenters on the CY 2010 OPPS/ASC proposed rule specifically requested that we add them to the CY 2010 bypass list. These three codes are: CPT code 77300 (Basic radiation dosimetry, central axis depth dose calculation, TDF, NSD, gap calculation, off axis factor, tissue inhomogeneity factors, calculation of non-ionizing radiation surface and depth dose, as required during course of treatment, only when prescribed by the treating physician); CPT code 77331 (Special dosimetry (e.g., TLD, microdosimetry)(specify), only when prescribed by the treating physician); and CPT code 77370 (Special medical radiation physics consultation).
Thus, the bypass list that we used to calculate the payment rates in this final rule with comment period does not include 11 of the 14 codes proposed for inclusion on the CY 2010 bypass list. These 11 HCPCS codes are identified in Table 1 of this final rule with comment period. In response to commenters' requests that we document additions to the bypass list, we have included a column in the list of bypass codes in Table 2 to identify additions for the CY 2010 update year, and we will continue to identify new additions in future rulemaking.
Comment: A few commenters noted that CMS removed radiation oncology HCPCS codes that did not meet the empirical criteria from the bypass list for the CY 2009 OPPS/ASC final rule with comment period. Observing that this action had an adverse effect on the median costs for those codes and services frequently billed with those codes, the commenters requested that a number of the radiation oncology CPT codes be added to the bypass list, including CPT codes 77295 (Therapeutic radiology simulation-aided field setting, 3-dimensional); 77299 (Unlisted procedure, therapeutic radiology clinical treatment planning); 77300 (Basic radiation dosimetry calculation, central axis depth dose calculation, TDF, NSD, gap calculation, off axis factor, tissue inhomogeneity factors, calculation of non-ionizing radiation surface and depth dose, as required during course of treatment, only when prescribed by treating physician); 77301 (Intensity modulated radiotherapy plan, including dose-volume histograms for target and critical structure partial tolerance specifications); 77310 (Teletherapy, isodose plan (whether hand or computer calculated); intermediate (three or more treatment ports directed to a single area of interest)); 77315 (Teletherapy. Isodose plan (whether hand or computer calculated); complex (mantle or inverted Y, tangential ports, the use of wedges, compensators, complex blocking, rotational beam, or special beam considerations)); 77327 (Brachytherapy isodose plan; intermediate (multiplane dosage calculations, application involving 5 to10 sources/ribbons, remote afterloading brachytherapy, 9 to 12 sources)); 77328 (Brachytherapy isodose plan; complex (multiplane isodose plan, volume implant calculations, over 10 sources/ribbons used, special spatial reconstruction, remote afterloading brachytherapy, over 12 sources)); 77331 (Special dosimetry (e.g., TLD, microdosimetry) (specify), only when prescribed by the treating physician); 77336 (Continuing medical physics consultation, including assessment of treatment parameters, quality assurance of dose delivery, and review of patient treatment documentation in support of the radiation oncologist, reporter per week of therapy); 77370 (Special medical radiation physics consultation); 77371 (Radiation treatment delivery, stereotactic radiosurgery (SRS), Start Printed Page 60329 complete course of treatment of cranial lesion(s) consisting of 1 session; multi-source Cobalt 60 based); 77401 (Radiation treatment delivery, superficial and/or ortho voltage); 77470 (Special treatment procedure (e.g., total body irradiation, hemibody radiation, per oral, endocavitary or intraoperative cone irradiation)); 77600 (Hyperthermia, externally generated; superficial (i.e., heating to a depth of 4 cm or less)); 77783 (Remote afterloading high intensity brachytherapy; 9–12 source positions or catheters); and 77789 (Surface application of radiation source).
Response: Some of the HCPCS codes that commenters suggested that we add to the bypass list are already included on the bypass list for this final rule with comment period, including CPT codes 77301, 77315, 77336, and 77401. These codes met the empirical criteria in earlier years and, because of our policy to retain codes once they have been added to the bypass list, these codes continue on the bypass list. However, many of the codes that commenters requested for addition the CY 2010 bypass list do not meet the empirical criteria because the percentage of “natural” single procedure claims with packaging exceeds 5 percent and, for some, the low volume of “natural” single claims prevents us from making an accurate assessment about packaging in the multiple procedure claims. Most of these codes have a low packaged median cost in the “natural” single procedure claims.
We examined the billing patterns for these HCPCS codes in the multiple major claims to better understand the potential impact that adding the recommended codes that do not meet the empirical criteria to the bypass list might have on the redistribution of packaged costs. We specifically analyzed the amount of packaged cost on the same date of service as the suggested bypass codes and other codes in the same clinical series as the recommended bypass codes in the multiple procedure claims, as well as the number of other procedures appearing on the same date of service, the APCs associated with these procedures, and whether any of these other procedures were already included on the bypass list. For three codes, specifically CPT codes 77600 (Hyperthermia, externally generated; superficial (i.e. heating to a depth of 4cm or less)); 77605 (Hyperthermia, externally generated; deep (i.e. heating to depths greater than 4 cm)); and 77610 (Hyperthermia generated by interstitial probe(s); 5 or fewer interstitial applicators), we did not observe a significant amount of additional packaging on the multiple procedure claims or many other services, so we believe that including these codes on the bypass list would result in a limited amount of redistributed packaged cost. Therefore, we added these three codes to the CY 2010 bypass list. We also observed packaged costs associated with CPT code 77327, but the amount was proportionally limited relative to the procedure costs on the same date of service, and we believe that we can appropriately add this code to the CY 2010 bypass list.
As discussed above in this section, we also are adding the radiation oncology codes that we proposed to include on the CY 2010 bypass list, specifically CPT codes 77300, 77331, and 77370, because these codes meet the empirical criteria, they were proposed for addition to the bypass list, and several commenters specifically requested these codes be included on the bypass list. However, several codes in the commenters' suggested additions to the bypass list not only failed the empirical criteria in the “natural” single procedure claims, but also were associated with significant packaged costs proportional to the costs of the other procedures appearing on the same date of service and the presence of many other separately paid procedures. Most of this packaged cost on claims for the candidate bypass codes was reported as revenue code charges without HCPCS codes, and we could not ascertain whether some of the packaging should be associated with the suggested bypass code or with one of the many other procedures appearing on the same date of service in the multiple claims. Because we would be unable to allocate the packaged cost among services or to determine that it was not associated with the candidate bypass list code, we believe it would be inappropriate to add these HCPCS codes to the bypass list. Although previous commenters have suggested that packaging of radiation guidance services in CY 2008 reduced the number of claims available for setting payment rates for radiation oncology services, it is notable that only a small portion of the packaged costs on the claims for radiation oncology services could be attributed to the radiation guidance services. In summary, we are not adding CPT codes 77295, 77299, 77310, 77328, 77371, 77470, 77783, and 77789 to the final CY 2010 bypass list.
We always appreciate the empirical information that commenters submitted regarding their suggested additions to the bypass list. However, we note that, due to the redistributive properties of the bypass list and our process for creating “pseudo” single procedure claims, we always must examine the redistributive impact of additions to the bypass list on all HCPCS code and APC median costs. Future recommendations from the public for additions to the bypass list should consider the global impact on APCs and HCPCS codes of changes to the bypass list in order to facilitate our evaluation of codes suggested for inclusion on the bypass list in the future.
Comment: Some commenters supported the inclusion of the HCPCS codes for additional hours of drug administration on the bypass list. In addition, several commenters requested that CPT 90768 (Intravenous infusion, for therapy, prophylaxis, or diagnosis (specify substance or drug); concurrent infusion (List separately in addition to code for primary procedure)) be made separately payable and added to the bypass list to ensure consistent treatment of codes for additional hours of drug administration under the bypass list.
Response: We appreciate the commenters' support and have continued to include the separately payable codes for additional hours of drug administration on the CY 2010 bypass list. Bypassing these drug administration codes, and associating all the packaging with the code for the initial hour of drug administration, enables us to use many correctly coded claims for initial drug administration services that would otherwise not be available for ratesetting. We did not include CPT 90768 on the CY 2010 bypass list because we proposed to unconditionally package its successor code (CPT code 96368 (Intravenous infusion, for therapy, prophylaxis, or diagnosis (specify substance or drug); concurrent infusion (List separately in addition to code for primary procedure))) in CY 2010 and, therefore, CPT code 90768 is not a candidate for the bypass list. Our final CY 2010 policy to package payment for CPT code 96368 is discussed in section VIII.B. of this final rule with comment period.
As discussed above, the bypass list consists of separately paid services with no or minimal packaging or separately paid services that CMS knowingly prices without including packaged costs and associates any packaging with the other service(s) billed on the same date of service. The purpose of the bypass list is to help develop better estimates of total resource costs for a given separately payable procedure through creating “pseudo” single procedure claims from the multiple procedure claims by removing line-items without Start Printed Page 60330 packaging from each claim's date of service. Including packaged codes on the bypass list would remove valid packaging from a multiple procedure claim and would not allow CMS to derive more estimates of a service's total resource costs from multiple procedure claims. We have previously discussed our reasons for packaging CPT code 90768 in the CY 2009 OPPS/ASC final rule with final period (73 FR 68674).
Comment: Several commenters supported the inclusion of HCPCS code G0340 (Image-guided robotic linear accelerator-based stereotactic radiosurgery, delivery including collimator changes and custom plugging, fractionated treatment, all lesion, per session, second through fifth session, maximum) on the bypass list.
Response: We appreciate the commenters' support and have continued to include HCPCS code G0340 on the CY 2010 bypass list.
Comment: One commenter requested that CMS examine whether changes to the bypass list or other edits included in CMS' ratesetting processes negatively affected the proposed CY 2010 payment rates for APC 0651 (Complex Interstitial Radiation Source Application) and composite APC 8001(LDR Prostate Brachytherapy Composite).
Response: In analyzing the impact of the final CY 2010 bypass list changes on APCs 0651 and 8001, we noted modest changes in both single procedure claim frequency and median costs. In the case of composite APC 8001, bypass list changes increased the single procedure claims available for ratesetting purposes and reduced the median cost by roughly 2 percent. APC 0651 experienced a modest increase of 3 percent in the single procedure claims available for ratesetting and its median cost also increased by about 3 percent. Neither APC 0651 nor composite APC 8001 experienced significant fluctuations in median cost or single procedure claim frequency due to the line-item trim discussed in section II.A.2.(a) of this final rule with comment period.
After consideration of the public comments received, we are adopting, as final, our proposed methodology to use a bypass list to create “pseudo” single claims. To ensure consistency between the CY 2010 proposed and final rules, we began our consideration of comments using the same list of bypass codes for this final rule with comment period that we used to calculate the median costs and payment rates that we proposed for CY 2010, which was the CY 2009 final rule bypass list. We added HCPCS codes to the CY 2010 bypass list based on whether they met the empirical criteria and, if they did not, whether we believe that the amount of redistributed packaged cost that their inclusion on the bypass list would generate would be appropriate. We ultimately added seven codes to the CY 2010 bypass list. The list of CY 2010 bypass code additions that we proposed in the CY 2010 OPPS/ASC proposed rule but did not implement in this final rule with comment period appears in Table 1. Table 2 below is the final list of bypass codes for CY 2010. “Overlap bypass codes” that are members of the multiple imaging composite APCs are identified by asterisks (*) in Table 2. HCPCS codes that have been added for CY 2010 are also identified by asterisks (*) in Table 2.
| CY 2010 HCPCS Code | CY 2010 Short descriptor |
|---|---|
| 57452 | Exam of cervix w/scope. |
| 76120 | Cine/video x-rays. |
| 76813 | Ob us nuchal meas, 1 gest. |
| 88314 | Histochemical stain. |
| 88367 | Insitu hybridization, auto. |
| 92700 | Ent procedure/service. |
| 94660 | Pos airway pressure, CPAP. |
| 95971 | Analyze neurostim, simple. |
| 99406 | Behav chng smoking 3–10 min. |
| 99407 | Behav chng smoking >10 min. |
| G0249 | Provide INR test mater/equip. |
c. Calculation of CCRs
(1) Development of the CCRs
We calculated hospital-specific overall ancillary CCRs and hospital-specific departmental CCRs for each hospital for which we had CY 2008 claims data from the most recent available hospital cost reports, in most cases, cost reports beginning in CY 2007. For the CY 2010 OPPS ratesetting, we used the set of claims processed during CY 2008. We applied the hospital-specific CCR to the hospital's charges at the most detailed level possible, based on a revenue code-to-cost center crosswalk that contains a hierarchy of CCRs used to estimate costs from charges for each revenue code. That crosswalk is available for review and continuous comment on the CMS Web site at: http://www.cms.hhs.gov/HospitalOutpatientPPS/03_crosswalk.asp#TopOfPage. We calculated CCRs for the standard and nonstandard cost centers accepted by the electronic cost report database. In general, the most detailed level at which we calculated CCRs was the hospital-specific departmental level. For a discussion of the hospital-specific overall ancillary CCR calculation, we refer readers to the CY 2007 OPPS/ASC final rule with comment period (71 FR 67983 through 67985).
In the CY 2010 OPPS/ASC proposed rule (74 FR 35253), we proposed to continue using the hospital-specific overall ancillary and departmental CCRs to convert charges on the claims reported under specific revenue codes to estimated costs through application of a revenue code-to-cost center crosswalk for CY 2010.
We did not receive any public comments on this proposal. Therefore, we are finalizing our proposal for CY 2010, without modification, to calculate hospital-specific overall and departmental CCRs as described above in this section.
(2) Charge Compression
Since the implementation of the OPPS, some commenters have raised concerns about potential bias in the OPPS cost-based weights due to “charge compression,” which is the practice of applying a lower charge markup to higher-cost services and a higher charge markup to lower-cost services. (We discuss our CCR calculation in section Start Printed Page 60343 II.A.1.c. of this final rule with comment period and how we use these CCRs to estimate cost on hospital outpatient claims in detail in section II.A.2.a. of this final rule with comment period). As a result, the cost-based weights incorporate aggregation bias, undervaluing high cost items and overvaluing low cost items when an estimate of average markup, embodied in a single CCR, is applied to items of widely varying costs in the same cost center. Commenters on previous rules have expressed increased concern about the impact of charge compression when CMS began setting the relative weights for payment under the IPPS based on the costs of inpatient hospital services, rather than the charges for the services.
To explore this issue, in August 2006 we awarded a contract to RTI International (RTI) to study the effects of charge compression in calculating the IPPS relative weights, particularly with regard to the impact on inpatient diagnosis-related group (DRG) payments, and to consider methods to capture better the variation in cost and charges for individual services when calculating costs for the IPPS relative weights across services in the same cost center. Of specific note was RTI's analysis of a regression-based methodology estimating an average adjustment for CCR by type of revenue code from an observed relationship between provider cost center CCRs and proportional billing of high and low cost services in the revenue codes associated with the cost center in the claims data. RTI issued a report in March 2007 with its findings on charge compression. The report is available on the CMS Web site at: http://www.cms.hhs.gov/reports/downloads/Dalton.pdf. Although this report was focused largely on charge compression in the context of the IPPS cost-based relative weights, several of the findings were relevant to the OPPS. Therefore, we discussed the findings and our responses to that report in the CY 2008 OPPS/ASC proposed rule (72 FR 42641 through 42643) and reiterated them in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66599 through 66602).
RTI noted in its 2007 report that its research was limited to IPPS DRG cost-based weights and that it did not examine potential areas of charge compression specific to hospital outpatient services. We were concerned that the analysis was too limited in scope because typically hospital cost report CCRs encompass both inpatient and outpatient services for each cost center. Further, because both the IPPS and OPPS rely on cost-based weights, we preferred to introduce any methodological adjustments to both payment systems at the same time. We believe that because charge compression affects the cost estimates for services paid under both IPPS and OPPS in the same way, it is appropriate that we would use the same or, at least, similar approaches to address the issue. Finally, we noted that we wished to assess the educational activities being undertaken by the hospital community to improve cost reporting accuracy in response to RTI's findings, either as an adjunct to or in lieu of regression-based adjustments to CCRs.
We expanded RTI's analysis of charge compression to incorporate outpatient services. In August 2007, we again contracted with RTI. Under this contract, we asked RTI to evaluate the cost estimation process for the OPPS relative weights. This research included a reassessment of the regression-based CCR models using hospital outpatient and inpatient charge data, as well as a detailed review of the OPPS revenue code-to-cost center crosswalk and the OPPS' hospital-specific CCR methodology. In evaluating cost-based estimation, in general, the results of RTI's analyses impact both the OPPS APC relative weights and the IPPS MS–DRG (Medicare severity) relative weights. The RTI final report can be found on RTI's Web site at: http://www.rti.org/reports/cms/HHSM-500-2005-0029I/PDF/Refining_Cost_to_Charge_Ratios_200807_Final.pdf. For a complete discussion of the RTI recommendations, public comments, and our responses, we refer readers to the CY 2009 OPPS/ASC final rule with comment period (73 FR 68519 through 68527).
In the FY 2009 IPPS final rule, we finalized our proposal for both the OPPS and IPPS to add one cost center to the cost report so that, in general, the costs and charges for relatively inexpensive medical supplies would be reported separately from the costs and charges for more expensive implantable devices (such as pacemakers and other implantable devices). Specifically, we created one cost center for “Medical Supplies Charged to Patients” and one cost center for “Implantable Devices Charged to Patients.” This change split the CCR for “Medical Supplies and Equipment” into one CCR for medical supplies and another CCR for implantable devices. In response to the majority of commenters on the proposal set forth in the FY 2009 IPPS proposed rule, we finalized a definition of the “Implantable Devices Charged to Patients” cost center as capturing the costs and charges billed with the following UB–04 revenue codes: 0275 (Pacemaker), 0276 (Intraocular lens), 0278 (Other implants), and 0624 (FDA investigational devices). We made this change to the cost report form for cost reporting periods beginning in the spring of 2009. Because there is generally a 3-year lag between the availability of cost report data for IPPS and OPPS ratesetting purposes in a given calendar year, we believe we will be able to use data from the revised cost report form to estimate costs from charges associated with UB–04 revenue codes 0275, 0276, 0278, and 0624 for implantable devices in order to more accurately estimate the costs of device-related procedures for the CY 2013 OPPS relative weights. For a complete discussion of the proposal, public comments, and our responses, we refer readers to the FY 2009 IPPS final rule (73 FR 48458 through 45467).
For the CY 2009 OPPS/ASC proposed rule, we made a similar proposal for drugs, proposing to split the “Drugs Charged to Patients” cost center into two cost centers: one for drugs with high pharmacy overhead costs and one for drugs with low pharmacy overhead costs (73 FR 41492). We noted that we expected that CCRs from the proposed new cost centers would be available in 2 to 3 years to refine OPPS drug cost estimates by accounting for differential hospital markup practices for drugs with high and low pharmacy overhead costs. However, after consideration of the public comments received and the APC Panel recommendations, we did not finalize our proposal to split the single standard “Drugs Charged to Patients” cost center into two cost centers, and instead indicated in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68659) that we would continue to explore other potential approaches to improve our drug cost estimation methodology. Unlike implantable devices, we do not currently have a policy to address charge compression in our cost estimation for expensive drugs and biologicals. In section V.B.3. of the CY 2010 OPPS/ASC proposed rule (74 FR 35326 through 35333), we proposed an adjustment to our cost estimation methodology for drugs and biologicals to address charge compression by proposing to shift a portion of the pharmacy overhead cost associated with packaged drugs and biologicals from those packaged drugs and biologicals to separately payable drugs and biologicals; proposing payment for separately payable drugs and biologicals at ASP+4 percent; and proposing a proportional reduction in the total amount of pharmacy overhead cost Start Printed Page 60344 associated with packaged drugs and biologicals prior to our estimating the total resource costs of individual OPPS services.
Finally, in the CY 2009 OPPS/ASC final rule with comment period, we indicated that we would be making some OPPS-specific changes in response to the RTI report recommendations. With regard to modifying the cost reporting preparation software in order to impose fixed descriptions for nonstandard cost centers, we indicated that the change would be made for the next release of the cost report software. We anticipate that these changes will be made to the cost reporting software in CY 2010 and will act as a quality check for hospitals to review their choice of nonstandard cost center code to ensure that the reporting of nonstandard cost centers is accurate, while not significantly increasing provider burden. In addition to improving the reporting mechanism for the nonstandard cost centers, we indicated in the CY 2009 OPPS/ASC final rule with comment period that we also planned to add the new nonstandard cost centers for Cardiac Rehabilitation, Hyperbaric Oxygen Therapy, and Lithotripsy. We expect that changes to add these nonstandard cost centers also will be made for cost reports beginning in CY 2010. Furthermore, we noted in the FY 2010 IPPS final rule (74 FR 43781 through 43782) that we are updating the cost report form to eliminate outdated requirements, in conjunction with the Paperwork Reduction Act (PRA), and that we had proposed actual changes to the cost reporting form, the attending cost reporting software, and the cost report instructions in Chapters 36 and 40 of the PRM–II. The comment period for this proposal (74 FR 31738) ended on August 31, 2009. We believe that improved cost report software, the incorporation of new nonstandard cost centers, and elimination of outdated requirements will improve the accuracy of the cost data contained in the electronic cost report data files and, therefore, the accuracy of our cost estimation processes for the OPPS relative weights. As has been described above, CMS has taken steps to address charge compression in the IPPS and OPPS, and continues to examine ways in which it can improve the accuracy of its cost estimation process.
Comment: Several commenters expressed support for the policy adopted in the FY 2009 IPPS final rule, with application to both the OPPS and IPPS, to create one cost center for “Medical Supplies Charged to Patients” and one cost center for “Implantable Devices Charged to Patients.” Some commenters recommended that CMS verify the accuracy of the CCRs derived from the new cost centers by comparing CCRs calculated from the new cost center against regression-based CCRs or by undertaking other activities to ensure that data reported in these revised cost centers are consistent and accurate.
One commenter stated that hospitals are reluctant to bill for devices that do not remain in the patient upon discharge, specifically cryoablation probes, under revenue code 0278 ( Medical/Surgical Supplies: Other Implants). The commenter requested that CMS work with hospitals to revise the common hospital practice of billing for cryoablation probes under revenue code 0272 ( Medical/Surgical Supplies: Sterile Supplies) rather than revenue code 0278. The commenter asserted that billing cryoablation probes under revenue code 0272 would result in estimating costs from charges using a CCR derived from the revised cost center for “Medical Supplies Charged to Patients,” rather than one derived from the “Implantable Devices Charged to Patients,” even though cryoablation probes are high cost implantable devices. The commenter believed that, without a change in the revenue code under which many hospitals report cryoablation probes, the recent cost center changes for medical supplies would negatively bias the estimated cost of cryoablation probes and the accuracy of the APC payment rates for cryoablation procedures.
Some commenters suggested that CMS engage in outreach and educational activities to hospitals on the changes to the cost report and the reporting of charges with respect to the medical device and medical supply cost centers so that hospitals can appropriately report data. The commenters recommended that the outreach activities go beyond the “distribution of bulletins that are used to inform providers about changes to the Medicare program.”
Response: We appreciate the commenters' support for our CY 2009 policy to split the “Medical Supplies Charged to Patients” into one cost center for “Medical Supplies Charged to Patients” and one cost center for “Implantable Devices Charged to Patients”. In the FY 2009 IPPS final rule (73 FR 48458 through 48467), we explained in detail the reasoning behind the development of the cost center split and our decision to ultimately have hospitals use the American Hospital Association's National Uniform Billing Committee (NUBC) revenue codes to determine what would be reported in the “Medical Supplies Charged to Patients” and the “Implantable Devices Charged to Patients” cost centers. In that discussion, we noted that while we require that the device broadly be considered implantable to have its costs and charges included in the new “Implantable Devices Charged to Patients” cost center, our final policy did not require the device to remain in the patient at discharge (73 FR 48462 through 48463). We typically do not specify a revenue code-to-cost center crosswalk that hospitals must adopt to prepare their cost report, recognizing hospitals' need to interpret the NUBC definitions and cost reporting requirements within the context of their own financial systems. In response to comments on our proposal to create the new cost center in the FY 2009 IPPS final rule, we did define the new “Implantable Devices Charged to Patients” cost center by the revenue codes that we believe would map to this cost center to facilitate ease of reporting by hospitals. We note that revenue code definitions are established by the NUBC, and we fully expect hospitals to follow existing guidelines regarding revenue code use. Specifically with regard to reporting cryoablation probes, we do not believe that the current NUBC definition of revenue code 0278 (Medical/Surgical Supplies and Devices (also see 062x, an extension of 027x); Other implants (a)) precludes reporting hospital charges for cryoablation probes under this revenue code. Therefore, we believe hospitals can report charges for cryoablation probes under the revenue code 0278 using the definitions in the official UB 04 Data Specifications Manual.
As discussed in the FY 2010 IPPS final rule (74 FR 43780), we reiterated that we had not proposed any policy changes with respect to the use of revenue codes or alternative ways of identifying high-cost devices. We refer readers to the discussion in the FY 2009 IPPS final rule concerning our current policy on these matters (73 FR 48462). Hospitals were able to report costs and charges for the new “Implantable Devices Charged to Patients” cost center for cost reporting periods beginning on or after May 1, 2009 as line 55.30 on Form 2552–96 and, at the time of development of this final rule with comment period, we anticipate that hospitals will be able to report costs and charges for the new cost center as line 69 on the revised draft Medicare hospital cost report form CMS–2552–10 beginning February 1, 2010.
In the FY 2009 IPPS final rule (73 FR 48463), we agreed that once the data reflecting the cost center changes become available for ratesetting, we Start Printed Page 60345 would evaluate the CCRs that we derive from the new “Medical Supplies Charged to Patients” and “Implantable Devices Charged to Patients” cost centers and that we would continue to analyze cost report data. In the FY 2010 IPPS final rule (74 FR 43782), we indicated that we might consider the results of regression analyses as one way to evaluate costs and charges reported in the new cost center. However, we point out that we do not believe it is appropriate to “pick and choose” between CCRs; rather, the determining factor should be payment accuracy, regardless of whether one method increases or decreases payment for devices (73 FR 48463). That is, the validity of the CCRs resulting from the newly implemented cost center cannot be determined to be accurate simply because they will result in higher overall cost estimates for procedures that rely on implantable devices and, therefore, higher APC payment rates.
As discussed in the FY 2010 IPPS rule, we believe it is early to plan specific outreach activities on the revised cost report form CMS–2552–10 and the new “Implantable Devices Charged to Patients” cost center, given that the comment period for the revised cost reporting forms closed on August 31, 2009. We agree that such educational activities are important, and we have been considering various options for educating the provider community that would involve fiscal intermediaries, Medicare administrative contractors, and cost report vendors. We look forward to working with the provider community on these initiatives.
Comment: A few commenters noted that two revenue codes became effective for reporting radiopharmaceuticals, specifically 0343 (Nuclear Medicine; Diagnostic Radiopharmaceuticals) for diagnostic preparations and 0344 (Nuclear Medicine; Therapeutic Radiopharmaceuticals) for therapeutic preparations in October 2004; and that this more specific revenue code reporting should help capture the unique costs and charges of radiopharmaceuticals. The commenters also pointed out that the costs and charges associated with these revenue codes likely would be reported by hospitals under the broader radiology cost center on the Medicare hospital cost report. They expressed concern that, because the CCR used to estimate charges for these revenue codes encompasses a large volume of many different services, the specificity of charge information in the claims data gained through use of the new revenue codes would not translate into better cost estimation for diagnostic and therapeutic radiopharmaceuticals under the OPPS. The commenters suggested that CMS require hospitals to report costs and charges for these two revenue codes as unique cost centers on the cost report.
Response: We agree with the commenters that the broader the range and volume of services included in a given cost center, the more the resulting CCR calculated from the costs and charges for that cost center represents a weighted average of included services. To the extent that the revenue codes implemented in October 2004, specifically 0343 (Nuclear Medicine; Diagnostic Radiopharmaceuticals) for diagnostic preparations and 0344 (Nuclear Medicine; Therapeutic Radiopharmaceuticals) for therapeutic preparations, have no specific associated cost center in which to capture their unique costs and charges and to the extent hospitals report these costs and charges in cost center 4100 “Radiology—Diagnostic” or 4200 “Radiology—Therapeutic,” the CCRs for cost centers 4100 and 4200 that CMS uses to estimate costs from charges on claims for specific radiopharmaceuticals will reflect the average cost and markup associated with all diagnostic and therapeutic radiology procedures. However, our policy for establishing new cost centers requires a public review process that allows commenters the opportunity to provide input on any changes, and many commenters historically have not been interested in adding cost centers to the cost report because of the associated hospital administrative burden.
As we have noted above, we have recently undertaken regulatory comment and response on our effort to update the cost report. The proposed draft hospital cost report Form CMS–2552–10 went on Federal Register public display at the Office of the Federal Register on July 2, 2009, for a 60-day review and comment period, which ended on August 31, 2009. As we stated in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68525 through 68526), that notice and comment procedure is the process by which we are considering public comments requesting additional cost centers. We will consider all comments for new cost centers submitted through that process as we work to improve and modify the hospital cost report. We also note that we make the revenue code-to-cost center crosswalk that we use to match Medicare hospital cost report information with claims data continually available for inspection and comment on the CMS Web site: http://www.cms.hhs.gov/HospitalOutpatientPPS.
Comment: One commenter believed that the proposed drug cost center split discussed in the CY 2009 OPPS/ASC proposed rule would represent an unnecessary burden for hospitals.
Response: While we welcome comments regarding OPPS policy, we note that the drug cost center proposal was a CY 2009 proposal which was not finalized (73 FR 68654 through 68657). We have not proposed a policy to split the drug cost center for CY 2010.
Comment: One commenter requested that CMS issue clarifying instructions for reporting computed tomography (CT) and magnetic resonance imaging (MRI) equipment and supported the creation of new cost centers to capture the unique costs and charges of CT scanning, MRI, and other radiology procedures.
Response: We did not propose to implement separate standard radiology cost centers for CT Scanning, MRI, and other radiology procedures due to the significant number of comments we received in response to our general request in the CY 2009 OPPS/ASC proposed rule for comments and reactions to RTI's recommendations. The commenters on the CY 2009 OPPS/ASC proposed rule were generally in favor of these cost centers in theory, but suggested that the allocation of capital cost across these cost centers was not consistent or consistently accurate across hospitals and that smaller hospitals might not have sufficiently sophisticated accounting systems to accurately allocate costs (73 FR 68526). In that discussion, we expressed our preference for establishing these cost centers as standard cost centers because standard cost centers constitute the minimum set of cost centers that a hospital is required to report, assuming that the hospital maintains separate departments for those services and reports the costs and charges for these departments in separate accounts within its own internal accounting systems. We believe this step would improve the accuracy of radiology payment by encouraging greater and more consistent reporting of the costs and charges specifically associated with advanced imaging services. However, we also noted that nonstandard cost centers already are available for CT Scanning and MRI and that hospitals that provide these services and maintain a separate account for each of these services in their internal accounting records to capture the costs and charges are currently required, in accordance with § 413.53(a)(1), to report these cost centers on the cost report, even if CMS Start Printed Page 60346 does not identify a nonstandard cost center code for the department(s).
As we stated in the CY 2009 OPPS/ASC final rule with comment (73 FR 68525 through 68526) and in response to an earlier comment in this section, we will consider public comments requesting additional cost centers in response to the PRA Federal Register notice for the proposed draft cost report form CMS–2552–10. The comment period for this proposal ended August 31, 2009.
Comment: A few commenters expressed concern about the timing for implementing the nonstandard cost center for cardiac rehabilitation, suggesting that a delay could limit beneficiary access to cardiac rehabilitation services because the proposed CY 2010 payment was too low. The commenters noted that the new CCRs would not be available for setting OPPS payment rates until CY 2013.
Response: While we understand the commenters' concern regarding the timing of implementing the cardiac rehabilitation nonstandard cost center, in our CY 2009 OPPS/ASC final rule with comment period discussion (73 FR 68524), we explained our preference for improving the accuracy of the APC relative weights through long-term changes to the cost report rather than implementing short-term statistical adjustments, in order to ensure that actual hospital data are used to set payment rates. As discussed above, we currently anticipate we will implement new nonstandard cost centers for Cardiac Rehabilitation, Hyperbaric Oxygen Therapy, and Lithotripsy with the revised Medicare hospital cost report form in CY 2010.
We have approximately 2.5 million CY 2008 claims from almost 2,000 hospitals for cardiac rehabilitation sessions available for setting the CY 2010 payment rates for these services. Given that the OPPS payment for the services has been highly stable for the past several years, we have no reason to believe that Medicare beneficiaries' access to cardiac rehabilitation will be limited in CY 2010 based on the final OPPS payment rates for the services. Further discussion of CY 2010 payment for traditional and intensive cardiac rehabilitation services is included in section XII.B. of this final rule with comment period.
Comment: One commenter believed that CMS continues to expand and complicate the antiquated Medicare cost report rather than to design a helpful tool. The commenter believes that the current “piecemeal” approach to revising the cost report is costly and burdensome. Based on that impression, the commenter recommended that CMS partner with the hospital industry to consider more comprehensive changes to the cost report.
Response: In the FY 2009 IPPS proposed and final rules (73 FR 23546 and 73 FR 48461) and CY 2009 OPPS/ASC proposed rule and final rule with comment period (73 FR 41431 and 73 FR 68526), we stated that we began a comprehensive review of the Medicare hospital cost report, and splitting the current cost center for “Medical Supplies Charged to Patients” into one line for “Medical Supplies Charged to Patients” and another line for “Implantable Devices Charged to Patients” is part of that initiative to update and revise the cost report. We also explained that in the context of the effort to update the cost report and eliminate outdated requirements, we would make changes to the cost report form and cost report instructions that would be available to the public for comment. Thus, the public would have an opportunity to suggest the more comprehensive reforms that one commenter on the CY 2010 OPPS/ASC proposed rule advocates. Similarly, the public would be able to offer suggestions for ensuring that these reforms are made in a manner that is not disruptive to hospitals' billing and accounting systems, and within the guidelines of General Accepted Accounting Principles (GAAP), which are consistent with the Medicare principles of reimbursement and sound accounting practices. The proposed draft hospital cost report Form CMS–2552–10 went on Federal Register public display at the Office of the Federal Register on July 2, 2009, for a 60-day review and comment period, which ended on August 31, 2009. We will consider comments from the public as we work to improve and modify the hospital cost report. The cost center for “Implantable Devices Charged to Patients” is available for use for cost reporting periods beginning on or after May 1, 2009. The revised hospital cost report Form CMS–2552–10 will be effective for cost reporting periods beginning on or after February 1, 2010 (74 FR 43781 through 43782).
2. Data Development Process and Calculation of Median Costs
In this section of this final rule with comment period, we discuss the use of claims to calculate final OPPS payment rates for CY 2010. The hospital OPPS page on the CMS Web site on which this final rule with comment period is posted provides an accounting of claims used in the development of the final payment rates at: http://www.cms.hhs.gov/HospitalOutpatientPPS. The accounting of claims used in the development of this final rule with comment period is included on the CMS Web site under supplemental materials for the CY 2010 OPPS/ASC final rule with comment period. That accounting provides additional detail regarding the number of claims derived at each stage of the process. In addition, below in this section we discuss the file of claims that comprise the data set that is available for purchase under a CMS data use agreement. Our CMS Web site, http://www.cms.hhs.gov/HospitalOutpatientPPS, includes information about purchasing the “OPPS Limited Data Set,” which now includes the additional variables previously available only in the OPPS Identifiable Data Set, including ICD–9–CM diagnosis codes and revenue code payment amounts. This file is derived from the CY 2008 claims that were used to calculate the final payment rates for the CY 2010 OPPS.
As proposed, we used the methodology described in sections II.A.2.b. through e. of this final rule with comment period to establish the relative weights used in calculating the final OPPS payment rates for CY 2010 shown in Addenda A and B to this final rule with comment period.
a. Claims Preparation
For the CY 2010 OPPS/ASC proposed rule, we used the CY 2008 hospital outpatient claims processed before January 1, 2009 to calculate the median costs of APCs, which in turn are used to set the proposed relative weights for CY 2010. To begin the calculation of the relative weights for CY 2010, we pulled all claims for outpatient services furnished in CY 2008 from the national claims history file. This is not the population of claims paid under the OPPS, but all outpatient claims (including, for example, critical access hospital (CAH) claims and hospital claims for clinical laboratory services for persons who are neither inpatients nor outpatients of the hospital). In the discussion that follows, we have updated the information to reflect the claims available for this final rule with comment period, specifically CY 2008 claims processed through June 30, 2009.
We then excluded claims with condition codes 04, 20, 21, and 77. These are claims that providers submitted to Medicare knowing that no payment would be made. For example, providers submit claims with a condition code 21 to elicit an official denial notice from Medicare and Start Printed Page 60347 document that a service is not covered. We then excluded claims for services furnished in Maryland, Guam, the U.S. Virgin Islands, American Samoa, and the Northern Mariana Islands because hospitals in those geographic areas are not paid under the OPPS.
We divided the remaining claims into the three groups shown below. Groups 2 and 3 comprise the 107 million claims that contain hospital bill types paid under the OPPS.
1. Claims that were not bill types 12X, 13X (hospital bill types), 14X (laboratory specimen bill types), or 76X (CMHC bill types). Other bill types are not paid under the OPPS and, therefore, these claims were not used to set OPPS payment.
2. Claims that were bill types 12X, 13X or 14X. Claims with bill types 12X and 13X are hospital outpatient claims. Claims with bill type 14X are laboratory specimen claims, of which we use a subset for the limited number of services in these claims that are paid under the OPPS.
3. Claims that were bill type 76X (CMHC). (These claims are later combined with any claims in item 2 above with a condition code 41 to set the per diem partial hospitalization rates determined through a separate process.)
To convert charges on the claims to estimated cost, we needed to multiply those charges by the CCR associated with each revenue code as discussed in section II.A.1.c.(1) of this final rule with comment period. For the CCR calculation process, we used the same general approach that we used in developing the final APC rates for CY 2007, using the revised CCR calculation that excluded the costs of paramedical education programs and weighted the outpatient charges by the volume of outpatient services furnished by the hospital. We refer readers to the CY 2007 OPPS/ASC final rule with comment period for more information (71 FR 67983 through 67985). We first limited the population of cost reports to only those for hospitals that filed outpatient claims in CY 2008 before determining whether the CCRs for such hospitals were valid.
We then calculated the CCRs for each cost center and the overall ancillary CCR for each hospital for which we had claims data. We did this using hospital-specific data from the Hospital Cost Report Information System. We used the most recent available cost report data, in most cases, cost reports with cost reporting periods beginning in CY 2007. As proposed, for this final rule with comment period, we used the most recently submitted cost reports to calculate the CCRs to be used to calculate median costs for the final CY 2010 OPPS payment rates. If the most recent available cost report was submitted but not settled, we looked at the last settled cost report to determine the ratio of submitted to settled cost using the overall ancillary CCR, and we then adjusted the most recent available submitted but not settled cost report using that ratio. We then calculated both an overall ancillary CCR and cost center-specific CCRs for each hospital. We used the overall ancillary CCR referenced in section II.A.1.c.(1) of this final rule with comment period for all purposes that require use of an overall ancillary CCR.
We then flagged CAH claims, which are not paid under the OPPS, and claims from hospitals with invalid CCRs. The latter included claims from hospitals without a CCR; those from hospitals paid an all-inclusive rate; those from hospitals with obviously erroneous CCRs (greater than 90 or less than .0001); and those from hospitals with overall ancillary CCRs that were identified as outliers (3 standard deviations from the geometric mean after removing error CCRs). In addition, we trimmed the CCRs at the cost center (that is, departmental) level by removing the CCRs for each cost center as outliers if they exceeded ±3 standard deviations from the geometric mean. We used a four-tiered hierarchy of cost center CCRs, which is the revenue code-to-cost center crosswalk, to match a cost center to every possible revenue code appearing in the outpatient claims that is relevant to OPPS services, with the top tier being the most common cost center and the last tier being the default CCR. If a hospital's cost center CCR was deleted by trimming, we set the CCR for that cost center to “missing” so that another cost center CCR in the revenue center hierarchy could apply. If no other cost center CCR could apply to the revenue code on the claim, we used the hospital's overall ancillary CCR for the revenue code in question. For example, if a visit was reported under the clinic revenue code but the hospital did not have a clinic cost center, we mapped the hospital-specific overall ancillary CCR to the clinic revenue code. The revenue code-to-cost center crosswalk is available for inspection and comment on the CMS Web site: http://www.cms.hhs.gov/HospitalOutpatientPPS. Revenue codes not used to set medians or to model impacts are identified with an “N” in the revenue code-to-cost center crosswalk.
As we proposed, we updated the revenue code-to-cost center crosswalk to more accurately reflect the current use of revenue codes. We indicated in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68531) that we intended to assess the NUBC revenue codes to determine whether any changes to the list of packaged revenue codes should be proposed for the CY 2010 OPPS. We expanded this evaluation to review all revenue codes in the revenue code-to-cost center crosswalk that we have used for OPPS ratesetting purposes in recent years against the CY 2008 NUBC definitions of revenue codes in place for CY 2008. As a result of that review, we proposed to revise the revenue code-to-cost center crosswalk as described in Table 2 of the CY 2010 OPPS/ASC proposed rule (74 FR 35256 through 35261).
Comment: Two commenters specifically addressed the proposed OPPS treatment of a number of revenue codes for CY 2010 and submitted identical, detailed recommendations. In general, the commenters agreed with the proposed treatment of revenue codes 0253 (Pharmacy; Take Home Drugs); 0290 (Durable Medical Equipment (other than renal); General Classification); 0291 (Durable Medical Equipment; Rental); 0292 (Durable Medical Equipment; Purchase of New DME); 0293 (Durable Medical Equipment; Purchase of Used DME); 0294 (Durable Medical Equipment; Supplies/Drugs for DME); 052x (Free-Standing Clinic; All Classifications); 066X (Respite Care; All Classifications); 0749, 0759, 0779, 0799, and 0910 (All Reserved); and 0948 (Other Therapeutic Services—Pulmonary Rehabilitation). The commenters disagreed with the proposed treatment of the revenue codes as displayed in Table 3 below, which provides the commenters' perspective on each revenue code.
Response: Specifically, our revenue proposal addressed: (1) Acknowledging that costs estimated from charges are associated with specific revenue codes when calculating OPPS payment rates; (2) identifying the appropriate cost center CCR that should be used to estimate costs for certain revenue codes; and (3) packaging of revenue center costs into the costs of separately paid procedures when revenue charges are reported without a HCPCS code. The commenters addressed some revenue codes that were explicitly identified and discussed in the CY 2010 OPPS/ASC proposed rule (74 FR 35256 through 35266), as well as some additional revenue codes. Table 3 below displays our response to each area where the commenters disagreed with our proposed treatment of the revenue code. Start Printed Page 60348
We note that we continually make our revenue code-to-cost center crosswalk available on the CMS Web site for review and comment, and we welcome further comments on the crosswalk from the public at any time.
Start Printed Page 60349 Start Printed Page 60350 Start Printed Page 60351 Start Printed Page 60352 Start Printed Page 60353Comment: One commenter suggested that CMS include dates in the revenue code-to-cost center crosswalk document to allow hospitals and CMS to easily track the effective dates for each change.
Response: We appreciate the desire to track changes to the revenue code-to-cost center crosswalk. However, rather than document changes to individual revenue codes in the crosswalk, we will provide the public with the current and past copies of the same revenue code-to-cost center crosswalk that we directly incorporate into our modeling of the OPPS payment rates each year.
Table 4 below shows the update to the revenue codes for which estimated costs on each claim for this final rule with comment period are based and incorporates the costs for those revenue codes into APC median cost estimates. Column A of Table 4 provides the 2008 revenue code and description. Column B indicates whether the charges reported with the revenue code will be converted to cost and incorporated into median cost estimates for CY 2010. Column C indicates whether the charges reported with the revenue code were converted to cost and incorporated into median cost estimates for the CY 2009 OPPS. In both columns, a “Y” indicates that the charges will be converted to cost in CY 2010 (or were converted for CY 2009), and an “N” indicates that charges reported under the revenue code will not be converted to cost and incorporated into median cost estimates. Finally, Column D provides our rationale for the CY 2010 final change.
Start Printed Page 60354 Start Printed Page 60355 Start Printed Page 60356 Start Printed Page 60357Also, as a result of our comprehensive review of the revenue codes included in the revenue code-to-cost center crosswalk, as we proposed, we are adding revenue codes to the hierarchy of primary, secondary, and tertiary hospital cost report cost centers that result in the departmental CCRs that we use to estimate cost from charges for some revenue codes or to revise the applicable cost centers associated with a given revenue code. Table 5 below lists the revenue codes for which we made changes to the revenue code-to-cost center crosswalk for CY 2010 ratesetting and our rationale for each change. With the exception of revenue code 0942 (Other Therapeutic Services; Education/Training), the revenue codes for which we made changes to the designated departmental CCRs are those identified in our comprehensive review that are also listed above in Table 4. Start Printed Page 60358
| 2008 Revenue code and description | Rationale for CY 2010 change |
|---|---|
| 0392—Administration, Processing and Storage for Blood and Blood Components; Processing and Storage | We crosswalked charges under revenue code 0392 to cost center 4700 (Blood Storing, Processing, & Transfusing) because we believe that cost center 4700 is the most likely departmental cost center to which hospitals would assign the costs of blood processing and storage. We made no secondary or tertiary cost centers because we believe that no other departmental cost centers are appropriate. |
| 0623—Medical Surgical Supplies—Extension of 027X; Surgical Dressings | We crosswalked the charges reported under revenue code 0623 to cost center 5500 (Medical Supplies Charged to Patients) as the primary cost center because we believe that the costs associated with the charges for surgical dressings are most likely to be assigned by hospitals to cost center 5500. We made no secondary or tertiary cost centers because we believe that no other departmental cost centers are appropriate. |
| 0942—Other Therapeutic Services (also see 095x, an extension of 094x); Educ/Training | We crosswalked the charges under revenue code 0942 to cost center 6000 (Clinic) as the primary cost center. Previously, the charges under revenue code 0942 were crosswalked to the overall ancillary CCR. As discussed above, we believe that cost center 6000 is a more appropriate primary cost center. We made no secondary or tertiary cost centers because we believe that no other departmental cost centers are appropriate. |
| 0948—Other Therapeutic Services (also see 095x, an extension of 094x); Pulmonary Rehabilitation | We crosswalked the charges under revenue code 0948 to cost center 4900 (Respiratory Therapy) as primary and to cost center 6000 (Clinic) as secondary because we believe that hospitals are most likely to assign the costs of these services to these cost centers. We are not establishing a tertiary cost center. |
Having revised the revenue code-to-cost center crosswalk, we then converted the charges to costs on each claim by applying the CCR that we believed was best suited to the revenue code indicated on the line with the charge. One exception to this general methodology for converting charges to costs on each claim is the calculation of median blood costs, as discussed in section II.A.2.d.(2) of the proposed rule and this final rule with comment period.
Thus, we applied CCRs as described above to claims with bill type 12X, 13X, or 14X, excluding all claims from CAHs and hospitals in Maryland, Guam, the U.S. Virgin Islands, American Samoa, and the Northern Mariana Islands and claims from all hospitals for which CCRs were flagged as invalid.
We identified claims with condition code 41 as partial hospitalization services of hospitals and moved them to another file. These claims were combined with the 76X claims identified previously to calculate the partial hospitalization per diem rates. We note that the separate file containing partial hospitalization claims is included in the files that are available for purchase as discussed above.
We then excluded claims without an HCPCS code. We moved to another file claims that contained nothing but influenza and pneumococcal pneumonia (PPV) vaccines. Influenza and PPV vaccines are paid at reasonable cost and, therefore, these claims are not used to set OPPS rates.
We next copied line-item costs for drugs, blood, and brachytherapy sources (the lines stay on the claim, but are copied onto another file) to a separate file. No claims were deleted when we copied these lines onto another file. These line-items are used to calculate a per unit mean and median cost and a per day mean and median cost for drugs, therapeutic radiopharmaceutical agents, and brachytherapy sources, as well as other information used to set payment rates, such as a unit-to-day ratio for drugs.
To implement our policy to redistribute some portion of total cost for packaged drugs and biologicals to the separately payable drugs and biologicals as acquisition and pharmacy overhead and handling costs discussed in section V.B.3. of this final rule with comment period, we used the line-item cost data for drugs and biologicals for which we had an HCPCS code with ASP pricing information to calculate the ASP+X values first for all drugs and biologicals, and then for separately payable drugs and biologicals and for packaged drugs and biologicals, respectively, by taking the ratio of total claim cost for each group relative to total ASP dollars (per unit of each drug or biological HCPCS code's July 2009 ASP amount multiplied by total units for each drug or biological in the CY 2008 claims data). These values are ASP+11 percent, ASP–3 percent, and ASP+259 percent, respectively. As we discuss in greater detail in section V.B.3. of this final rule with comment period, we are finalizing a policy to redistribute $150 million of the total cost in our claims data for packaged drugs and biologicals that have an associated ASP from packaged drugs with an ASP to separately payable drugs and biologicals. The $150 million is, roughly, one-third of the difference of $445 million between the total cost of packaged drugs and biologicals with an associated ASP in our CY 2008 claims data ($616 million) and ASP for the same drugs and biologicals ($171 million). In response to comments that CMS excluded valid overhead and handling costs associated with drugs lacking ASP information, largely costs estimated from uncoded charges reported under pharmacy revenue codes, we also are finalizing a policy to redistribute an additional $50 million of the total cost in our claims data for drugs and biologicals lacking an ASP, largely for estimated costs associated with uncoded charges billed under pharmacy revenue code series 025X (Pharmacy (also see 063X, an extension of 025X)), 026X (IV Therapy), and 063X (Pharmacy—Extension of 025X). As we state in section V.B.3. of this final rule with comment period, because we do not know ASP for this subset of drug costs, we do not know the amount of associated pharmacy overhead. We observe about $656 million for drugs lacking an ASP in our CY 2008 claims data. This total excludes the cost of diagnostic and therapeutic radiopharmaceuticals because they are not reported under pharmacy revenue codes or under the pharmacy cost center on the cost report.
Removing a total of $150 million in pharmacy overhead cost from packaged drugs and biologicals reduces the $616 million to $466 million, a 24 percent reduction. Removing $50 million from the cost of drugs lacking an ASP reduces the $656 million to $606 million, an 8 percent reduction. To implement our final CY 2010 policy to redistribute $150 million in claim cost from Start Printed Page 60359 packaged drugs and biologicals with an ASP to separately payable drugs and biologicals and $50 million in claim cost from packaged drugs and biologicals lacking an ASP, including uncoded pharmacy revenue code charges, we multiplied the cost of each packaged drug or biological with an HCPCS code and ASP pricing information in our CY 2008 claims data by 0.76, and we multiplied all other packaged drug costs in our CY 2008 claims data, excluding those for diagnostic radiopharmaceuticals, by 0.92. We also added the redistributed $200 million to the total cost of separately payable drugs and biologicals in our CY 2008 claims data, which increased the relationship between the total cost for separately payable drugs and biologicals and ASP dollars for the same drugs and biologicals to ASP+4 percent.
For CY 2010, we added an additional trim in our claims preparation to remove line-items that were not paid during claim processing, presumably for a line-item rejection or denial. The number of edits for valid OPPS payment in the Integrated Outpatient Code Editor (I/OCE) and elsewhere has grown significantly in the past few years, especially with the implementation of the full spectrum of National Correct Coding Initiative (NCCI) edits. To ensure that we are using valid claims that represent the cost of payable services to set payment rates, we removed line-items with an OPPS status indicator for the claim year (CY 2008) and a status indicator of “S,” “T,” “V,” or “X” when separately paid under the final CY 2010 payment system. This logic preserves charges for services that would not have been paid in the claim year but for which some estimate of cost is needed for the prospective year, such as services newly proposed to come off the inpatient list for CY 2010 which were assigned status indicator “C” in the claim year.
Using February 2009 APC Panel data, we estimate that the impact of removing line-items with valid status indicators that received no CY 2008 payment was limited to approximately 1.4 percent of all line-items for separately paid services. This additional trim reduced the number of single bills available for ratesetting by 1.5 percent. For approximately 92 percent of procedural APCs, we observed a change in the APC median cost of less than 1 percent. A handful of APCs experienced greater changes in median cost. For example, APC 0618 (Trauma Response with Critical Care) experienced declines in both the number of single bills used to set the median cost and the estimated median cost itself. This occurred because the I/OCE has an edit to ensure that HCPCS code G0390 (Trauma response team activation associated with hospital critical care service), which is assigned to APC 0618, receives payment only when one unit of G0390 appears with both a revenue code in the 68x series and CPT code 99291 (Critical care, evaluation and management of the critically ill or critically injured patient; first 30–74 minutes) on the claim for the same date of service, as described in the CY 2007 OPPS/ASC final rule with comment period (71 FR 68134). If the I/OCE criteria are not met, HCPCS code G0390 is not separately paid, and we found that a number of CY 2008 claims including HCPCS code G0390 did not meet the criteria for payment. On the other hand, a few APCs had greater estimated median costs and greater numbers of single bills as a result of this additional trim, presumably because removing lines from the claim allowed us to identify more single bills. We believe that removing lines with valid status indicators that were edited and not paid during claims processing increases the accuracy of the single bills used to determine the APC median costs for ratesetting.
Comment: One commenter claimed that the removal of charges and costs from denied lines was in contrast to longstanding policy for hospital inpatient services. A few commenters expressed concern about APC 0312 (Radioelement Applications), noting that there has been significant fluctuation in the payment rates for this APC in the past. They believe that implementing the proposed line-item trim, which removed a significant number of single claims, may have contributed to that instability. The commenters suggested that historical data would not indicate any reason for significant line-items to be trimmed. One commenter believed that the payment rates for low dose rate prostate brachytherapy were arbitrary and unfair. Based on the commenters' impression that the purpose of the line-item trim was to act as a quality check, the commenters requested that the line-item trim be suppressed for APC 0312.
Response: While payment systems such as the IPPS do not remove charge and cost data, this is largely due to the differences in the fundamental structures of the two payment systems. The IPPS is a system based on DRGs that relies on significant bundling of services under common clinical scenarios, while the OPPS is largely based on payment for a specific individual service. These differences in payment approach under each system are reflected in the way that data are used to establish the payment weights, from the CCRs used to reduce charges to cost to the structure of how charge and cost information is classified. One byproduct of the differences between the IPPS and the OPPS is the level of editing in each system to ensure that a correct payment is made. Similarly, there are many NCCI edits to ensure that payment is made to hospitals for outpatient services only when there is correct coding because there are hundreds of APCs that may contribute to inappropriate unbundling of services when those services are reported for a hospital outpatient encounter. In the CY 2010 OPPS/ASC proposed rule (74 FR 35262), we indicated that removing lines with valid status indicators that were edited and not paid during claims processing increases the accuracy of the single bills used for ratesetting. Doing so allows the single bills used for ratesetting purposes to be representative of those services as they would be paid in the prospective year.
In studying the billing patterns for HCPCS codes that are assigned to APC 0312, we noted that the line-item trim removes a number of unpaid single bills for this APC, as the commenters had suggested. However, we also observed a general decline in the reporting of services assigned to this APC that was unrelated to the line-item trim, suggesting that a portion of the observed decline in the number of single bills available for ratesetting is due to an actual reduction in the frequency that the services assigned to APC 0312 are furnished. While we understand the commenters' concern regarding the reduction of single bills used in ratesetting for APC 0312, the data suggest that the reduction is due in part to a decline in the billing of individual services assigned to the APC. Further, we believe that removing these line-items which have likely been rejected or denied is appropriate in light of the goal of using accurate single procedure claims for ratesetting under the OPPS.
After consideration of the public comments received relating to our CY 2010 proposal for claims preparation, we are adopting it as final, with modification to the treatment of certain revenue codes as described in Table 4 in this section.
b. Splitting Claims and Creation of “Pseudo” Single Claims
(1) Splitting Claims
We then split the remaining claims into five groups: single majors, multiple majors, single minors, multiple minors, and other claims. (Specific definitions Start Printed Page 60360 of these groups follow below.) In the CY 2010 OPPS/ASC proposed rule (74 FR 35262), we proposed to continue our current policy of defining major procedures as any HCPCS code having a status indicator of “S,” “T,” “V,” or “X;” defining minor procedures as any code having a status indicator of “F,” “G,” “H,” “K,” “L,” “R,” “U,” or “N,” and classifying “other” procedures as any code having a status indicator other than one that we have classified as major or minor. For CY 2010, we proposed to continue assigning status indicator “R” to blood and blood products; status indicator “U” to brachytherapy sources; status indicator “Q1” to all “STVX-packaged codes;” status indicator “Q2” to all “T-packaged codes;” and status indicator “Q3” to all codes that may be paid through a composite APC based on composite-specific criteria or paid separately through single code APCs when the criteria are not met. As discussed in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68709), we established status indicators “Q1,” “Q2,” and “Q3” to facilitate identification of the different categories of codes. We proposed to treat these codes in the same manner for data purposes for CY 2010 as we have treated them since CY 2008. Specifically, we proposed to continue to evaluate whether the criteria for separate payment of codes with status indicator “Q1” or “Q2” are met in determining whether they are treated as major or minor codes. As discussed earlier in this section, because we proposed to treat CPT code 76098 as conditionally packaged, this logic now includes the addition of CPT code 76098 as a “Q2” code. Codes with status indicator “Q1” or “Q2” are carried through the data either with status indicator “N” as packaged or, if they meet the criteria for separate payment, they are given the status indicator of the APC to which they are assigned and are considered as “pseudo” single major codes. Codes assigned status indicator “Q3” are paid under individual APCs unless they occur in the combinations that qualify for payment as composite APCs and, therefore, they carry the status indicator of the individual APC to which they are assigned through the data process and are treated as major codes during both the split and “pseudo” single creation process. The calculation of the median costs for composite APCs from multiple major claims is discussed in section II.A.2.e. of this final rule with comment period.
Specifically, we divided the remaining claims into the following five groups:
1. Single Major Claims: Claims with a single separately payable procedure (that is, status indicator “S,” “T,” “V,” or “X,” which includes codes with status indicator “Q3”); claims with one unit of a status indicator “Q1” code (“STVX-packaged”) where there was no code with status indicator “S,” “T,” “V,” or “X” on the same claim on the same date; or claims with one unit of a status indicator “Q2” code (“T-packaged”) where there was no code with a status indicator “T” on the same claim on the same date.
2. Multiple Major Claims: Claims with more than one separately payable procedure (that is, status indicator “S,” “T,” “V,” or “X,” which includes codes with status indicator “Q3”), or multiple units of one payable procedure. These claims include those codes with a status indicator “Q2” code (“T-packaged”) where there was no procedure with a status indicator “T” on the same claim on the same date of service but where there was another separately paid procedure on the same claim with the same date of service (that is, another code with status indicator “S,” “V,” or “X”). We also include in this set, claims that contained one unit of one code when the bilateral modifier was appended to the code and the code was conditionally or independently bilateral. In these cases, the claims represented more than one unit of the service described by the code, notwithstanding that only one unit was billed.
3. Single Minor Claims: Claims with a single HCPCS code that was assigned status indicator “F,” “G,” “H,” “K,” “L,” “R,” “U,” or “N” and not status indicator “Q1” (“STVX-packaged”) or status indicator “Q2” (“T-packaged”) code.
4. Multiple Minor Claims: Claims with multiple HCPCS codes that are assigned status indicator “F,” “G,” “H,” “K,” “L,” “R,” “U,” or “N;” claims that contain more than one code with status indicator “Q1” (“STVX-packaged”) or more than one unit of a code with status indicator “Q1” but no codes with status indicator “S,” “T,” “V,” or “X” on the same date of service; or claims that contain more than one code with status indicator “Q2” (T-packaged), or “Q2” and “Q1,” or more than one unit of a code with status indicator “Q2” but no code with status indicator “T” on the same date of service.
5. Non-OPPS Claims: Claims that contain no services payable under the OPPS (that is, all status indicators other than those listed for major or minor status). These claims were excluded from the files used for the OPPS. Non-OPPS claims have codes paid under other fee schedules, for example, durable medical equipment or clinical laboratory tests, and do not contain a code for a separately payable or packaged OPPS service. Non-OPPS claims include claims for therapy services paid sometimes under the OPPS but billed, in these non-OPPS cases, with revenue codes indicating that the therapy services would be paid under the Medicare Physician Fee Schedule (MPFS).
The claims listed in numbers 1, 2, 3, and 4 above are included in the data file that can be purchased as described above. Claims that contain codes to which we have assigned status indicators “Q1” (“STVX-packaged”) and “Q2” (“T-packaged”) appear in the data for the single major file, the multiple major file, and the multiple minor file used in this final rule with comment period. Claims that contain codes to which we have assigned status indicator “Q3” (composite APC members) appear in both the data of the single and multiple major files used in this final rule with comment period, depending on the specific composite calculation.
Because we did not receive any public comments on our proposed process of organizing claims by type, we are finalizing our CY 2010 proposal without modification.
(2) Creation of “Pseudo” Single Claims
As we proposed, to develop “pseudo” single claims for this final rule with comment period, we examined both the multiple major claims and the multiple minor claims. We first examined the multiple major claims for dates of service to determine if we could break them into “pseudo” single procedure claims using the dates of service for all lines on the claim. If we could create claims with single major procedures by using dates of service, we created a single procedure claim record for each separately payable procedure on a different date of service (that is, a “pseudo” single).
We also used the bypass codes listed earlier in Table 1 and discussed in section II.A.1.b. of this final rule with comment period to remove separately payable procedures that we determined contained limited or no packaged costs or that were otherwise suitable for inclusion on the bypass list from a multiple procedure bill. As discussed above, we ignore the “overlap bypass codes,” that is, those HCPCS codes that are both on the bypass list and are members of the multiple imaging Start Printed Page 60361 composite APCs, in this initial assessment for “pseudo” single claims. The CY 2010 “overlap bypass codes” are listed in Table 1 in section II.A.1.b. of this final rule with comment period. When one of the two separately payable procedures on a multiple procedure claim was on the bypass list, we split the claim into two “pseudo” single procedure claim records. The single procedure claim record that contained the bypass code did not retain packaged services. The single procedure claim record that contained the other separately payable procedure (but no bypass code) retained the packaged revenue code charges and the packaged HCPCS code charges. We also removed lines that contained multiple units of codes on the bypass list and treated them as “pseudo” single claims by dividing the cost for the multiple units by the number of units on the line. Where one unit of a single, separately payable procedure code remained on the claim after removal of the multiple units of the bypass code, we created a “pseudo” single claim from that residual claim record, which retained the costs of packaged revenue codes and packaged HCPCS codes. This enabled us to use claims that would otherwise be multiple procedure claims and could not be used.
We then assessed the claims to determine if the criteria for the multiple imaging composite APCs, discussed in section II.A.2.e.(5) of this final rule with comment period, were met. Where the criteria for the imaging composite APCs were met, we created a “single session” claim for the applicable imaging composite service and determined whether we could use the claim in ratesetting. For HCPCS codes that are both conditionally packaged and are members of a multiple imaging composite APC, we first assessed whether the code would be packaged and if so, the code ceased to be available for further assessment as part of the composite APC. Because the packaged code would not be a separately payable procedure, we considered it to be unavailable for use in setting the composite APC median cost. Having identified “single session” claims for the imaging composite APCs, we reassessed the claim to determine if, after removal of all lines for bypass codes, including the “overlap bypass codes,” a single unit of a single separately payable code remained on the claim. If so, we attributed the packaged costs on the claim to the single unit of the single remaining separately payable code other than the bypass code to create a “pseudo” single claim. We also identified line-items of overlap bypass codes as a “pseudo” single claim. This allowed us to use more claims data for ratesetting purposes.
We also examined the multiple minor claims to determine whether we could create “pseudo” single procedure claims. Specifically, where the claim contained multiple codes with status indicator “Q1” (“STVX-packaged”) on the same date of service or contained multiple units of a single code with status indicator “Q1,” we selected the status indicator “Q1” HCPCS code that had the highest CY 2008 relative weight, set the units to one on that HCPCS code to reflect our policy of paying only one unit of a code with a status indicator of “Q1.” We then packaged all costs for the following into a single cost for the “Q1” HCPCS code that had the highest CY 2008 relative weight to create a “pseudo” single claim for that code: additional units of the status indicator “Q1” HCPCS code with the highest CY 2008 relative weight; other codes with status indicator “Q1;” and all other packaged HCPCS codes and packaged revenue code costs. We changed the status indicator for selected codes from the data status indicator of “N” to the status indicator of the APC to which the selected procedure was assigned for further data processing and considered this claim as a major procedure claim. We used this claim in the calculation of the APC median cost for the status indicator “Q1” HCPCS code.
Similarly, where a multiple minor claim contained multiple codes with status indicator “Q2” (“T-packaged”) or multiple units of a single code with status indicator “Q2,” we selected the status indicator “Q2” HCPCS code that had the highest CY 2008 relative weight, set the units to one on that HCPCS code to reflect our policy of paying only one unit of a code with a status indicator of “Q2.” We then packaged all costs for the following into a single cost for the “Q2” HCPCS code that had the highest CY 2008 relative weight to create a “pseudo” single claim for that code: additional units of the status indicator “Q2” HCPCS code with the highest CY 2008 relative weight; other codes with status indicator “Q2;” and other packaged HCPCS codes and packaged revenue code costs. We changed the status indicator for the selected code from a data status indicator of “N” to the status indicator of the APC to which the selected code was assigned, and we considered this claim as a major procedure claim.
Lastly, where a multiple minor claim contained multiple codes with status indicator “Q2” (“T-packaged”) and status indicator “Q1” (“STVX-packaged”), we selected the status indicator “Q2” HCPCS code (“T-packaged”) that had the highest relative weight for CY 2008 and set the units to one on that HCPCS code to reflect our policy of paying only one unit of a code with a status indicator of “Q2.” We then packaged all costs for the following into a single cost for the selected (“T packaged”) HCPCS code to create a “pseudo” single claim for that code: additional units of the status indicator “Q2” HCPCS code with the highest CY 2008 relative weight; other codes with status indicator “Q2;” codes with status indicator “Q1” (“STVX-packaged”); and other packaged HCPCS codes and packaged revenue code costs. We favor status indicator “Q2” over “Q1” HCPCS codes because “Q2” HCPCS codes have higher CY 2008 relative weights. If a status indicator “Q1” HCPCS code had a higher CY 2008 relative weight, it would become the primary code for the simulated single bill process. We changed the status indicator for the selected status indicator “Q2” (“T-packaged”) code from a data status indicator of “N” to the status indicator of the APC to which the selected code was assigned and we considered this claim as a major procedure claim.
We excluded those claims that we were not able to convert to single claims even after applying all of the techniques for creation of “pseudo” singles to multiple major and to multiple minor claims. As has been our practice in recent years, we also excluded claims that contained codes that were viewed as independently or conditionally bilateral and that contained the bilateral modifier (Modifier 50 (Bilateral procedure)) because the line-item cost for the code represented the cost of two units of the procedure, notwithstanding that the code appeared with a unit of one.
Comment: One commenter noted that the bilateral procedure logic did not appear to appropriately exclude claims with bilateral codes from the single major claims, having observed bilateral procedure codes in that claims subset. Also, the commenter suggested that the conditional packaging of the status indicator “Q2” (“T-packaged”) codes did not appear to be treated consistently with the policy we proposed, which was that a “Q2” procedure with the highest scaled weight would be paid separately when there is no status indicator “T” procedure on the claim and that the costs of any other “Q2” codes on the claim would be packaged.
Response: In seeking to address the commenter's observations, we discovered that the bilateral logic was Start Printed Page 60362 not processed correctly as we proposed. Similarly, inaccurate program logic in the weight comparison for status indicator “Q2” (“T-packaged”) codes caused the packaging to be assigned based on order of precedence rather than by weight.
For this final rule with comment period, we accurately applied the bilateral and status indicator “Q2” (“T-packaged”) weight comparison packaging logic, consistent with the proposed and final policy. The national unadjusted payments for CY 2010 accurately reflect the policy that we proposed to continue for CY 2010 OPPS and that we are finalizing in this final rule with comment period.
After consideration of the public comment received, we are finalizing our CY 2010 proposal, without modification, for the process by which we develop “pseudo” single procedure claims.
c. Completion of Claim Records and Median Cost Calculations
We then packaged the costs of packaged HCPCS codes (codes with status indicator “N” listed in Addendum B to this final rule with comment period and the costs of those lines for codes with status indicator “Q1” or “Q2” when they are not separately paid), and the costs of packaged revenue codes into the cost of the single major procedure remaining on the claim. For CY 2010, this packaging also included the redistributed packaged pharmacy overhead cost relative to the units of separately payable drugs on each single procedure claim.
As noted in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66606), for the CY 2008 OPPS, we adopted an APC Panel recommendation that requires CMS to review the final list of packaged revenue codes for consistency with OPPS policy and ensure that future versions of the I/OCE edit accordingly. We compared the packaged revenue codes in the I/OCE to the final list of packaged revenue codes for the CY 2009 OPPS (73 FR 68531 through 68532) that we used for packaging costs in median calculation. As a result of that analysis, we proposed to use the packaged revenue codes for CY 2010 that were displayed in Table 4 of the CY 2010 OPPS/ASC proposed rule (74 FR 35265 through 35266).
As noted in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68531), we replaced the NUBC standard abbreviations for the revenue codes listed in Table 2 of the CY 2009 OPPS/ASC proposed rule with the most current NUBC descriptions of the revenue code categories and subcategories to better articulate the meanings of the revenue codes without actually changing the proposed list of revenue codes. In the course of making the changes in labeling for the revenue codes in Table 2 of the CY 2009 OPPS/ASC final rule with comment period, we noticed some changes to revenue categories and subcategories that we believed warranted further review for future OPPS updates. Although we finalized the list of packaged revenue codes in Table 2 for CY 2009, we indicated in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68531) that we intended to assess the NUBC revenue codes to determine whether any changes to the list of packaged revenue codes should be proposed for the CY 2010 OPPS. We specifically requested public input and discussion on this issue during the comment period of the CY 2009 OPPS/ASC final rule with comment period. We did not receive any public comments on this issue. As we discuss in section II.A.2.a. of this final rule with comment period, we have completed that analysis for all revenue codes in the revenue code-to-cost center crosswalk. As discussed in the CY 2010 OPPS/ASC proposed rule (74 FR 35264 through 35265), as a result, we proposed to add several revenue codes to the list of packaged revenue codes for the CY 2010 OPPS. Specifically, we believe that the costs derived from charges reported under revenue codes 0261 (IV Therapy; Infusion Pump); 0392 (Administration, Processing and Storage for Blood and Blood Components; Processing and Storage); 0623 (Medical Supplies—Extension of 027X, Surgical Dressings); 0943 (Other Therapeutic Services (also see 095X, an extension of 094X), Cardiac Rehabilitation); and 0948 (Other Therapeutic Services (also see 095X, an extension of 094X), Pulmonary Rehabilitation) are appropriately packaged into payment for other OPPS services when charges appear on lines with these revenue codes but no HCPCS code appears on the line. Revenue codes that we proposed to add to the CY 2010 packaged revenue code list were identified by asterisks (*) in Table 4 of the CY 2010 OPPS/ASC proposed rule.
The public comments that we received that resulted in our changing the list of packaged revenue codes for CY 2010 are discussed in section II.A.2.a. of this final rule with comment period. Thus, we are finalizing the proposed packaged revenue codes for CY 2010, with modification. The final CY 2010 packaged revenue codes are listed in Table 6 below. Revenue codes that we are adding to the CY 2010 packaged revenue code list are identified by asterisks (*) in Table 6.
| Revenue code | Description |
|---|---|
| 0250 | Pharmacy; General Classification. |
| 0251 | Pharmacy; Generic Drugs. |
| 0252 | Pharmacy; Non-Generic Drugs. |
| 0254 | Pharmacy; Drugs Incident to Other Diagnostic Services. |
| 0255 | Pharmacy; Drugs Incident to Radiology. |
| 0257 | Pharmacy; Non-Prescription. |
| 0258 | Pharmacy; IV Solutions. |
| 0259 | Pharmacy; Other Pharmacy. |
| 0260 | IV Therapy; General Classification. |
| *0261 | IV Therapy; Infusion Pump. |
| 0262 | IV Therapy; IV Therapy/Pharmacy Svcs. |
| 0263 | IV Therapy; IV Therapy/Drug/Supply Delivery. |
| 0264 | IV Therapy; IV Therapy/Supplies. |
| 0269 | IV Therapy; Other IV Therapy. |
| 0270 | Medical/Surgical Supplies and Devices; General Classification. |
| 0271 | Medical/Surgical Supplies and Devices; Non-sterile Supply. |
| 0272 | Medical/Surgical Supplies and Devices; Sterile Supply. |
| 0275 | Medical/Surgical Supplies and Devices; Pacemaker. |
| 0276 | Medical/Surgical Supplies and Devices; Intraocular Lens. |
| 0278 | Medical/Surgical Supplies and Devices; Other Implants. |
| 0279 | Medical/Surgical Supplies and Devices; Other Supplies/Devices. |
| 0280 | Oncology; General Classification. |
| 0289 | Oncology; Other Oncology. |
| 0343 | Nuclear Medicine; Diagnostic Radiopharmaceuticals. |
| 0344 | Nuclear Medicine; Therapeutic Radiopharmaceuticals. |
| 0370 | Anesthesia; General Classification. |
| 0371 | Anesthesia; Anesthesia Incident to Radiology. |
| 0372 | Anesthesia; Anesthesia Incident to Other DX Services. |
| 0379 | Anesthesia; Other Anesthesia. |
| 0390 | Administration, Processing and Storage for Blood and Blood Components; General Classification. |
| Start Printed Page 60363 | |
| *0392 | Administration, Processing and Storage for Blood and Blood Components; Processing and Storage. |
| 0399 | Administration, Processing and Storage for Blood and Blood Components; Other Blood Handling. |
| 0621 | Medical Surgical Supplies—Extension of 027X; Supplies Incident to Radiology. |
| 0622 | Medical Surgical Supplies—Extension of 027X; Supplies Incident to Other DX Services. |
| *0623 | Medical Supplies—Extension of 027X, Surgical Dressings. |
| 0624 | Medical Surgical Supplies—Extension of 027X; FDA Investigational Devices. |
| 0630 | Pharmacy—Extension of 025X; Reserved. |
| 0631 | Pharmacy—Extension of 025X; Single Source Drug. |
| 0632 | Pharmacy—Extension of 025X; Multiple Source Drug. |
| 0633 | Pharmacy—Extension of 025X; Restrictive Prescription. |
| 0681 | Trauma Response; Level I Trauma. |
| 0682 | Trauma Response; Level II Trauma. |
| 0683 | Trauma Response; Level III Trauma. |
| 0684 | Trauma Response; Level IV Trauma. |
| 0689 | Trauma Response; Other. |
| 0700 | Cast Room; General Classification. |
| 0710 | Recovery Room; General Classification. |
| 0720 | Labor Room/Delivery; General Classification. |
| 0721 | Labor Room/Delivery; Labor. |
| 0732 | EKG/ECG (Electrocardiogram); Telemetry. |
| 0762 | Specialty Room—Treatment/Observation Room; Observation Room. |
| 0801 | Inpatient Renal Dialysis; Inpatient Hemodialysis. |
| 0802 | Inpatient Renal Dialysis; Inpatient Peritoneal Dialysis (Non-CAPD). |
| 0803 | Inpatient Renal Dialysis; Inpatient Continuous Ambulatory Peritoneal Dialysis (CAPD). |
| 0804 | Inpatient Renal Dialysis; Inpatient Continuous Cycling Peritoneal Dialysis (CCPD). |
| 0809 | Inpatient Renal Dialysis; Other Inpatient Dialysis. |
| 0810 | Acquisition of Body Components; General Classification. |
| 0819 | Inpatient Renal Dialysis; Other Donor. |
| 0821 | Hemodialysis-Outpatient or Home; Hemodialysis Composite or Other Rate. |
| 0824 | Hemodialysis-Outpatient or Home; Maintenance—100%. |
| 0825 | Hemodialysis-Outpatient or Home; Support Services. |
| 0829 | Hemodialysis-Outpatient or Home; Other OP Hemodialysis. |
| 0942 | Other Therapeutic Services (also see 095X, an extension of 094x); Education/Training. |
| *0943 | Other Therapeutic Services (also see 095X, an extension of 094X), Cardiac Rehabilitation. |
| *0948 | Other Therapeutic Services (also see 095X, an extension of 094X), Pulmonary Rehabilitation. |
In addition, we excluded: (1) claims that had zero costs after summing all costs on the claim, and (2) claims containing packaging flag number 3. Effective for services furnished on or after July 1, 2004, the I/OCE assigned packaging flag number 3 to claims on which hospitals submitted token charges for a service with status indicator “S” or “T” (a major separately payable service under the OPPS) for which the fiscal intermediary or MAC was required to allocate the sum of charges for services with a status indicator equaling “S” or “T” based on the relative weight of the APC to which each code was assigned. We do not believe that these charges, which were token charges as submitted by the hospital, are valid reflections of hospital resources. Therefore, we deleted these claims. We also deleted claims for which the charges equaled the revenue center payment (that is, the Medicare payment) on the assumption that where the charge equaled the payment, to apply a CCR to the charge would not yield a valid estimate of relative provider cost.
For the remaining claims, we then standardized 60 percent of the costs of the claim (which we have previously determined to be the labor-related portion) for geographic differences in labor input costs. We made this adjustment by determining the wage index that applied to the hospital that furnished the service and dividing the cost for the separately paid HCPCS code furnished by the hospital by that wage index. As has been our policy since the inception of the OPPS, we proposed to use the pre-reclassified wage indices for standardization because we believe that they better reflect the true costs of items and services in the area in which the hospital is located than the post-reclassification wage indices and, therefore, would result in the most accurate unadjusted median costs.
We also excluded claims that were outside 3 standard deviations from the geometric mean of units for each HCPCS code on the bypass list (because, as discussed above, we used claims that contain multiple units of the bypass codes).
After removing claims for hospitals with error CCRs, claims without HCPCS codes, claims for immunizations not covered under the OPPS, and claims for services not paid under the OPPS, approximately 58 million claims were left. Using these 58 million claims, we created approximately 99 million single and “pseudo” single claims, of which we used 99 million single bills (after trimming out approximately 657,000 claims as discussed above in this section) in the CY 2010 median development and ratesetting.
We used these claims to calculate the CY 2010 median costs for each separately payable HCPCS code and each APC. The comparison of HCPCS code-specific and APC medians determines the applicability of the 2 times rule. Section 1833(t)(2) of the Act provides that, subject to certain exceptions, the items and services within an APC group cannot be considered comparable with respect to the use of resources if the highest median (or mean cost, if elected by the Secretary) for an item or service in the group is more than 2 times greater than the lowest median cost for an item or service within the same group (the 2 times rule). Finally, we reviewed the median costs for this final rule with comment period and reassigned HCPCS codes to different APCs where we believed that it was appropriate. Section III. of this final rule with comment period includes a discussion of certain HCPCS code assignment changes that resulted from examination of the median costs, review of the public comments, and for other reasons. The APC medians were recalculated after we reassigned the affected HCPCS codes. Both the HCPCS code-specific medians and the APC medians were weighted to account for the inclusion of multiple units of the bypass codes in the creation of “pseudo” single bills. Start Printed Page 60364
Comment: Several commenters objected to the volatility of the OPPS rates from year to year. The commenters asserted that the absence of stability in the OPPS rates creates budgeting, planning, and operating problems for hospitals, and that as more care is provided on an outpatient, rather than inpatient basis, the need for stable payment rates from one year to the next becomes more important to hospitals. Some commenters suggested that CMS limit reductions in APC payments to a set percentage, with one commenter noting that CMS dampened payment decreases for blood and blood products to mitigate large payment fluctuations in order to limit provider losses. One commenter suggested that the median costs from claims be adjusted to limit changes from year to year. Another commenter suggested that CMS perform a thorough examination of the payment rates and examine billed charges, costs, median and mean costs, and CCRs to isolate the source of the fluctuations as well as mandate a review of all APCs that fluctuate above a certain percentage, similar to the 2 times rule.
Response: There are a number of factors pertinent to the OPPS that may cause median costs to change from one year to the next. Some of these are a reflection of hospital behavior, and some of them are a reflection of fundamental characteristics of the OPPS as defined in statute. For example, the OPPS payment rates are based on hospital cost report and claims data. However, hospital costs and charges change each year and this results in both changes to the CCRs taken from the most currently available cost reports and also differences in the charges on the claims that are the basis of the calculation of the median costs on which OPPS rates are based. Similarly, hospitals adjust their mix of services from year to year by offering new services and ceasing to furnish services and changing the proportion of the various services they furnish, which have an impact on the CCRs that we derive from their cost reports. CMS cannot stabilize these hospital-driven fundamental inputs to the calculation of OPPS payment rates.
Moreover, there are other essential elements of the OPPS which contribute to the changes in relative weights each year. These include, but are not limited to, reassignments of HCPCS codes to APCs to rectify 2 times violations as required by the law, to address the costs of new services, to address differences in hospitals' costs that may result from changes in medical practice, and to respond to public comments. Our efforts to improve payment accuracy may also contribute to payment volatility in the short run, as may be the case when we are eventually able to use more specific CCRs to estimate the costs of implantable devices, based on the final policy that we adopted to disaggregate the single cost center for medical supplies into two more specific cost centers, as described in the FY 2009 IPPS final rule (73 FR 48458 through 48467). Moreover, for some services, we cannot avoid using small numbers of claims, either because the volume of services is naturally low or because the claims data do not facilitate the calculation of a median cost for a single service. Where there are small numbers of claims that are used in median calculation, there is more volatility in the median cost from one year to the next. Lastly, changes to OPPS payment policy (for example, changes to packaging) also contribute to some extent to the fluctuations in the OPPS payment rates for the same services from year to year.
We cannot avoid the naturally occurring volatility in the cost report and claims data that hospitals submit and on which the payment rates are based. Moreover (with limited exceptions), we reassign HCPCS codes to APCs where it is necessary to avoid 2 times violations. However, we have made other changes to resolve some of the other potential reasons for instability from year to year. Specifically, we continue to seek ways to use more claims data so that we have fewer APCs for which there are small numbers of single bills used to set the APC median costs. Moreover, we have tried to eliminate APCs with very small numbers of single bills where we could do so. We recognize that changes to payment policies, such as the packaging of payment for ancillary and supportive services and the implementation of composite APCs, may contribute to volatility in payment rates in the short term, but we believe that larger payment packages and bundles should help to stabilize payments in future years by enabling us to use more claims data and by establishing payments for larger groups of services.
While we recognize the reasoning behind a policy that would dampen both increases and decreases in the weights or payment rates of the OPPS, this would not be as simple or beneficial as commenters have implied. Implementing such a dampening policy would require the assumption that payment policy is static from year to year. Based on the commenters' own acknowledgement, and the data used to develop the OPPS, we know that this is not true. Further, in seeking to mitigate fluctuations in the OPPS, implementing such a system would make payments less reflective of the true service costs. Dampening payments across all APCs in this way could unfairly harm those hospitals whose true cost for a service increases significantly, while inappropriately benefiting those hospitals whose true cost for a service decreases significantly. While one commenter requested that CMS adopt a policy to investigate any APCs that fluctuate above a certain threshold, this mandate would be unnecessary since we already examine all APCs that experience significant median cost fluctuations, as described in the CY 2010 OPPS/ASC proposed rule (74 FR 35626 through 35627).
Comment: Some commenters asked that CMS provide an adjustment for medical education costs under the OPPS because many of the costs of teaching services are now incurred in the HOPD as services previously furnished only in the inpatient setting are now being furnished in the HOPD. They also noted that the OPPS did not have a teaching adjustment while many of the other Medicare payment systems, such as inpatient, psychiatric, and rehabilitation facilities, already include one. These commenters stated that CMS indicated that it would study the costs and payment differential among different classes of providers in the April 7, 2000 OPPS final rule but has not done so. They recommended that CMS study whether the hospital outpatient costs of teaching hospitals are higher than the costs of other hospitals for purposes of determining whether there should be a teaching hospital adjustment. The commenters explained that analysis of 2007 Medicare cost reports showed that the average outpatient margins were −30.4 for major teaching hospitals, −13.8 for other teaching hospitals, and −14.4 for nonteaching hospitals. They believed that these findings demonstrated that the hospital outpatient costs of major teaching hospitals are significantly greater than the costs of other hospitals. The commenters requested that CMS conduct its own analysis and that if that analysis showed a difference due to the unique missions of teaching hospitals, CMS should add a teaching adjustment to the OPPS.
Response: Unlike payment under the IPPS, the law does not provide for payment for indirect medical education costs to be made under the OPPS. Section 1833(t)(2)(E) of the Act states that the Secretary shall establish, in a budget neutral manner “* * * other adjustments as determined to be necessary to ensure equitable payments, Start Printed Page 60365 such as adjustments for certain classes of hospitals.” We have not found such an adjustment to be necessary to ensure equitable payments to teaching hospitals and, therefore, have not developed such an adjustment. Furthermore, in this final rule with comment period, we have developed payment weights that we believe provide appropriate and adequate payment for the complex medical services, such as new technology services and device-dependent procedures, which we understand are furnished largely by teaching hospitals. We note that teaching hospitals benefit from the recalibration of the APCs in this final rule with comment period. The final CY 2010 impacts by class of hospital are displayed in Table 73 in section XXI.B. of this final rule with comment period.
After consideration of the public comments we received, we are finalizing our proposed CY 2010 methodology for calculating the median costs upon which the CY 2010 OPPS payment rates are based, with modifications as discussed throughout this section.
In some cases, APC median costs are calculated using variations of the process outlined above. Section II.A.2.d. of this final rule with comment period that follows addresses the calculation of single APC criteria-based median costs. Section II.A.2.e. of this final rule with comment period discusses the calculation of composite APC criteria-based median costs. Section X.B. of this final rule with comment period addresses the methodology for calculating the median cost for partial hospitalization services.
At the February 2009 APC Panel Meeting, the APC Panel recommended that CMS study the claims data for any APC in which the calculated payment reduction would be greater than 10 percent. The APC Panel also recommended that CMS provide a list of APCs to the APC Panel at the next meeting with a proposed payment rate change of greater than 10 percent. While we recognize the concerns the APC Panel expressed with regards to cost variability in the system, we already engage in a standard review process for all APCs that experience significant changes in median costs. We study all significant changes in estimated cost to determine the effect that proposed and final payment policies have on the APC payment rates and ensure that these policies are appropriate and that the intended cost estimation methodologies have been correctly applied. We note that there are a number of factors that cause APC median costs to change from one year to the next. Some of these are a reflection of hospital behavior, and some of them are a reflection of fundamental characteristics of the OPPS as defined in the statute. With limited exceptions, we are required by law to reassign HCPCS codes to APCs where it is necessary to avoid 2 times violations. Thus, there are various mechanisms already in place to ensure that we assess changes in cost and adjust APC weights accordingly or justify why we have not made adjustments. We plan to continue our examination of all APCs that experience changes of greater than 10 percent. In the CY 2010 OPPS/ASC proposed rule (74 FR 35267), we indicated that we would provide the APC Panel with a list of the APCs with proposed changes in costs of more than 10 percent for CY 2010 at the next CY 2009 APC Panel meeting. Accordingly, we accepted this recommendation of the APC Panel in full.
At the August 2009 meeting of the APC Panel, we provided the APC Panel a list of all APCs fluctuating by more than 10 percent when comparing the CY 2010 proposed rule APC median costs to those based on CY 2009 final rule data. We found that the median costs for 7 APCs decreased by 10 percent or more and the median costs for 63 APCs increased by 10 percent or more. These changes occurred due to some of the reasons described earlier, including reassignment of HCPCS codes from one APC to another to resolve 2 times violations, modeling changes such as the removal of lines for codes that were not payable in CY 2008 under the OPPS payment rules, low volumes of services influencing the claims used to determine APC median costs, and updated cost and charge information from hospital claims and cost reports. We noted that the median costs for 63 APCs increased by 10 percent or more and that the reasons for the increases were similar to the reasons for the decreases of more than 10 percent but, in general, we found nothing that raised concern regarding the data process we used to calculate the proposed median costs. The APC Panel discussed the different APCs on the list but did not express any significant concern with the fluctuations. As a result, they did not make any further recommendations related to the list of APCs with median costs fluctuating by greater than 10 percent.
At the February 2009 APC Panel meeting, we reviewed and examined the data process in preparation for the CY 2010 rulemaking cycle. At this meeting, the APC Panel recommended that the Data Subcommittee continue its work and we accepted that recommendation. The APC Panel further recommended at the August 2009 meeting that the Data Subcommittee continue its work. We are accepting this most recent recommendation, and we will continue to work closely with the APC Panel's Data Subcommittee to prepare and review data and analyses relevant to the APC configurations and OPPS payment policies for hospital outpatient items and services.
d. Calculation of Single Procedure APC Criteria-Based Median Costs
(1) Device-Dependent APCs
Device-dependent APCs are populated by HCPCS codes that usually, but not always, require that a device be implanted or used to perform the procedure. For a full history of how we have calculated payment rates for device-dependent APCs in previous years and a detailed discussion of how we developed the standard device-dependent APC ratesetting methodology, we refer readers to the CY 2008 OPPS/ASC final rule with comment period (72 FR 66739 through 66742). Overviews of the procedure-to-device edits and device-to-procedure edits used in ratesetting for device-dependent APCs are available in the CY 2005 OPPS final rule with comment period (69 FR 65761 through 65763) and the CY 2007 OPPS/ASC final rule with comment period (71 FR 68070 through 68071).
In the CY 2010 OPPS/ASC proposed rule (74 FR 35267), we proposed to revise our standard methodology for calculating median costs for device-dependent APCs, which utilizes claims data that generally represent the full cost of the required device, to exclude claims that contain the “FC” modifier. Specifically, we proposed to calculate the median costs for device-dependent APCs for CY 2010 using only the subset of single procedure claims from CY 2008 claims data that pass the procedure-to-device and device-to-procedure edits; do not contain token charges (less than $1.01) for devices; do not contain the “FB” modifier signifying that the device was furnished without cost to the provider, supplier, or practitioner, or where a full credit was received; and do not contain the “FC” modifier signifying that the hospital received partial credit for the device. The “FC” modifier became effective January 1, 2008, and is present for the first time on claims that would be used in OPPS ratesetting for CY 2010. We stated in the CY 2010 OPPS/ASC proposed rule (74 FR 35267) that we believe the standard methodology for calculating median costs for device- Start Printed Page 60366 dependent APCs, further refined to exclude claims with the “FC” modifier, gives us the most appropriate median costs for device-dependent APCs in which the hospital incurs the full cost of the device.
The median costs for the majority of device-dependent APCs that were calculated using the CY 2010 proposed rule claims data were generally stable, with most median costs increasing moderately compared to the median costs upon which the CY 2009 OPPS payment rates were based. However, the median costs for APC 0225 (Implantation of Neurostimulator Electrodes, Cranial Nerve) and APC 0418 (Insertion of Left Ventricular Pacing Electrode) demonstrated significant fluctuation. Specifically, the proposed CY 2010 median cost for APC 0225 increased approximately 49 percent compared to the final CY 2009 median cost, although this APC median cost had declined by approximately the same proportion from CY 2008 to CY 2009. The proposed CY 2010 median cost for APC 0418, which had decreased approximately 45 percent from CY 2008 to CY 2009, showed an increase of approximately 56 percent based on the claims data available for the CY 2010 proposed rule. As indicated in the CY 2010 OPPS/ASC proposed rule (74 FR 35267), we believe the fluctuations in median costs for these two APCs are a consequence of the small number of single bills upon which the median costs are based and the small number of providers of these services. As we have stated in the past, some fluctuation in relative costs from year to year is to be expected in a prospective payment system for low volume device-dependent APCs, particularly where there are small numbers of single bills from a small number of providers.
At the February 2009 meeting of the APC Panel, one presenter stated that the assignment of the single-array cranial neurostimulator pulse generator implantation procedure described by CPT code 61885 (Insertion or replacement of cranial neurostimulator pulse generator or receiver, direct or inductive coupling; with connection to a single electrode array) to APC 0039 (Level I Implantation of Neurostimulator Generator), along with the peripheral/gastric neurostimulator pulse generator implantation procedure described by CPT code 64590 (Insertion or replacement of peripheral or gastric neurostimulator pulse generator or receiver, direct or inductive coupling) is not appropriate, given the clinical and cost differences between the two procedures. According to the presenter, the cranial procedure described by CPT code 61885 is more similar clinically and in terms of resource utilization to the spinal neurostimulator pulse generator implantation procedure described by CPT code 63685 (Insertion or replacement of spinal neurostimulator pulse generator or receiver, direct or inductive coupling), which is the only CPT code assigned to APC 0222 (Level II Implantation of Neurostimulator) for CY 2009. The presenter requested that the APC Panel recommend that CMS restructure the existing configuration of neurostimulator pulse generator implantation APCs for CY 2010 by splitting APC 0039, so that procedures involving peripheral/gastric neurostimulators and cranial neurostimulators would be in distinct APCs, or by reassigning the cranial neurostimulator pulse generator implantation procedure described by CPT code 61885 from APC 0039 to APC 0222. In response to this request, the APC Panel recommended that CMS combine APC 0039 and APC 0222 for CY 2010, given the overall similarity in median costs among the cranial, peripheral/gastric, and spinal neurostimulator pulse generator implantation procedures assigned to these two APCs. The APC Panel also recommended that CMS maintain the configuration of APC 0315 (Level III Implantation of Neurostimulator Generator) as it currently exists in CY 2009 for CY 2010. The dual-array cranial neurostimulator pulse generator implantation procedure described by CPT code 61886 (Insertion or replacement of cranial neurostimulator pulse generator or receiver, direct or inductive coupling; with connection to two or more electrode arrays) is currently the only procedure assigned to APC 0315.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35267 through 35268), we stated that we agree with the APC Panel that the median costs of the procedures described by CPT codes 61885, 63685, and 64590 are sufficiently similar to warrant placement of the CPT codes into a single APC, rather than two APCs. We accepted the APC Panel's recommendation and, therefore, proposed to reassign CPT code 63685 to APC 0039, to delete APC 0222, and to maintain the current configuration of APC 0315 for CY 2010. We also proposed to change the title of APC 0315 to “Level II Implantation of Neurostimulator Generator” to reflect the proposed two-level, rather than three-level, structure of the neurostimulator pulse generator implantation APCs.
In reviewing the APC Panel recommendation for consolidating APC 0039 and APC 0222, we observed that the median costs of the procedures assigned to APC 0425 (Level II Arthroplasty or Implantation with Prosthesis) and APC 0681 (Knee Arthroplasty) also are sufficiently similar to warrant combining these two APCs into one APC. The proposed median cost for the only procedure currently assigned to APC 0681, described by CPT code 27446 (Arthroplasty, knee, condyle and plateau; medial OR lateral compartment), was approximately $7,464 based on the claims data available for the CY 2010 OPPS/ASC proposed rule. This proposed median cost was very similar to the proposed median cost of approximately $7,852 calculated for APC 0425, which included other procedures involving the implantation of prosthetic devices into bone, similar to the procedure described by CPT code 27446. Given the shared resource and clinical characteristics of the procedures included in APC 0425 and the only procedure assigned to APC 0681 for CY 2009, in the CY 2010 OPPS/ASC proposed rule, we proposed to consolidate these two APCs by reassigning CPT code 27446 to APC 0425, and deleting APC 0681. We also noted that, over the past several years, the median cost for CPT code 27446 has fluctuated due to a low volume of services being performed by a small number of providers, and to a single provider performing the majority of services (73 FR 68535). We indicated in the CY 2010 OPPS/ASC proposed rule (74 FR 35268) that we believe by reassigning CPT code 27446 to APC 0425 and deleting APC 0681, we can maintain greater stability from year to year in the payment rate for this knee arthroplasty service, while also paying appropriately for the service.
At its August 2009 meeting, the APC Panel heard a joint presentation from neurostimulator manufacturers who asserted that CMS' proposal to consolidate spinal, peripheral/gastric, and single-array cranial neurostimulator pulse generator implantation procedures into a single APC does not adequately capture facility resources associated with the different types of neurostimulator pulse generators involved in these procedures and would undermine access to rechargeable neurostimulators. The neurostimulator manufacturers asked the APC Panel to recommend to CMS a revised, three-level APC configuration for neurostimulator pulse generator implantation procedures that would Start Printed Page 60367 differentiate payment for procedures involving rechargeable and nonrechargeable neurostimulators. Following discussion of this request, the APC Panel recommended that CMS adopt the two-level neurostimulator pulse generator implantation APC configuration proposed by CMS for CY 2010.
Comment: Many commenters supported CMS' proposal to continue using the standard methodology for calculating median costs for device-dependent APCs, revised to exclude claims that contain the “FC” modifier. The commenters stated that the exclusion of partial credit claims would result in APC median costs that more appropriately reflect true hospital costs. Some commenters also supported the mandatory reporting of all HCPCS device C-codes to encourage hospitals to remain vigilant in reporting the costs of performing services involving devices. The commenters urged CMS to continue educating hospitals on the importance of accurate coding for devices, supplies, and other technologies to help ensure these items are more appropriately reflected in future years' payment rates for outpatient services.
Some commenters recommended CMS continue examining and refining the ratesetting methodology for procedures involving devices in order to encourage the continued development and proliferation of new technology. The commenters also encouraged CMS to develop mechanisms for capturing the costs of devices included on multiple procedure claims.
Response: We appreciate the commenters' support of the standard device-dependent APC ratesetting methodology, including our proposal to refine the methodology to exclude claims that contain the “FC” modifier. As we have stated in the past (73 FR 68535 through 68536), we agree that accurate reporting of device, supply, and technology charges will help to ensure that these items are appropriately accounted for in future years' OPPS payment rates. We encourage stakeholders to carefully review HCPCS code descriptors, as well as any guidance CMS may have provided for specific HCPCS codes. In addition, we have provided further instructions on the billing of medical and surgical supplies in the October 2008 OPPS update (Transmittal 1599, Change Request 6196, dated September 19, 2008) and the April 2009 OPPS update (Transmittal 1702, Change Request 6416, dated March 13, 2009). For HCPCS codes that are paid under the OPPS, providers may also submit inquiries to the AHA Central Office on HCPCS, which serves as a clearinghouse on the proper use of Level I HCPCS codes for hospitals and certain Level II HCPCS codes for hospitals, physicians, and other health professionals. Inquiries must be submitted using the approved form, which may be downloaded from the AHA Web site ( http://www.ahacentraloffice.org) and either faxed to 312–422–4583 or mailed directly to the AHA Central Office: Central Office on HCPCS, American Hospital Association, One North Franklin, Floor 29, Chicago, IL 60606.
We agree with the commenters that we should continue to encourage the development and proliferation of new technology under the OPPS. We have special mechanisms to provide payment for new technologies and services under the OPPS, including new technology APCs and transitional pass-through payments for certain devices. We refer readers to sections III.C. and IV.A., respectively, of this final rule with comment period for more information on these payment methodologies. For all OPPS services, we continue our efforts to use the data from as many multiple procedure claims as possible, through approaches such as use of the bypass list and date splitting of claims as described further in section II.A. of this final rule with comment period, and through methodologies such as increased packaging and composite APCs. We refer readers to section II.A.2.e. of this final rule with comment period for a detailed summary of the public comments related to the establishment of a composite payment methodology for procedures involving cardiac resynchronization therapy defibrillators and pacemakers and our responses.
Comment: Many commenters responded to CMS' proposal to revise the APC configuration for neurostimulator pulse generator implantation procedures from a three-level structure to a two-level structure. While one commenter supported the proposal to combine the single-array cranial neurostimulator pulse generator implantation procedure, described by CPT code 61885 and used for vagus nerve stimulation, with the spinal neurostimulator pulse generator implantation procedure, described by CPT code 63685, many commenters argued that the proposed two-level configuration for neurostimulator pulse generator implantation procedures would threaten patient access to rechargeable spinal neurostimulators. These commenters asserted that hospitals may be unable to offer rechargeable spinal neurostimulator pulse generators at the proposed CY 2010 payment rate for APC 0039, which, according to the commenters, is substantially less than the cost of the device and the CY 2009 payment rate for the procedure. Some commenters presented an analysis of CY 2008 OPPS claims data available for the CY 2010 OPPS/ASC proposed rule that demonstrated a $4,132 difference in costs for spinal neurostimulator pulse generator implantation procedures involving rechargeable devices compared to the same procedures involving nonrechargeable devices. According to these commenters, this difference in cost warrants a separate APC for rechargeable spinal neurostimulator pulse generator procedures. They argued that while the cost difference does not violate the 2 times rule, it is large enough to influence hospitals to choose the lower cost nonrechargeable spinal neurostimulator pulse generators instead of the rechargeable devices if hospitals receive the same payment for the implantation procedure, regardless of the type of technology that is used. Several commenters noted that the threat to patient access to rechargeable spinal neurostimulators should be of particular concern to CMS, given the Agency's past recognition of the technology's ability to reduce the need for device replacements and the associated surgical risks, thereby reducing costs while providing optimal therapy.
Some commenters also stated that the consolidation of APC 0039 and APC 0222 would result in a disproportionately small number of single claims for procedures involving spinal neurostimulator pulse generators being used in ratesetting compared to the number of single claims for other types of neurostimulator pulse generator implantation procedures (specifically, peripheral/gastric and single-array cranial), further reducing the payment for these procedures relative to their costs. The commenters pointed out that, because spinal neurostimulator pulse generator implantation procedures are almost always performed with permanent lead placement procedures, rather than being staged as is common with other neurostimulator implantation procedures, they are typically not captured in the single claims used to calculate the median cost for consolidated APC 0039, upon which payment for that APC would be based. Many commenters argued that the proposed policy would be inconsistent with CMS' rationale in the CY 2008 OPPS/ASC final rule with comment period for implementing the current Start Printed Page 60368 APC configuration for neurostimulator pulse generator implantation procedures, which places the spinal neurostimulator pulse generator implantation procedure in its own APC. According to the commenters, CMS implemented a separate APC for this procedure because, unlike other neurostimulator pulse generator implantation procedures that involve only the less costly nonrechargeable devices, spinal neurostimulator pulse generator implantation procedures utilize either the more costly rechargeable device or the less costly nonrechargeable device. The commenters summarized CMS' assessment in the CY 2008 OPPS/ASC final rule with comment period that the placement of the procedure described by CPT code 63685 as the only procedure in APC 0222 would enable CMS to calculate payment rates for spinal neurostimulator implantation procedures that reflect changes in surgical practice based on clinical, rather than financial, considerations.
Many commenters asserted that CMS' proposed APC configuration for neurostimulator pulse generator implantation procedures would result in APC 0039 being overly broad and clinically heterogeneous. The commenters stated that the spinal, peripheral/gastric, and single-array cranial neurostimulator pulse generator implantation procedures proposed for assignment to APC 0039 are clinically disparate and involve widely diverse neurostimulator technologies (including vagus nerve stimulators for epilepsy, sacral nerve stimulators for urinary incontinence, gastric pacemakers for chronic nausea and vomiting, and spinal neurostimulators for chronic neuropathic pain). One commenter requested that the CY 2010 proposal for neurostimulator pulse generator implantation procedures be reviewed by a pain management physician and a certified coder working in pain management.
According to the commenters, in order to address these concerns, CMS should differentiate payment for procedures involving rechargeable and nonrechargeable neurostimulators by revising the current (CY 2009) three-level APC payment structure for neurostimulator pulse generator implantation procedures. The commenters stated that their recommended configuration would group peripheral/gastric and spinal neurostimulator pulse generator implantation procedures (described by CPT codes 64590 and 63685, respectively) involving nonrechargeable devices in Level 1; single-array cranial neurostimulator pulse generator implantation procedures (described by CPT code 61885) involving nonrechargeable devices in Level 2; and dual-array cranial neurostimulator pulse generator implantation procedures (described by CPT code 61886) and any neurostimulator pulse generator implantation procedure involving rechargeable devices in Level 3. According to the commenters, this APC configuration for neurostimulator pulse generator implantation procedures could be implemented by assigning APCs based on the presence of HCPCS device C-codes present on claims or through the creation of new Level II HCPCS G-codes that would distinguish procedures performed to implant nonrechargeable neurostimulator pulse generators from those performed to implant rechargeable neurostimulator pulse generators. The commenters asserted that CMS has shown a willingness to use alternative mapping schemes in the past to differentiate resource costs for procedures involving technologies such as drug-eluting coronary stents, implantable cardioverter defibrillators (ICDs), and linear accelerator-based stereotactic radiosurgery (LINAC–SRS), when there are important technology and facility resource cost differences that cannot be identified through the use of existing CPT codes.
The commenters urged CMS to maintain the current neurostimulator pulse generator implantation APC configuration as adopted in CY 2008 if the Agency decides not to implement their recommended three-level technology-specific APC configuration, or to create a four-level APC configuration in which the existing APC 0039 is split, with one APC for single-array cranial neurostimulator pulse generator implantation procedures and a separate APC for peripheral/gastric neurostimulator pulse generator implantation procedures. According to the commenters, either approach would yield more accurate payment rates than CMS' proposal for CY 2010.
Response: We do not agree with the commenters who argued that we should not implement our CY 2010 proposal to revise the APC configuration of neurostimulator pulse generator implantation procedures from a three-level structure to a two-level structure. We are finalizing our CY 2010 proposal to reassign CPT code 63685 to APC 0039, to delete APC 0222, and to maintain the current configuration of APC 0315. We believe that the final CY 2010 median costs for the neurostimulator pulse generator implantation procedures, described by CPT codes 61885, 63685, and 64590, are sufficiently similar to warrant their placement in a single APC, as demonstrated in Table 7 below. The difference between the procedure with the highest median cost in APC 0039, described by CPT code 63685, and the procedure with the lowest median cost in APC 0039, described by CPT code 64590, is approximately $3,000. Even if we were to consider the difference in costs between spinal neurostimulator pulse generator implantation procedures described by CPT code 63685 when they are performed with a rechargeable device compared to when they are performed with a nonrechargeable device, estimated by the commenters to be approximately $4,000, the grouping of these procedures in the same APC would not violate the 2 times rule. We also point out that, as demonstrated in Table 7, we use a similar number of single claims with each of the CPT codes assigned to APC 0039 to calculate the median cost upon which the final CY 2010 payment rate for APC 0039 is based.
We do not agree with the commenters that these modest differences in costs, either among the various types of neurostimulator pulse generator implantation procedures assigned to APC 0039 or among the same types of procedures involving rechargeable versus nonrechargeable devices, are sufficiently substantial to result in hospitals denying access to the limited subset of patients for whom the more expensive rechargeable technology is clinically indicated. We note that payment based on a measure of central tendency is a principle of any prospective payment system. As we have stated in the past (73 FR 68562), in some individual cases, payment exceeds the average cost, and in other cases, payment is less than the average cost. On balance, however, payment should approximate the relative cost of the average case, recognizing that, as a prospective payment system, the OPPS is a system of averages.
In addition to being similar in terms of resource utilization, we believe the procedures described by CPT codes 61885, 63685, and 64590 are comparable from a clinical perspective because they all involve the subcutaneous placement of a neurostimulator pulse generator. We do not agree with the commenters who argued that these procedures should be considered clinically disparate because they use widely diverse technologies for very different clinical indications. It is not uncommon under the OPPS to group procedures described by relatively general HCPCS codes that Start Printed Page 60369 may utilize a wide variety of technologies and may be performed to treat different patient populations in the same APC. Furthermore, as stated in the CY 2009 OPPS/ASC final rule with comment period (73 FR 66537), the standard device-dependent APC ratesetting methodology does not take into consideration patient diagnoses. In response to the commenter who requested that the CY 2010 proposal for neurostimulator pulse generator implantation procedures be reviewed by a pain management physician and a certified coder working in pain management, we note that the CMS staff involved in reviewing the clinical characteristics of the APC groups include medical advisors from a variety of specialties as well as certified coders.
We also do not agree that we should not implement the two-level APC configuration for neurostimulator pulse generator implantation procedures as proposed for CY 2010 because, as argued by some commenters, it would be inconsistent with our rationale in the CY 2008 OPPS/ASC final rule with comment period to maintain a separate APC solely for spinal neurostimulator pulse generator implantation procedures. It is our standard process under the OPPS to reassess the composition of APCs, including reviewing the median costs of individual HCPCS codes, annually when we have the most current claims and Medicare cost report data, and to propose through our annual rulemaking cycle changes that we believe are necessary to maintain and improve the clinical and resource homogeneity of APCs based on the updated data. In CY 2008, the median costs for the single-array cranial and peripheral/gastric neurostimulator pulse generator implantation procedures described by CPT codes 61885 and 64590 of $12,799 and $10,954, respectively, were more divergent from the median cost calculated for the spinal neurostimulator pulse generator implantation procedure of $15,150 using the CY 2006 claims and cost report data available at that time, compared to the median costs for these procedures calculated from the CY 2008 claims and cost report data available for this CY 2010 OPPS/ASC final rule with comment period, as demonstrated in Table 7 below.
Finally, we do not agree with the commenters that we should differentiate payment for neurostimulator pulse generator implantation procedures based on the type of technology that is implanted (that is, rechargeable or nonrechargeable), nor do we agree with the commenters that past CMS policy to use alternative mapping schemes to differentiate resource costs for certain procedures, such as those involving drug-eluting stents, ICDs, and LINAC–SRS, serves as a precedent to do so. As we have stated in the past (72 FR 66715 through 66716 and 73 FR 68538), a policy to provide different payments for the same procedures according to the types of devices implanted would not be consistent with our overall strategy under the OPPS to encourage hospitals to use resources more efficiently by increasing the size of the payment bundles. The circumstances surrounding the payment policies and coding configurations for drug-eluting stents (67 FR 66732 through 66734), ICDs (72 FR 66702 through 66703), and LINAC–SRS (72 FR 66734 through 66737) are markedly different from the circumstances surrounding neurostimulator pulse generator implantation procedures. We developed HCPCS G-codes to distinguish payment for procedures involving drug-eluting stents from procedures involving non-drug-eluting stents because drug-eluting stents did not meet the criteria for transitional pass-through payment or for payment under a New Technology APC. Unlike drug-eluting stents, rechargeable spinal neurostimulators were granted pass-through status under the OPPS in CY 2006, which lasted until December 31, 2007. In the case of ICDs, we created HCPCS G-codes to gather cost data on single and dual chamber ICDs, but we did not differentiate payment for ICD insertion based on the type of technology that was used (72 FR 66703). Finally, our policy to utilize HCPCS G-codes rather than CPT codes for payment under the OPPS for LINAC–SRS treatment delivery services recognizes the vastly different capital equipment costs required for various LINAC–SRS services, rather than differences in the costs of single-use devices implanted in patients during the same procedure.
Comment: Some commenters disagreed with CMS” presentation at the August 2009 APC Panel meeting of the proposed CY 2010 line-item median costs for the two device HCPCS C-codes that describe neurostimulator pulse generators, specifically HCPCS code C1767 (Generator, neurostimulator (implantable), nonrechargeable) and HCPCS code C1820 (Generator, neurostimulator (implantable), with rechargeable battery and charging system). The commenters disputed the accuracy of the data presented by CMS, specifically that the line-item median costs for HCPCS codes C1767 and C1820 are $9,606 and $9,636, respectively, based on CY 2008 claims available for the CY 2010 OPPS/ASC proposed rule. According to the commenters, these line-item median costs are inconsistent with the commenters” analyses of CY 2010 OPPS/ASC proposed rule data, which indicated that the line-item median costs for HCPCS codes C1767 and C1820 are $10,580 and $13,587, respectively. One commenter urged CMS to reanalyze the data and to disregard the APC Panel's support of the proposed CY 2010 APC configuration for neurostimulator pulse generator implantation procedures if the data were found to be erroneous. Another commenter characterized CMS” presentation of the line-item median costs for HCPCS codes C1767 and C1820 as incomplete because OPPS payment rates are based upon median costs that include all packaged items and services associated with providing a procedure as they appear on single claims, and not the line-item median costs for individual devices. The commenter asked CMS to ensure that all data presented to the APC Panel in the future is full and appropriate information for decisionmaking.
Response: In response to the commenters” concerns, we reassessed our methodology for calculating the proposed CY 2010 line-item median costs for HCPCS codes C1767 and C1820 and verified that the information presented to the APC Panel is accurate based on the CY 2008 claims data available for the CY 2010 OPPS/ASC proposed rule. The line-item statistics for these HCPCS codes, along with all other HCPCS codes recognized under the OPPS, are released to the public as part of the OPPS limited data set. We do not agree with the commenters that the presentation of these data was incomplete or inappropriate. We frequently consider line-item median costs for devices and other packaged items and services as one data element among several when we evaluate the clinical and resource homogeneity of APCs, particularly when stakeholders voice concerns that the costs of different items are driving procedure costs or influencing hospitals” decisions to provide certain services. An advantage of the line-item median costs is that they represent data from all OPPS claims, and not just the single claims that we are able to use in ratesetting for procedures. Therefore, we believe that a comparison of line-item costs is particularly appropriate for different types of neurostimulator pulse generators because one of the commenters” concerns was that there are relatively few single claims available Start Printed Page 60370 for ratesetting for the implantation of spinal neurostimulator pulse generators. We would expect the device costs on multiple procedure claims to be reflective of the hospital costs of these neurostimulator pulse generators, because commenters stated that multiple procedure claims resulted from the most typical spinal neurostimulator implantation procedures. Furthermore, we would not expect there to be significant packaged costs associated with the neurostimulator pulse generators described by these device HCPCS codes. Therefore, we would expect the line-item median costs to accurately reflect the differential costs of non-rechargeable and rechargeable neurostimulator technology. We note that the APC Panel members are well-acquainted with the OPPS ratesetting methodology, including the use of single procedure claims and not line-item median costs for individual items, to calculate the median costs upon which OPPS payment rates are based.
| CY 2010 APC | Revised APC Title for CY 2010 | CY 2010 CPT Code | CY 2010 CPT Code Descriptor | CY 2010 CPT Code Median Cost | CY 2010 CPT Code Single Claims | CY 2010 APC Median Cost |
|---|---|---|---|---|---|---|
| 0039 | Level I Implantation of Neurostimulator Generator | 61885 | Insertion or replacement of cranial neurostimulator pulse generator or receiver, direct or inductive coupling; with connection to a single electrode array | $14,141 | 1,260 | $13,766 |
| 63685 | Insertion or replacement of spinal neurostimulator pulse generator or receiver, direct or inductive coupling | 15,802 | 1,262 | 13,766 | ||
| 64590 | Insertion or replacement of peripheral or gastric neurostimulator pulse generator or receiver, direct or inductive coupling | 12,726 | 1,978 | 13,766 | ||
| 0315 | Level II Implantation of Neurostimulator Generator | 61886 | Insertion or replacement of cranial neurostimulator pulse generator or receiver, direct or inductive coupling; with connection to two or more electrode arrays | 18,350 | 1,004 | 18,350 |
Comment: Several commenters expressed support for the proposed CY 2010 payment rate for the implantation of auditory osseointegrated devices, described by CPT codes 69714 (Implantation, osseointegrated implant, temporal bone, with percutaneous attachment to external speech processor/cochlear stimulator; without mastoidectomy); 69715 (Implantation, osseointegrated implant, temporal bone, with percutaneous attachment to external speech processor/cochlear stimulator; with mastoidectomy); 69717 (Replacement (including removal of existing device), osseointegrated implant, temporal bone, with percutaneous attachment to external speech processor/cochlear stimulator; without mastoidectomy); and 69718 (Replacement (including removal of existing device), osseointegrated implant, temporal bone, with percutaneous attachment to external speech processor/cochlear stimulator; with mastoidectomy) and assigned to APC 0425. Other commenters, however, stated that the proposed payment rate for APC 0425 is less than hospitals' device and service-related costs associated with the procedures described by these CPT codes and urged CMS to consider a slight increase in the payment for APC 0425.
Response: We agree with the commenters that the payment rate for APC 0425, calculated from the standard device-dependent APC ratesetting methodology, appropriately reflects hospitals' relative costs for providing the procedures assigned to APC 0425 as reported to us in the claims and cost report data. We used 1,410 single claims from CY 2008 to calculate the median cost upon which the final CY 2010 payment rate for APC 0425 is based. The final CY 2010 median cost for APC 0425 is approximately $7,932, slightly higher than the final CY 2009 median cost for APC 0425 of $7,863. We note that we were able to use significantly more single claims in ratesetting for APC 0425 for CY 2010 compared to CY 2009 (1,410 single claims from CY 2008 compared to 668 single claims from CY 2007). We disagree with the commenters who requested an additional increase in the payment rate for APC 0425, because this would artificially and inaccurately inflate payment rates. A fundamental principle of the OPPS is that it is based on relative weights, and as we have stated in the past (73 FR 68541), it is the relativity of the costs to one another, rather than absolute cost, that is important in setting payment rates. To deviate from our standard OPPS ratesetting methodology and increase the payment rates for certain procedures beyond their relatives costs as derived from claims and cost report data would skew this relativity.
Comment: Some commenters supported CMS' proposal to reassign CPT code 27446 to APC 0425 and to delete APC 0681. Other commenters, however, opposed the consolidation of these two APCs, arguing that the procedure described by CPT code 27446 is clinically dissimilar from the arthroplasty procedures currently assigned to APC 0425. The commenters recommended that CMS continue to maintain APC 0681 for CY 2010 and to add other total knee arthroplasty procedures to this APC, along with the procedure described by CPT code 27446.
Response: We disagree with the commenters who argued that it is necessary to maintain APC 0681 specifically for knee arthroplasty procedures because we do not believe it is appropriate to maintain an APC that is not necessary to classify services into groups that are similar clinically and in terms of resource utilization. We continue to believe that CPT code 27446 is most appropriately assigned to APC 0425 for CY 2010, as we proposed, based on consideration of the procedure's clinical and resource characteristics. As described in section XI.B. of this final rule with comment Start Printed Page 60371 period, we are not removing any total knee arthroplasty procedures from the inpatient list.
Comment: Several commenters supported the proposed payment rate for the implantation of cochlear implants, described by CPT code 69930 (Cochlear device implantation, with or without mastoidectomy) and assigned to APC 0259 (Level VII ENT Procedures). These commenters stated that while hospitals' device and service-related costs for these procedures likely still exceed the proposed payment rate for APC 0259, they represent an improvement in payment relative to CY 2009 that may lead to better access to care for Medicare beneficiaries.
Response: We appreciate the commenters' support of the proposed payment rate for APC 0259. We believe that the standard device-dependent APC ratesetting methodology results in a payment rate that reflects hospitals' relative costs for providing the procedure assigned to this APC as reported to us in the claims and cost report data.
Comment: One commenter concurred with CMS' proposal that APC 0385 (Level I Prosthetic Urological Procedures) and APC 0386 (Level II Prosthetic Urological Procedures) continue to be recognized as device-dependent APCs. The commenter supported CMS' continued application of procedure-to-device edits for procedures assigned to these APCs.
Response: We appreciate the commenter's support of the continued recognition of APC 0385 and 0386 as device-dependent APCs. We agree that claims processing edits for devices that are integral to the performance of procedures assigned to device-dependent APCs are an important element of the standard device-dependent APC ratesetting methodology.
Comment: One commenter urged CMS not to reduce the payment for the procedure described by CPT code 62361 (Implantation or replacement of device for intrathecal or epidural drug infusion; nonprogrammable pump), which is assigned to APC 0227 (Implantation of Drug Infusion Device). The commenter stated that patient access to this procedure is limited due to recent payment cuts.
Response: The final CY 2010 median cost for APC 0227 of approximately $13,268 is approximately 10 percent higher than the median cost of $12,006, upon which the final CY 2009 payment rate was based, and approximately 13 percent higher than the median cost of $11,569, upon which the final CY 2008 payment rate was based. We believe that the final CY 2010 median cost for APC 0227 of $13,268, which is calculated using the standard device-dependent APC methodology, results in a final CY 2010 payment rate that accurately and appropriately reflects hospitals” costs for providing the service described by CPT code 62361 and will not result in any barriers to patient care.
In summary, after consideration of the public comments we received, we are finalizing our proposed CY 2010 payment policies for device-dependent APCs, without modification. The CY 2010 OPPS payment rates for device-dependent APCs are based on their median costs calculated from CY 2008 claims and the most recent cost report data, using only claims that pass the device edits, do not contain token charges for devices, and do not have a modifier signifying that the device was furnished without cost or with full or partial credit. We continue to believe that the median costs calculated from the single claims that meet these criteria represent the most valid estimated relative costs of these services to hospitals when they incur the full cost of the devices required to perform the procedures. The CY 2010 device-dependent APCs are listed in Table 8 below.
| CY 2010 APC | CY 2010 Status indicator | CY 2010 APC Title |
|---|---|---|
| 0039 | S | Level I Implantation of Neurostimulator Generator |
| 0040 | S | Percutaneous Implantation of Neurostimulator Electrodes |
| 0061 | S | Laminectomy, Laparoscopy, or Incision for Implantation of Neurostimulator Electrodes |
| 0082 | T | Coronary or Non-Coronary Atherectomy |
| 0083 | T | Coronary or Non-Coronary Angioplasty and Percutaneous Valvuloplasty |
| 0084 | S | Level I Electrophysiologic Procedures |
| 0085 | T | Level II Electrophysiologic Procedures |
| 0086 | T | Level III Electrophysiologic Procedures |
| 0089 | T | Insertion/Replacement of Permanent Pacemaker and Electrodes |
| 0090 | T | Insertion/Replacement of Pacemaker Pulse Generator |
| 0104 | T | Transcatheter Placement of Intracoronary Stents |
| 0106 | T | Insertion/Replacement of Pacemaker Leads and/or Electrodes |
| 0107 | T | Insertion of Cardioverter-Defibrillator |
| 0108 | T | Insertion/Replacement/Repair of Cardioverter-Defibrillator Leads |
| 0115 | T | Cannula/Access Device Procedures |
| 0202 | T | Level VII Female Reproductive Procedures |
| 0225 | S | Implantation of Neurostimulator Electrodes, Cranial Nerve |
| 0227 | T | Implantation of Drug Infusion Device |
| 0229 | T | Transcatheter Placement of Intravascular Shunts |
| 0259 | T | Level VII ENT Procedures |
| 0293 | T | Level V Anterior Segment Eye Procedures |
| 0315 | S | Level II Implantation of Neurostimulator Generator |
| 0384 | T | GI Procedures with Stents |
| 0385 | S | Level I Prosthetic Urological Procedures |
| 0386 | S | Level II Prosthetic Urological Procedures |
| 0418 | T | Insertion of Left Ventricular Pacing Electrode |
| 0425 | T | Level II Arthroplasty or Implantation with Prosthesis |
| 0427 | T | Level II Tube or Catheter Changes or Repositioning |
| 0622 | T | Level II Vascular Access Procedures |
| 0623 | T | Level III Vascular Access Procedures |
| 0648 | T | Level IV Breast Surgery |
| Start Printed Page 60372 | ||
| 0652 | T | Insertion of Intraperitoneal and Pleural Catheters |
| 0653 | T | Vascular Reconstruction/Fistula Repair with Device |
| 0654 | T | Insertion/Replacement of a Permanent Dual Chamber Pacemaker |
| 0655 | T | Insertion/Replacement/Conversion of a Permanent Dual Chamber Pacemaker |
| 0656 | T | Transcatheter Placement of Intracoronary Drug-Eluting Stents |
| 0674 | T | Prostate Cryoablation |
| 0680 | S | Insertion of Patient Activated Event Recorders |
(2) Blood and Blood Products
Since the implementation of the OPPS in August 2000, we have made separate payments for blood and blood products through APCs rather than packaging payment for them into payments for the procedures with which they are administered. Hospital payments for the costs of blood and blood products, as well as for the costs of collecting, processing, and storing blood and blood products, are made through the OPPS payments for specific blood product APCs.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35269), we proposed to continue to establish payment rates for blood and blood products using our blood-specific CCR methodology, which utilizes actual or simulated CCRs from the most recently available hospital cost reports to convert hospital charges for blood and blood products to costs. This methodology has been our standard ratesetting methodology for blood and blood products since CY 2005. It was developed in response to data analysis indicating that there was a significant difference in CCRs for those hospitals with and without blood-specific cost centers, and past comments indicating that the former OPPS policy of defaulting to the overall hospital CCR for hospitals not reporting a blood-specific cost center often resulted in an underestimation of the true hospital costs for blood and blood products. Specifically, in order to address the differences in CCRs and to better reflect hospitals' costs, we proposed to continue to simulate blood CCRs for each hospital that does not report a blood cost center by calculating the ratio of the blood-specific CCRs to hospitals' overall CCRs for those hospitals that do report costs and charges for blood cost centers. We would then apply this mean ratio to the overall CCRs of hospitals not reporting costs and charges for blood cost centers on their cost reports in order to simulate blood-specific CCRs for those hospitals. We calculated the median costs upon which the proposed CY 2010 payment rates for blood and blood products were based using the actual blood-specific CCR for hospitals that reported costs and charges for a blood cost center and a hospital-specific simulated blood-specific CCR for hospitals that did not report costs and charges for a blood cost center.
We stated in the CY 2010 OPPS/ASC proposed rule (74 FR 35269) that we continue to believe the hospital-specific, blood-specific CCR methodology better responds to the absence of a blood-specific CCR for a hospital than alternative methodologies, such as defaulting to the overall hospital CCR or applying an average blood-specific CCR across hospitals. Because this methodology takes into account the unique charging and cost accounting structure of each provider, we believe that it yields more accurate estimated costs for these products. We indicated that we believe continuing with this methodology in CY 2010 would result in median costs for blood and blood products that appropriately reflect the relative estimated costs of these products for hospitals without blood cost centers and, therefore, for these blood products in general.
Comment: One commenter expressed appreciation for CMS' recognition of the complexities of calculating payment rates for blood and blood products and the accommodations CMS has made through the blood and blood product ratesetting methodology to ensure the calculated rates are as fair as possible. However, several commenters stated that the proposed payment rates for many blood and blood products are less than the costs hospitals incur acquiring, managing, and processing them, and that the claims-based cost data for blood and blood products are error-prone and subject to significant and unexplained fluctuations. They noted that the payment decreases for several blood and blood products seem inexplicable because prices for blood have been increasing due to new technologies and tests required to ensure the continued safety of the blood supply and increasingly expensive donor recruitment and retention efforts. According to the commenters, a comparison of the proposed APC payment changes for blood and blood products to the producer price index (PPI) for blood and organ banks, which increased 3.1 percent from July 2008 to July 2009, indicates that the blood product payment rates in the CY 2010 OPPS/ASC proposed rule do not reflect overall pricing trends in the blood banking industry. The commenters asked CMS to adjust the CY 2010 payment rates for blood and blood products by increasing all of the CY 2009 payment rates by 3.1 percent, or by implementing a 3.1 percent payment floor for CY 2010 payment rates compared to CY 2009 payment rates for blood and blood products. One commenter particularly urged CMS to apply a 3 percent minimum increase in payment for the highest volume blood product, described by HCPCS code P9016 (Red blood cells, leukocytes reduced, each unit). The commenters asserted that the use of the PPI for blood and organ banks in calculating hospital payment is not unprecedented. They stated that in the CY 2005 OPPS final rule, CMS used the PPI for blood and derivatives for human use in calculating the payment rates for low-volume blood products. They also pointed out that CMS recognized the value of the PPI for blood and organ banks by using it to update blood and blood product prices in the market basket under the IPPS for CY 2010.
Response: We continue to believe that using blood-specific CCRs applied to hospital claims data results in payments that appropriately reflect hospitals' relative costs of providing blood and blood products as reported to us by hospitals. We do not believe it is necessary or appropriate to use the PPI for blood and organ banks as a benchmark for updating the payment rates for blood and blood products from year to year, because it is not our standard process under the OPPS for any item or service to update payment rates by implementing across-the-board, product-specific inflation updates to the payment rates that were in place the year before. Rather, we annually update Start Printed Page 60373 payment groups and payment weights using the most recently available hospital claims and cost report data. This process allows us to recalibrate the payment groups and payment weights in response to changes in hospitals' costs from year to year. A fundamental principle of the OPPS is that it is based on relative weights, and as we have stated in the past (73 FR 68541), it is the relativity of the costs to one another, rather than absolute cost, that is important in setting payment rates. To deviate from our standard OPPS ratesetting methodology and update the payment rates for blood and blood products by the PPI would skew this relativity.
We also note that, as discussed in section II.B. of this final rule with comment period, we are required by law to update the conversion factor used to determine payment rates under the OPPS. For CY 2010, the update is equal to the hospital inpatient market basket increase. The PPI for blood and organ banks is one of several price proxies used to calculate the hospital inpatient market basket (74 FR 43847), which represents the change in price over time of the same mix (quantity and intensity) of goods and services purchased to provide hospital services. In this way, the PPI for blood and blood products is already incorporated in the CY 2010 payment rates for blood and blood products.
Comment: One commenter noted that the proposed CY 2010 median costs for several blood and blood products fluctuated significantly relative to CY 2009. The commenter expressed concern about potentially large payment decreases and noted that, in the past, CMS dampened payment decreases for blood and blood products to limit product losses. The commenter requested that CMS disclose the source of the fluctuations in CY 2010 median costs for blood and blood products and implement a dampening policy to mitigate significant payment fluctuations, not only for blood and blood products but for all other services.
Response: As stated previously, we continue to believe that using blood-specific CCRs applied to hospital claims data results in payments that appropriately reflect hospitals' relative costs of providing blood and blood products as reported to us by hospitals. We do not believe it is necessary or appropriate to implement a dampening policy to mitigate significant payment fluctuations, for blood and blood products or for any other items and services payable under the OPPS, as described in section II.A.2.c. of this final rule with comment period. As we have stated in the past (73 FR 68541), it is our common practice to review significant changes in median costs from year to year and from the proposed rule to the final rule for a given calendar year. The final CY 2010 median costs for more than two-thirds of all blood and blood products changed by a margin of less than 10 percent compared to the CY 2009 median costs. Of the remaining blood and blood products, 8 demonstrated decreases in median costs of greater than 10 percent, and 5 demonstrated increases in median costs of greater than 10 percent. We determined that the fluctuations in median costs for these 13 blood and blood products were due to contributions of additional claims, the addition or removal of individual hospitals furnishing particular blood and blood products, and revised cost report data. For all APCs whose payment rates are based upon relative payment weights, we note that the quality and accuracy of reported units and charges significantly influence the median costs that are the basis for our payment rates, especially for low volume items and services. Beyond our standard OPPS trimming methodology (described in section II.A.2. of this final rule with comment period) that we apply to those claims that have passed various types of claims processing edits, it is not our general policy to judge the accuracy of hospital coding and charging for purposes of ratesetting.
Comment: One commenter recommended that CMS recognize plasma protein fraction (PPF) products as drugs under the OPPS and assign status indicator “K” (Nonpass-Through Drugs and Nonimplantable Biologicals, Including Therapeutic Radiopharmaceuticals) to HCPCS codes P9043 (Infusion, plasma protein fraction (human), 5%, 50 ml) and P9048 (Infusion, plasma protein fraction (human), 5%, 250 ml), rather than assigning them status indicator “R” (Blood and Blood Products). The commenter also requested that CMS instruct providers to use the appropriate infusion CPT codes for administration of PPF, rather than blood transfusion codes. According to the commenter, PPF is similar clinically to albumin in terms of how it is derived and the patients for whom it is indicated. The commenter also stated that, according to the AABB, both albumin and PPF are blood derivatives that should be billed with pharmacy revenue codes. According to the commenter, the AABB also indicates that the administration of blood derivatives, including PPF, should be billed with injection or infusion CPT codes rather than blood transfusion CPT codes.
Response: We did not propose to change the status indicators for the PPF products described by HCPCS codes P9043 and P9048 from “R” to “K” for CY 2010. Because changing the status indicators for these products as the commenter recommended could have significant payment implications, we believe we should not consider such a potential change in policy without seeking input from all interested stakeholders through our annual rulemaking cycle. Specifically, changing the status indicator from “R” to “K” would require us to calculate the payment rates for PPF using mean unit cost from hospital claims, as we currently do for albumin products, rather than using our standard blood-specific CCR methodology for blood and blood products.
We last addressed the issue of whether plasma-derived therapies and their recombinant analogs should be considered blood and blood products for purposes of payment under the OPPS in the CY 2003 OPPS final rule with comment period (67 FR 66774) and the CY 2004 OPPS final rule with comment period (68 FR 63455). We stated that, because these products are highly processed and not manufactured by local blood banks, they do not have the same access and safety concerns as other blood and blood products. Therefore, we did not consider any plasma-derived products and their recombinant analogs, including albumin and immune globulins, to fall under the category of blood and blood products (67 FR 66774).
We are requesting comments on this final rule with comment period that address whether PPF should be recognized as a blood and blood product, designated with status indicator “R,” or as a nonpass-through drug and biological, designated with status indicator “K.” Specifically, we are interested in how PPF is derived and manufactured, and whether the same access and safety concerns that apply to the blood and blood products recognized under the OPPS for payment purposes also apply to PPF. Finally, we are interested in the relationship between albumin and PPF, from clinical, manufacturing, and safety perspectives, and whether there would be a rationale for treating these products similarly for payment purposes under the OPPS. We will consider these comments as we prepare for the CY 2011 annual rulemaking cycle.
Comment: One commenter asked if the product “prepooled cryoprecipitate” Start Printed Page 60374 would be added to the list of blood and blood products.
Response: The existing HCPCS code that describes cryoprecipitate products, P9012 (Cryoprecipitate, each unit), is recognized under the OPPS for payment purposes as a blood and blood product. We note there is an established process in place for requesting a revision to the Level II HCPCS codes if stakeholders believe the current codes cannot adequately address all clinical circumstances. The Level II HCPCS coding system is a comprehensive and standardized system that classifies similar products that are medical in nature into categories for the purpose of efficient claims processing. The process and criteria for revising Level II HCPCS codes is available on the CMS Web site at: http://www.cms.hhs.gov/MedHCPCSGenInfo/02_HCPCSCODINGPROCESS.asp#TopOfPage.
After consideration of the public comments we received, we are finalizing, without modification, our proposal to calculate the median costs upon which the CY 2010 payment rates for blood and blood products are based using the blood-specific CCR methodology that we have utilized since CY 2005. We believe that continuing this methodology in CY 2010 results in median costs for blood and blood products that appropriately reflect the relative estimated costs of these products for hospitals without blood cost centers and, therefore, for these products in general.
We refer readers to Addendum B to this final rule with comment period for the final CY 2010 payment rates for blood and blood products, which are identified with status indicator “R.” For more detailed discussion of the blood-specific CCR methodology, we refer readers to the CY 2005 OPPS proposed rule (69 FR 50524 through 50525). For a full history of OPPS payment for blood and blood products, we refer readers to the CY 2008 OPPS/ASC final rule with comment period (72 FR 66807 through 66810).
(3) Single Allergy Tests
In the CY 2010 OPPS/ASC proposed rule (74 FR 35269), we proposed to continue with our methodology of differentiating single allergy tests (“per test”) from multiple allergy tests (“per visit”) by assigning these services to two different APCs to provide accurate payments for these tests in CY 2010. Multiple allergy tests are currently assigned to APC 0370 (Allergy Tests), with a median cost calculated based on the standard OPPS methodology. We provided billing guidance in CY 2006 in Transmittal 804 (issued on January 3, 2006) specifically clarifying that hospitals should report charges for the CPT codes that describe single allergy tests to reflect charges “per test” rather than “per visit” and should bill the appropriate number of units of these CPT codes to describe all of the tests provided. However, as noted in the CY 2010 OPPS/ASC proposed rule (74 FR 35269), our CY 2008 claims data available for that proposed rule for APC 0381 did not reflect improved and more consistent hospital billing practices of “per test” for single allergy tests. The median cost of APC 0381, calculated for the proposed rule according to the standard single claims OPPS methodology, was approximately $55, significantly higher than the CY 2009 median cost of APC 0381 of approximately $23 calculated according to the “per unit” methodology, and greater than we would expect for these procedures that are to be reported “per test” with the appropriate number of units. Some claims for single allergy tests still appear to provide charges that represent a “per visit” charge, rather than a “per test” charge. Therefore, consistent with our payment policy for single allergy tests since CY 2006, we proposed to calculate a “per unit” median cost for APC 0381, based upon 530 claims containing multiple units or multiple occurrences of a single CPT code. The proposed CY 2010 median cost for APC 0381 using the “per unit” methodology was approximately $29. For a full discussion of this methodology, we refer readers to the CY 2008 OPPS/ASC final rule with comment period (72 FR 66737).
We did not receive any public comments on our CY 2010 proposal for payment of single allergy tests. Therefore, we are finalizing our CY 2010 proposal, without modification, to calculate a “per unit” median cost for APC 0381 as described above in this section. The final CY 2010 median cost of APC 0381 is approximately $29.
(4) Echocardiography Services
In CY 2008, we implemented a policy whereby payment for all contrast agents is packaged into the payment for the associated imaging procedure, regardless of whether the contrast agent met the OPPS drug packaging threshold. Section 1833(t)(2)(G) of the Act requires us to create additional APC groups of services for procedures that use contrast agents to classify them separately from those procedures that do not utilize contrast agents. To reconcile this statutory provision with our final policy of packaging all contrast agents, for CY 2008, we calculated HCPCS code-specific median costs for all separately payable echocardiography procedures that may be performed with contrast agents by isolating single and “pseudo” single echocardiography claims with the following CPT codes where a contrast agent was also billed on the claim:
- 93303 (Transthoracic echocardiography for congenital cardiac anomalies; complete);
- 93304 (Transthoracic echocardiography for congenital cardiac anomalies; follow-up or limited study);
- 93307 (Echocardiography, transthoracic, real-time with image documentation (2D) with or without M-mode recording; complete);
- 93308 (Echocardiography, transthoracic, real-time with image documentation (2D) with or without M-mode recording; follow-up or limited study);
- 93312 (Echocardiography, transesophageal, real time with image documentation (2D) (with or without M-mode recording); including probe placement, image acquisition, interpretation and report);
- 93315 (Transesophageal echocardiography for congenital cardiac anomalies; including probe placement, image acquisition, interpretation and report);
- 93318 (Echocardiography, transesophageal (TEE) for monitoring purposes, including probe placement, real time 2-dimensional image acquisition and interpretation leading to ongoing (continuous) assessment of (dynamically changing) cardiac pumping function and to therapeutic measures on an immediate time basis); and
- 93350 (Echocardiography, transthoracic, real-time with image documentation (2D), with or without M-mode recording, during rest and cardiovascular stress test using treadmill, bicycle exercise and/or pharmacologically induced stress, with interpretation and report).
After reviewing HCPCS code-specific median costs, we determined that all echocardiography procedures that may be performed with contrast agents are reasonably similar both clinically and in terms of resource use. In CY 2008, we created APC 0128 (Echocardiogram with Contrast) to provide payment for echocardiography procedures that are performed with a contrast agent. We refer readers to the CY 2008 OPPS/ASC final rule with comment period (72 FR 66643 through 66646) for more information on this methodology.
In order for hospitals to identify and receive appropriate payment for echocardiography procedures performed with contrast beginning in CY 2008, we created eight new HCPCS codes (C8921 Start Printed Page 60375 through C8928) that corresponded to the related CPT echocardiography codes and assigned them to the newly created APC 0128. We instructed hospitals to report the CPT codes when performing echocardiography procedures without contrast and to report the new HCPCS C-codes when performing echocardiography procedures with contrast, or without contrast followed by with contrast. As is our standard policy with regard to new codes, the APC assignment of these codes was then open to comment in that final rule.
We used the same process to calculate median costs for these codes for CY 2009 as we used for CY 2008 to separately identify echocardiography services provided with contrast and those provided without contrast because the data reported under these new codes were not yet available for CY 2009 ratesetting.
In addition, for CY 2009, the American Medical Association (AMA) revised several CPT codes in the 93000 series to more specifically describe particular services provided during echocardiography procedures. The CY 2009 descriptor for new CPT code 93306 (Echocardiography, transthoracic real-time with image documentation (2D), includes M-mode recording, when performed, complete, with spectral Doppler echocardiography, and with color flow Doppler echocardiography) includes the services described in CY 2008 by three CPT codes: 93307; 93320 (Doppler echocardiography, pulsed wave and/or continuous wave with spectral display; complete); and 93325 (Doppler echocardiography color flow velocity mapping). Therefore, the service described in CY 2009 by new CPT code 93306 was reported in the CY 2008 data with three CPT codes, specifically CPT codes 93307, 93320, and 93325. In CY 2008, the hospital received separate payment for CPT code 93307 through APC 0269 (Level II Echocardiogram without Contrast Except Transesophageal), into which payment for the other two services was packaged. The revised CY 2009 descriptor of CPT code 93307 explicitly excludes services described by CPT codes 93320 and 93325.
To estimate the hospital costs of CPT codes 93306 and 93307 based on their CY 2009 descriptors and the corresponding HCPCS codes C8929 and C8923 for CY 2009, we used claims data from CY 2007. As described in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68542 through 68544), we manipulated our CY 2007 single and “pseudo” single claims data to simulate the new CY 2009 definitions of these services. Specifically, we selected claims for CPT code 93307 on which CPT codes 93320 and 93325 were also present and we treated the summed costs on these claims as if they were a single procedure claim for CPT code 93306. Similarly, we selected single claims for CPT code 93307 to reflect the newly revised descriptor for CY 2009; that is, we included those claims where CPT code 93307 was not billed with packaged CPT code 93320 or CPT code 93325 on the same claim. We then applied our CY 2009 methodology for calculating HCPCS code-specific median costs for these echocardiography procedures with and without contrast by dividing the new set of claims for CPT codes 93306 and 93307 into those billed with and without contrast agents. We assigned the costs for simulated CPT codes 93306 and 93307 reported without contrast to those CPT codes. We then assigned the costs for simulated CPT codes 93306 and 93307 reported with contrast to new HCPCS code C8929 (Transthoracic echocardiography with contrast, or without contrast followed by with contrast, real-time with image documentation (2D), includes M-mode recording, when performed, complete, with spectral Doppler echocardiography, and with color flow Doppler echocardiography) and revised HCPCS code C8923 (Transthoracic echocardiography with contrast, or without contrast followed by with contrast, real-time with image documentation (2D), includes M-mode recording, when performed, complete, without spectral or color Doppler echocardiography), respectively. In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68542 through 68544), we assigned these CPT and HCPCS codes to APCs for CY 2009 based on their simulated median costs and clinical characteristics. New CY 2009 CPT code 93306 and HCPCS code C8929 were assigned comment indicator “NI” in that final rule with comment period, to signify that they were new codes whose interim final OPPS treatment was open to comment on that final rule with comment period.
The CY 2010 OPPS/ASC proposed rule was the first opportunity to have claims data available from hospitals for echocardiography services performed with contrast (or without contrast followed by with contrast) and reported with HCPCS codes C8921 through C8928. With the exception of HCPCS code C8923, which had a significant change in its code descriptor for CY 2009, in the CY 2010 OPPS/ASC proposed rule (74 FR 35271), we proposed to use our standard methodology to set the CY 2010 OPPS payment rates for these echocardiography services performed with contrast, taking into consideration their HCPCS code-specific median costs from CY 2008 claims.
For CY 2010 ratesetting, we proposed to employ an alternative ratesetting methodology for CPT codes 93306 and 93307 and HCPCS codes C8929 and C8923 that is similar to the approach we used for CY 2009 in order to account for the new codes and revised code descriptors for which CY 2008 data are unavailable. However, in the case of the proposed CY 2010 cost estimation, our CY 2008 claims for CPT code 93307 were only for services performed without contrast, and we have CY 2008 claims for HCPCS C8923 for the comparable services performed with contrast. Specifically, we selected claims for CPT code 93307 on which CPT codes 93320 and 93325 were also present and we treated the summed costs on these claims as if they were a single procedure claim for CPT code 93306 in order to simulate the median cost for CPT code 93306, for which CY 2008 claims data are not available. We then selected single claims for CPT code 93307 to reflect the newly revised descriptor for CY 2009; that is, we included those claims where CPT code 93307 was not billed with either packaged CPT code 93320 or CPT code 93325 on the same claim in order to simulate an appropriate CY 2010 proposed median cost for CPT code 93307. We assigned the costs of HCPCS code C8923 when reported with CPT codes 93320 and 93325 to HCPCS code C8929 and the costs of HCPCS code C8923 when reported without CPT code 93320 or 93325 to HCPCS code C8923.
Following publication of the CY 2009 OPPS/ASC final rule with comment period, several stakeholders brought a number of concerns to our attention, including the interim APC assignment of new CPT code 93351 (Echocardiography, transthoracic, real-time with image documentation (2D), includes M-mode recording, when performed, during rest and cardiovascular stress test using treadmill, bicycle exercise and/or pharmacologically induced stress, with interpretation and report; including performance of continuous electrocardiographic monitoring, with physician supervision) and the corresponding new HCPCS code C8930 (Transthoracic echocardiography, with contrast, or without contrast followed by with contrast, real-time with image documentation (2D), includes M-mode recording, when performed, during rest and cardiovascular stress test using Start Printed Page 60376 treadmill, bicycle exercise and/or pharmacologically induced stress, with interpretation and report; including performance of continuous electrocardiographic monitoring, with physician supervision). These stakeholders noted that new CY 2009 CPT code 93351 was created to include the services reported previously by CPT codes 93015 (Cardiovascular stress test using maximal or submaximal treadmill or bicycle exercise, continuous electrocardiographic monitoring, and/or pharmacological stress; with physician supervision, with interpretation and report) and 93350 (Echocardiography, transthoracic, real-time with image documentation (2D), includes M-mode recording, when performed, during rest and cardiovascular stress test using treadmill, bicycle exercise and/or pharmacologically induced stress, with interpretation and report). Because new CY 2009 CPT code 93351 was meant to include the services previously reported with both the CPT codes for a transthoracic echocardiogram during rest and stress (CPT code 93350 is recognized under the OPPS) and a cardiovascular stress test (CPT code 93017 is recognized under the OPPS, rather than CPT code 93015), these stakeholders disagreed with our assignments of both CPT codes 93350 and 93351 to APC 0269 for CY 2009.
Upon review of these concerns and our CY 2008 data, in the CY 2010 OPPS/ASC proposed rule (74 FR 35271), we proposed for CY 2010 to use an alternative methodology to simulate median costs for CPT code 93351 and corresponding HCPCS code C8930, for which CY 2008 claims data are unavailable, and for CPT code 93350 and corresponding HCPCS code C8928 (Transthoracic echocardiography with contrast, or without contrast followed by with contrast, real-time with image documentation (2D), includes M-mode recording, when performed, during rest and cardiovascular stress test using treadmill, bicycle exercise and/or pharmacologically induced stress, with interpretation and report). That is, we proposed to use claims that contain both CPT codes 93350 and 93017 (Cardiovascular stress test using maximal or submaximal treadmill or bicycle exercise, continuous electrocardiographic monitoring, and/or pharmacological stress; tracing only, without interpretation and report) to simulate the median cost for CPT code 93351. We also proposed to use the remaining claims that contain CPT code 93350 but that do not contain CPT code 93017 to develop the proposed CY 2010 median cost for CPT code 93350. For our proposed rule analysis, we identified over 74,000 CY 2008 claims with both CPT code 93350 and CPT code 93017 on the same date of service and no other separately paid services appearing on the same date after applying our bypass processing logic, discussed in section II.A.1.b. of the proposed rule (74 FR 35240 through 35241). We treated these modified claims containing both CPT codes 93350 and 93017 as a single service and we calculated a proposed median cost of approximately $604. Therefore, for CY 2010, we proposed to reassign CPT code 93351 to revised APC 0270 (Level III Echocardiogram without Contrast), which had a proposed APC median cost of approximately $596. We proposed to continue to assign CPT code 93350 to APC 0269, which had a proposed APC median cost of approximately $456, based on its proposed HCPCS code-specific median cost of approximately $406 based on approximately 11,000 single claims. Furthermore, we proposed to use claims for HCPCS code C8928 that are reported with CPT code 93017 on the same claim to simulate the CY 2010 median cost for HCPCS code C8930. We identified over 4,000 claims in the proposed rule data with both HCPCS code C8930 and CPT code 93017 on the same date of service and no other separately paid services appearing on the same date after applying our bypass processing logic, discussed in section II.A.1.b. of the proposed rule (74 FR 35240 through 35241), that we modified to treat HCPCS code C8930 and CPT code 93017 as a single service. We calculated a HCPCS code-specific proposed median cost of approximately $706. Therefore, we proposed to continue to assign HCPCS code C8930 to APC 0128 with a proposed APC median cost of approximately $660. We also proposed to continue to assign HCPCS code C8928 to APC 0128, based on its HCPCS code-specific proposed median cost of approximately $595 based on approximately 1,000 single claims.
Comment: One commenter on the CY 2009 OPPS/ASC final rule with comment period addressed the interim final treatment of new CPT code 93306 for CY 2009. The commenter requested that CMS not recognize CPT code 93306 under the OPPS because this code represents the combination of three services already described by existing CPT codes 93307, 93320, and 93325. Alternatively, the commenter recommended that CMS could instruct hospitals to continue billing CPT codes 93320 and 93325 in association with CPT code 93306 in order to encourage consistent reporting of services described by CPT codes 93320 and 93325 when they are furnished with any echocardiography service. The commenter believed that requiring the use of CPT code 93306 may confuse hospitals, as other echocardiography services require the separate reporting of CPT codes 93320 and 93325 when these additional procedures are performed. Because there are already existing codes for the services described by CPT code 93306 and hospitals could inappropriately stop reporting CPT codes 93320 and 93325 in association with other echocardiography services, the commenter requested that CMS not recognize CPT code 93306 for payment under the OPPS. According to the commenter, under all circumstances, hospitals would continue to report CPT code 93320 or CPT code 93325 when they are performed with any echocardiography procedure, a practice preferred by the commenter. Similarly, the commenter recommended that CMS not recognize the corresponding HCPCS code C8929 that describes CPT code 93306 when furnished with contrast because the contrast echocardiography procedure could also be reported using existing HCPCS code C8921 and CPT codes 93320 and 93325.
Response: As is our standard methodology, we review new CPT codes annually and assign status indicators to all new codes and provide APC assignments, if applicable, for codes that describe services that may be performed in the hospital outpatient department (which includes provider-based clinics located on and off campus). We consider CPT code 93306 to be part of the standard CPT code set hospitals use for reporting services under the OPPS, and the service described by the code is one that we believe could be furnished to a hospital outpatient and potentially covered and, therefore, paid by Medicare under the OPPS. We incorporated CPT code 93306 in the CY 2009 OPPS/ASC final rule with comment period, assigning it a separately payable status indicator and APC, consistent with our belief that the service described by this code could be appropriately reported by hospitals when they furnish the service in the HOPD. Furthermore, as described in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68543), we used a special cost estimation methodology to estimate the expected cost of CPT code 93306 based on hospital claims data for the individual predecessor codes in order to inform our interim final assignment of CPT code 93306 to a clinical APC for CY 2009. Start Printed Page 60377
Regarding the commenter's alternative suggestion that we instruct hospitals to continue to report CPT codes 93320 and 93325 when performed in association with the procedure described by CPT code 93306, we will not instruct hospitals to continue to report CPT codes 93320 and 93325 when billing for CPT code 93306 because CPT code 93306 incorporates the services described by CPT codes 93320 and 93325 in its code descriptor. Billing separately for these services when reporting CPT 93306 would not be consistent with correct coding principles and could create greater confusion and unnecessary burden for hospitals. Whenever possible, hospitals have repeatedly encouraged us to follow standard coding guidelines in order to reduce their administrative burden in reporting services differently for Medicare, and our recognition of CPT code 93306 for payment under the OPPS is consistent with hospitals' general request to us.
Finally, as we are continuing to instruct hospitals to use CPT code 93306 for CY 2010, it continues to be appropriate for hospitals to bill using HCPCS code C8929 when furnishing the service described by CPT code 93306 with contrast. In the case of CPT code 93306 and other CPT codes for echocardiography services, we have developed parallel HCPCS C-codes to report each procedure when furnished with contrast in order to provide payment through separate APCs for those echocardiography services furnished with and without contrast.
Comment: Several commenters on the CY 2010 OPPS/ASC proposed rule expressed support for the revisions to the echocardiography APCs included in the CY 2010 OPPS/ASC proposed rule. One commenter noted appreciation for the proposed reassignment of CPT code 93351 from APC 0269 to APC 0270. However, one commenter on the CY 2009 OPPS/ASC final rule with comment period suggested that the new CY 2009 CPT code 93351 should not be recognized for payment under the OPPS. The commenter reasoned that the comprehensive service described by CPT code 93351 is comprised of two services previously reported with CPT codes 93350 and 93015: CPT code 93015 includes physician supervision and interpretation, which are not hospital outpatient services; and CPT code 93015 is reported by nonhospital practitioners and is not recognized for payment under the OPPS.
In addition, a commenter on the CY 2010 OPPS/ASC proposed rule stated that a more appropriate treatment of CPT code 93351 under the OPPS would be to not recognize this code for payment under the OPPS but, rather, to continue to recognize for payment several existing CPT codes which, when reported in combination, would describe the service that would otherwise be reported with CPT code 93351 alone. The commenter believed that CPT code 93351 was created specifically for services performed in nonfacility settings and that the intent of the CPT Editorial Committee was to limit the use of the code to nonfacility settings only. The commenter stated that correspondence from CMS indicated that CPT code 93351 would be billable only when provided in a physician's office or independent laboratory settings.
Response: As is our standard methodology, we review new CPT codes annually and assign status indicators to all new codes and provide APC assignments, if applicable, for codes that describe services that may be performed in the HOPD (which includes provider-based clinics located on and off campus). The CPT code descriptor for CPT code 93351 makes no mention that the code is restricted from use in the HOPD, or that its use is limited to nonfacility settings. Further, there are no additional CPT instructions that would limit the reporting of CPT code 93351 to nonfacility or nonhospital settings. We consider this CPT code to be part of the standard CPT code set hospitals use for reporting services under the OPPS, and the service described by the code is one that we believe could be furnished to a hospital outpatient and potentially covered and, therefore, paid by Medicare under the OPPS. CPT code 93351 describes a service that would previously have been reported with CPT codes 93350 and 93017 under the OPPS. While the commenter was correct that we do not recognize CPT code 93015 for payment under the OPPS, a code that describes a cardiovascular stress test with interpretation and report, we do recognize CPT code 93017, which describes the tracing only for the cardiovascular stress test. We incorporated CPT code 93351 in the CY 2009 OPPS/ASC final rule with comment period, assigning it a separately payable status indicator and APC, consistent with our belief that the service described by this code could be appropriately reported by hospitals when they furnish the service in the HOPD. Furthermore, we established professional component (PC) and technical component (TC) payments under the MPFS for CPT code 93351, also consistent with our belief that the CPT code may be reported for services in facility settings, such as independent laboratory settings. We have communicated no information to the public that states that Medicare hospital outpatient payment would not be made if this CPT code were reported by a hospital for services furnished to hospital outpatients.
We proposed a methodology for identifying the hospital outpatient claims and isolating the hospital charges that would be associated with this procedure for CY 2010 in order to develop an appropriate hospital outpatient payment for the associated facility resources for the existing services that would be reported and paid under the new CPT code. Specifically, we proposed to use claims that contain both CPT codes 93350 and 93017 to simulate the median cost for CPT code 93351 and proposed to reassign CPT code 93351 from APC 0269 to revised APC 0270 for CY 2010 based on its simulated median cost. We continue to believe that this CPT code may be reported for OPPS services described by the code, and that our proposed CY 2010 cost estimation methodology accurately simulates a median cost for this new code that reflects the associated hospital resources for the component services that are newly described by this single CPT code.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to reassign CPT code 93351 to APC 0270 based on a simulated CPT-specific median cost identified from over 80,000 CY 2008 claims with both CPT code 93350 and CPT code 93017 on the same date of service and no other separately paid services appearing on the same date after applying our bypass processing logic, as discussed above. We calculated a final CPT-specific median cost of approximately $605 for CPT code 93351 and a final CY 2010 APC median cost for APC 0270 of approximately $591.
Comment: One commenter on the CY 2009 OPPS/ASC final rule with comment period requested that CMS delete HCPCS code C8930 (Transthoracic echocardiography, with contrast, or without contrast followed by with contrast, real-time with image documentation (2D), includes M-mode recording, when performed, during rest and cardiovascular stress test using treadmill, bicycle exercise and/or pharmacologically induced stress, with interpretation and report; including performance of continuous electrocardiographic monitoring, with physician supervision) as the services Start Printed Page 60378 described by this code could be reported using CPT code 93017 (Cardiovascular stress test using maximal or submaximal treadmill or bicycle exercise, continuous electrocardiographic monitoring, and/or pharmacological stress; tracing only, without interpretation and report) and HCPCS code C8928 (Transthoracic echocardiography with contrast, or without contrast followed by with contrast, real-time with image documentation (2D), includes M-mode recording, when performed, during rest and cardiovascular stress test using treadmill, bicycle exercise and/or pharmacologically induced stress, with interpretation and report).
Response: As described above, we are continuing to recognize the service described by CPT code 99351, which is the noncontrast echocardiography procedure that is parallel to HCPCS code C8930 for the same procedures provided with contrast. As previously noted, we have developed parallel HCPCS C-codes to report each echocardiography procedure when furnished with contrast in order to provide payment through separate APCs for those echocardiography services furnished with and without contrast. While we understand that the service reported under HCPCS code C8930 may be reported using a combination of a CPT code and a HCPCS C-code, we do not believe that this would be appropriate because the noncontrast echocardiography service is reported with a single CPT code. Hospitals are generally instructed to use the HCPCS code that most appropriately and specifically describes the service that was provided, including not unbundling component services that could otherwise be separately reported. In this instance, HCPCS code C8930 would be the most specific code that describes the full service provided when the component services that would otherwise be reported by CPT code 93017 and HCPCS code C8928 are provided together. Our CY 2010 ratesetting methodology for HCPCS code C8928 is based on claims data and specifically excludes those cases when the service was furnished along with the procedure described by CPT code 93017. On the other hand, our CY 2010 ratesetting methodology for HCPCS code C8930 specifically includes cases where the services described by HCPCS code C8928 and CPT code 93017 were provided together. In that way, we are able to base CY 2010 payment for all of these services on their actual or simulated hospital costs in the context of the CPT and HCPCS C-codes that will be reported in CY 2010.
After consideration of the public comment we received, we are finalizing our CY 2010 proposal, without modification, to continue to recognize HCPCS code C8930 for OPPS payment. For CY 2010, HCPCS code C8930 continues to be assigned to APC 0128, with a final CY 2010 APC median cost of approximately $645.
Comment: One commenter on the CY 2009 OPPS/ASC final rule with comment period requested that CMS not recognize CPT code 93352 (Use of echocardiographic contrast agent during stress echocardiography), as the OPPS has already developed Level II HCPCS C-codes to identify echocardiography procedures performed with contrast.
Response: During our review of CPT code 93352 for the CY 2009 OPPS/ASC final rule with comment period, we assigned an interim final status indicator “M” (Not paid under the OPPS) to CPT code 93352 for CY 2009. In our CY 2010 OPPS/ASC proposed rule, we proposed to continue this status indicator assignment for CY 2010.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to assign status indicator “M” to CPT code 93352.
Comment: A few commenters requested that CPT code 93318 (Echocardiography, transesophageal (TEE) for monitoring purposes, including probe placement, real time 2-dimensional image acquisition and interpretation leading to ongoing (continuous) assessment of (dynamically changing) cardiac pumping function and to therapeutic measures on an immediate time basis) not be reassigned to APC 0269 as proposed for CY 2010. Instead, these commenters requested that CPT code 93318 continue to be assigned to APC 0270 for CY 2010. Commenters stated that CPT code 93318 is clinically similar to CPT code 93312 (Echocardiography, transesophageal, real time with image documentation (2D) (with or without M-mode recording); including probe placement, image acquisition, interpretation and report), and because CPT code 93312 is assigned to APC 0270, CPT code 93318 should be assigned to APC 0270 as well. While these commenters noted that the reassignment of CPT code 93318 to APC 0269 would be most consistent with its CPT-specific median cost presented in the proposed rule, they stated that the unexplained volatility in the cost of CPT code 93318 suggests that clinical homogeneity should be the deciding factor when assigning this service to an APC.
Response: As is our standard process, for the CY 2010 proposed rule, we reviewed each APC for clinical cohesiveness and resource homogeneity. As the commenters noted, we proposed to reassign CPT code 93318 to APC 0269 as we believed that the proposed CPT-specific median cost more closely matched the median cost of APC 0269. While we continue to believe that the CPT-specific median cost of CPT 93318 (approximately $472) closely resembles the median cost of APC 0269 (approximately $447), upon further review, we agree with the commenter that the clinical characteristics of the procedure described by CPT code 93318 are similar to the procedure described by CPT code 93312. We also note that we have only 344 single and 593 total claims for CPT code 93318 from only 188 providers in comparison to 29,987 single and 52,342 total claims for CPT code 93312 from 2,093 providers. We believe the limited claims data from relatively few providers contribute to the variability in cost observed for CPT code 93318 and agree with the commenters that this procedure should remain assigned to APC 0270 for CY 2010.
After consideration of the public comments we received, we are not finalizing our proposal to reassign CPT code 93318 to APC 0269. Instead, for CY 2010, we are continuing to assign CPT code 93318 to APC 0270, with a final CY 2010 APC median cost of approximately $591.
Comment: Several commenters supported the continuation of separate APCs for payment of echocardiography procedures with contrast and without contrast. While these commenters were generally supportive of the proposed ratesetting methodology, they were concerned that the proposed payment rate for APC 0128 of approximately $683 was insufficient to cover the costs associated with providing the echocardiogram and the related contrast materials and services for HCPCS codes C8921 (Transthoracic echocardiography with contrast, or without contrast followed by with contrast, for congenital cardiac anomalies; complete); C8925 (Transesophageal echocardiography (TEE) with contrast, or without contrast followed by with contrast, real time with image documentation (2D) (with or without M-mode recording); including probe placement, image acquisition, interpretation and report); C8926 (Transesophageal echocardiography (TEE) with contrast, or without contrast followed by with contrast, for congenital cardiac anomalies; including probe Start Printed Page 60379 placement, image acquisition, interpretation and report); and C8930 (Transthoracic echocardiography, with contrast, or without contrast followed by with contrast, real-time with image documentation (2D), includes M-mode recording, when performed, during rest and cardiovascular stress test using treadmill, bicycle exercise and/or pharmacologically induced stress, with interpretation and report; including performance of continuous electrocardiographic monitoring, with physician supervision). Specifically, the commenters noted that the noncontrast equivalent procedures (described by CPT codes 93303, 93312, 93315, and 93351) were all proposed for assignment to APC 0270, with a proposed payment rate of approximately $600. The commenters believed that the difference between the proposed payment rate for these procedures with contrast and without contrast is too small to cover the cost of the contrast material used in these procedures. The commenters suggested that CMS reassign HCPCS codes C8921, C8925, C8926, and C8930 to a new APC for echocardiography procedures performed with contrast or that CMS provide separate payment for the contrast material used in these procedures.
Response: The final payment differential between APC 0270, where CPT codes 99303, 99312, 99315, and 99351 are assigned, and APC 0128, where the corresponding HCPCS codes for the same procedures with contrast (HCPCS codes C8921, C8925, C8926, and C8930) are assigned, is the difference between approximately $645 and approximately $591 of $54. We believe this differential provides an appropriate higher payment to those hospitals that furnish these procedures with contrast and appropriately accounts for the cost of the contrast material, which is required for all of the services assigned to APC 0128. HCPCS codes C8921, C8925, C8926, and C8930 have median costs that range from a low of approximately $178 to a high of approximately $712. Each of these HCPCS codes was reported by fewer than 170 providers in CY 2008. The median costs of these services span most of the range of median costs of HCPCS codes assigned to APC 0128, and they do not form a cluster of high cost procedures in the APC such that they would warrant assignment to a new clinical APC. In contrast, the median costs of CPT codes 99303, 99312, 99315, and 99351 span a much narrower range, from a low of approximately $505 to a high of approximately $605. Two of these CPT codes were reported by more than 1,500 providers in CY 2008. Clearly, fewer providers are reporting the echocardiogram procedures with contrast, and we expect that the hospital cost distribution for that subset of hospitals could be different than the cost distribution of the large number of providers reporting the procedures without contrast. Therefore, no conclusions can be drawn about the aggregate OPPS payment to that subset of hospitals for all of their echocardiogram services in comparison to the aggregate echocardiogram costs of the subset of hospitals specifically based on the payment rates for APCs 0128 and 0270. The OPPS is a prospective payment system that relies on hospital charge and cost report data from the hospitals that furnish the services in order to determine relative costs. Therefore, we believe that our prospective payment rates calculated based on the costs of those providers furnishing the procedures in CY 2008 provide appropriate payment to the providers that will furnish the services in CY 2010.
In summary, after consideration of the public comments we received, we are finalizing our CY 2010 proposals for payment of echocardiography procedures with and without contrast, with modifications. We are finalizing our proposed methodologies for simulating the median costs of CPT codes 93306, 93307, 93351, and 93350 for which there are no CY 2008 hospital claims data for these specific CPT codes, as discussed above. In addition, we are finalizing our proposed methodologies for simulating the median costs of HCPCS codes C8929, C8923, C8930, and C8928 for which there are no CY 2008 hospital claims data for these specific HCPCS codes, as discussed above. We are not finalizing our proposal to reassign CPT code 93318 to APC 0269; instead, we are maintaining the assignment of CPT code 93318 to APC 0270 for CY 2010. Finally, we are finalizing our proposal to assign HCPCS codes C8921, C8925, C8926, and C8930 to APC 0128 for CY 2010.
Table 9 below shows CY 2010 CPT codes for billing echocardiography services without contrast, their final APC assignments for CY 2010, and the corresponding HCPCS codes for use when echocardiography services are performed with contrast (or without contrast followed by with contrast), along with their final APC assignments for CY 2010.
Start Printed Page 60380 Start Printed Page 60381 Start Printed Page 60382 Start Printed Page 60383Finally, in the CY 2010 OPPS/ASC proposed rule (74 FR 35275), for CY 2010, based upon our proposed APC configurations, we also proposed to revise the titles of our existing series of echocardiography APCs to more accurately describe the groups of services identified by CPT codes 93303 through 93352 and HCPCS codes C8921 through C8930 that are assigned to these APCs. We proposed to rename APCs 0269, 0270, and 0697 as described in Table 7 of the proposed rule.
Comment: One commenter supported the proposed revisions to the echocardiography APC titles and configurations.
Response: We appreciate the support for our proposal.
We are finalizing our proposal to rename APCs 0269, 0270, and 0697 without modification. Therefore, we are adopting as final the titles of these APCs as reflected in Table 10 below:
| Final CY 2010 APC | CY 2010 APC title | Final CY 2010 approximate APC median cost |
|---|---|---|
| 0128 | Echocardiogram With Contrast | $645 |
| 0269 | Level II Echocardiogram Without Contrast | 447 |
| Start Printed Page 60384 | ||
| 0270 | Level III Echocardiogram Without Contrast | 591 |
| 0697 | Level I Echocardiogram Without Contrast | 262 |
(5) Nuclear Medicine Services
In CY 2008, we began packaging payment for diagnostic radiopharmaceuticals into the payment for the associated nuclear medicine procedure. (For a discussion regarding the distinction between diagnostic and therapeutic radiopharmaceuticals, we refer readers to the CY 2008 OPPS/ASC final rule with comment period at 72 FR 66636.) Prior to the implementation of this policy, diagnostic radiopharmaceuticals were subject to the standard OPPS drug packaging methodology whereby payments are packaged when the estimated mean per day product costs fall at or below the annual packaging threshold for drugs, biologicals (other than implantable biologicals), and radiopharmaceuticals.
Packaging costs into a single aggregate payment for a service, encounter, or episode-of-care is a fundamental principle that distinguishes a prospective payment system from a fee schedule. In general, packaging the costs of supportive items and services into the payment for the independent procedure or service with which they are associated encourages hospital efficiencies and also enables hospitals to manage their resources with maximum flexibility. All nuclear medicine procedures require the use of at least one radiopharmaceutical or other radiolabeled product, and there are only a small number of radiopharmaceuticals that may be appropriately billed with each diagnostic nuclear medicine procedure. For the OPPS, we distinguish diagnostic radiopharmaceuticals from therapeutic radiopharmaceuticals for payment purposes, and this distinction is recognized in the Level II HCPCS codes for diagnostic radiopharmaceuticals that include the term “diagnostic” along with a radiopharmaceutical in their HCPCS code descriptors. As we stated in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66635), we believe that our policy to package payment for diagnostic radiopharmaceuticals (other than those already packaged when their per day costs are below the packaging threshold for OPPS drugs, biologicals, and radiopharmaceuticals) is consistent with OPPS packaging principles, provides greater administrative simplicity for hospitals, and encourages hospitals to use the most clinically appropriate and cost efficient diagnostic radiopharmaceutical for each study. For more background on this policy, we refer readers to discussions in the CY 2008 OPPS/ASC proposed rule (72 FR 42667 through 42672) and the CY 2008 OPPS/ASC final rule with comment period (72 FR 66635 through 66641).
For CY 2008 ratesetting, we used only claims for nuclear medicine procedures that contained a diagnostic radiopharmaceutical in calculating the median costs for APCs that include nuclear medicine procedures (72 FR 66639). This is similar to the established methodology used for device-dependent APCs before claims reflecting the procedure-to-device edits were included in our claims data. For CY 2008, we also implemented claims processing edits (called procedure-to-radiolabeled product edits) requiring the presence of a radiopharmaceutical (or other radiolabeled product) HCPCS code when a separately payable nuclear medicine procedure is present on a claim. Similar to our practice regarding the procedure-to-device edits that have been in place for some time, we continually review comments and requests for changes related to these edits and, based on our review, may update the edit list during our quarterly update process if necessary. The radiolabeled product and procedure HCPCS codes that are included in these edits can be viewed on the CMS Web site at: http://www.cms.hhs.gov/HospitalOutpatientPPS/01_overview.asp.
The CY 2008 OPPS claims that are subject to the procedure-to-radiolabeled product edits were not available for setting payment rates in CY 2009. Therefore, as described in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68545), we continued to use our established CY 2008 methodology for setting the payment rates for APCs that included nuclear medicine procedures for CY 2009. We used an updated list of radiolabeled products, including but not limited to diagnostic radiopharmaceuticals, from the procedure-to-radiolabeled product edit file to identify single and “pseudo” single claims for nuclear medicine procedures that also included at least one eligible radiolabeled product. Using this subset of claims, we followed our standard OPPS ratesetting methodology to calculate median costs for nuclear medicine procedures and their associated APCs. As in CY 2008, when we set APC median costs based on single and “pseudo” single claims that also included at least one radiolabeled product on our edit file, we observed an equivalent or higher median cost than that calculated from all single and “pseudo” single bills. We believe that this methodology appropriately ensured that the costs of diagnostic radiopharmaceuticals were included in the CY 2009 ratesetting process for these APCs.
As discussed in section II.A.4.b.(1) of the proposed rule (74 FR 35287) and this final rule with comment period, during the September 2007 APC Panel meeting, the APC Panel requested that CMS evaluate the impact of expanded packaging on beneficiaries. Also, during the March 2008 APC Panel meeting, the APC Panel requested that CMS report to the APC Panel at the first meeting in CY 2009 the impact of packaging on net payments for patient care. In response to these requests, we shared data with the APC Panel at the February 2009 APC Panel meeting that compared the frequency of the billing of diagnostic radiopharmaceuticals billed under the OPPS in CY 2007, before the packaging of all diagnostic radiopharmaceuticals went into effect, to the frequency of the billing of those same products in CY 2008, their first year of packaged payment. We also reviewed information about the aggregate payment for diagnostic radiopharmaceuticals and nuclear medicine procedures during those same 2 years. A summary of these data analyses is provided in section II.A.4.b.(1) of this final rule with comment period.
In addition to these aggregate analyses of total frequency and payment, we also presented our analyses of the number of hospitals performing nuclear medicine scans and the specific diagnostic radiopharmaceuticals appearing with Start Printed Page 60385 cardiac and tumor imaging nuclear medicine procedures, excluding positron emission tomography (PET) scans, by classes of hospitals between the CY 2007 claims processed through September 30, 2007 and the CY 2008 claims processed through September 30, 2008. At the March 2008 APC Panel meeting, the APC Panel also recommended that we evaluate the usage and frequency, geographic distribution, and size and type of hospitals performing nuclear medicine studies using radioisotopes to assess beneficiaries' access and that we present these analyses at the first APC Panel meeting in CY 2009. The number of all hospitals reporting any nuclear medicine procedure declined by 2 percent between the CY 2007 claims data and the CY 2008 claims data. Across several classes of hospitals (urban and rural, teaching and nonteaching, and small and large OPPS service volume), the number of hospitals billing any nuclear medicine procedure declined by up to 4 percent over that same time period. With regard to the specific diagnostic radiopharmaceuticals reported with cardiac and tumor imaging nuclear medicine procedure, we generally observed comparable distributions of radiopharmaceuticals between the CY 2007 claims data and the CY 2008 claims data. However, the utility of this analysis was limited due to the introduction of the procedure-to-radiolabeled product claims processing edits discussed above. There are nuclear medicine procedures reported with a diagnostic radiopharmaceutical HCPCS code on the CY 2008 claims that would have not necessarily been billed with a diagnostic radiopharmaceutical HCPCS code on the CY 2007 claims. Specifically, we observed an increase in billing for many radiopharmaceuticals, some new and costly, between the CY 2007 claims data and the CY 2008 claims data. We do not know how much of this was attributable to changes in hospitals' use of radiopharmaceuticals or to the CY 2008 introduction of the procedure-to-radiolabeled product edits that require a radiolabeled product on the claim for payment of the nuclear medicine procedure. With the exception of the notable increases in the frequencies of certain radiopharmaceutical HCPCS codes that potentially resulted from the introduction of these edits, in general, hospital billing patterns for diagnostic radiopharmaceuticals associated with cardiac and tumor imaging nuclear medicine scans did not change dramatically between CY 2007 and CY 2008 for all hospitals and classes of hospitals. We concluded that very few hospitals stopped providing nuclear medicine procedures as a result of our CY 2008 policy to package payment for diagnostic radiopharmaceuticals and that, in general, hospitals did not decrease their use of expensive radiopharmaceuticals.
As a result of the discussions of the APC Panel following our presentation of the analyses of the impact of packaging payment for all diagnostic radiopharmaceuticals in the OPPS, the APC Panel further recommended that CMS continue to analyze the impact on beneficiaries of increased packaging of diagnostic radiopharmaceuticals and provide more detailed analyses at the next APC Panel meeting. Further, the APC Panel requested that, in the more detailed analyses of packaging of diagnostic radiopharmaceuticals by type of nuclear medicine scan, CMS analyze the data according to the specific CPT codes billed with the diagnostic radiopharmaceuticals. We stated in the CY 2010 OPPS/ASC proposed rule (74 FR 35277) that we are accepting the APC Panel's recommendation and would provide additional data to the APC Panel at an upcoming meeting. We did not share additional data related to diagnostic radiopharmaceuticals with the APC Panel at the most recent August 2009 meeting because we believe the APC Panel's discussions would benefit from analyses of an additional year of claims data after CY 2008. Therefore, we plan to incorporate analysis of CY 2009 claims into the information we will present to the APC Panel for its review at the winter 2010 meeting.
At the February 2009 meeting of the APC Panel, the Panel commended CMS for its effort to date to tailor the resource-based APC system to facilitate appropriate payment for diagnostic and therapeutic radiopharmaceuticals. The APC Panel recommended that CMS continue its dialogue with professional societies, vendors, and other stakeholders to improve the accuracy of APC payments for these complex items and services, including consideration of developing composite APCs. We appreciate the support of the APC Panel, and we are accepting the APC Panel's recommendation to continue to communicate with interested stakeholders regarding payment for radiopharmaceuticals and the associated procedures. We regularly accept meetings from interested parties throughout the year, and we encourage stakeholders to continue a dialogue with us during the rulemaking cycle and throughout the year. Our response to the APC Panel's recommendation regarding composite APCs is included in our response to the public comments summarized below.
For CY 2010 ratesetting, we are able to use CY 2008 OPPS claims that were subject to the procedure-to-radiolabeled product claims processing edits incorporated into the I/OCE prior to payment of claims in order to develop single and “pseudo” single claims for nuclear medicine procedures according to our standard methodology. We believe that using the CY 2008 claims data for these services without further editing for the presence of a radiolabeled product is now appropriate for CY 2010 because these claims reflect all possible relationships between the nuclear medicine procedures and their associated radiolabeled products that we have accommodated for payment of nuclear medicine procedures. Moreover, as we indicated in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68548 through 68549), in the rare circumstance where a diagnostic radiopharmaceutical is not provided in association with a nuclear medicine procedure, for example, because a beneficiary receives a therapeutic radiopharmaceutical as part of a hospital inpatient stay and then returns to the HOPD for a nuclear medicine scan without needing a diagnostic radiopharmaceutical to be administered again for the study, we believe it is appropriate to use these claims for ratesetting purposes. We believe that just as these situations are representative of the performance of a nuclear medicine scan, it is also appropriate to include them for ratesetting purposes.
Comment: A number of commenters opposed CMS' proposed policy to package payment for all diagnostic radiopharmaceuticals into payment for their associated nuclear medicine procedures. They noted that the majority of diagnostic radiopharmaceuticals are not interchangeable and, for that reason, CMS' policy of packaging payment for all diagnostic radiopharmaceuticals into their associated nuclear medicine procedures does not foster hospital efficiencies. Some commenters expressed concern that packaging diagnostic radiopharmaceuticals into payment for associated nuclear medicine procedures results in overpayment of many procedures, especially those using existing low-cost radiopharmaceuticals, while the bundled payment would be insufficient for newer, and likely more expensive, radiopharmaceuticals.
In addition, the commenters requested that, if CMS continues to Start Printed Page 60386 package payment for diagnostic radiopharmaceuticals into payment for their associated nuclear medicine procedures, CMS revise the nuclear medicine APCs to provide differential payments for nuclear medicine procedures when used with different radiopharmaceuticals. Several commenters identified the series of tumor/infection imaging APCs, including APCs 0406 (Level I Tumor/Infection Imaging), 0408 (Level III Tumor/Infection Imaging), and 0414 (Level II Tumor/Infection Imaging), for CMS' attention to ensure appropriate payment for low volume, high cost radiopharmaceuticals. One commenter specifically suggested a composite APC for certain combinations of a tumor imaging scan and specific diagnostic radiopharmaceuticals.
Several commenters noted that there is wide variation in the costs of diagnostic radiopharmaceuticals, and that composite APCs for specific combinations of procedures and diagnostic radiopharmaceuticals would be necessary to ensure adequate payment to hospitals using expensive diagnostic radiopharmaceuticals. Other commenters suggested that the significant clinical and resource diversity of radiopharmaceuticals packaged into nuclear imaging procedures amounted to a violation of the 2 times rule. The commenters explained that, just as diagnostic radiopharmaceuticals are not interchangeable, certain radiopharmaceuticals are indicated for particular types of diseases, such as cancer, and are not clinically similar to other radiopharmaceuticals used for other purposes, such as tumor imaging.
Response: As we discussed in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68547), we understand that the selection of a diagnostic radiopharmaceutical for a particular nuclear medicine procedure is a complex decision based on many factors, including patient-specific factors, and that not every diagnostic radiopharmaceutical is fully interchangeable with others. However, as stated in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66617) and in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68546), we believe that nonspecific packaging (as opposed to selected code packaging) based on combinations of items and services observed on hospital claims is fully appropriate because of the myriad combinations of items and services that can be appropriately provided together. Under the OPPS, we package payment for ancillary, supportive, and interrelated items and services into payment for the independent services they accompany. As we discuss in section II.A.4. of this final rule with comment period, packaging promotes hospital efficiencies through numerous means, not only just through the choice of which radiopharmaceutical to use for a specific nuclear medicine scan. While all diagnostic radiopharmaceuticals may not be interchangeable, we believe that packaging the costs of diagnostic radiopharmaceuticals, however differential those costs may be, into the payment for nuclear medicine services that use these products is appropriate, whether there is one product or multiple products that could be used to furnish the particular service provided to an individual patient. The OPPS has a history of packaging items that are not necessarily interchangeable. It is our longstanding practice to package payment for nonpass-through implantable medical devices into payment for the procedure in which they are used, notwithstanding that there may be different devices or combinations of devices that could be used to furnish a service. (For a more complete discussion of the history of packaging items, we refer readers to the CY 2008 OPPS/ASC final rule with comment period (72 FR 66639).) Therefore, in accordance with our understanding that a diagnostic radiopharmaceutical is never provided without an accompanying nuclear medicine scan, we believe that it is appropriate to package the payment for all diagnostic radiopharmaceuticals into the payment for the associated nuclear medicine procedure.
With regard to suggested composites or other revisions designed to isolate specific nuclear medicine scans with a subset of diagnostic radiopharmaceuticals, as we discussed in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68546), we do not believe that the inability to substitute one diagnostic radiopharmaceutical for another is a compelling reason for creating composite APCs, as explained below. We developed composite APCs to provide a single payment for two or more services that are typically performed together during a single clinical encounter and that result in the provision of a complete service. Composite APCs differ from packaging. Composite APCs provide a single payment for specific combinations of independent services that would otherwise be separately payable if they were not provided together, while packaging entails associating the cost of ancillary, supportive, and interrelated services and supplies with a distinct service or composite service. Composite APCs are intended to expand the OPPS payment bundles to encourage hospital efficiencies. Providing a single payment for a specific combination of a diagnostic radiopharmaceutical with a particular nuclear medicine procedure would not constitute a composite APC and would provide no incentives for hospital efficiency. Specifically, a diagnostic radiopharmaceutical would never be separately payable under the OPPS when furnished alone, so the combination of a diagnostic radiopharmaceutical and a nuclear medicine procedure would not meet the definition of a composite APC as described above. From the perspective of value-based purchasing, we see no benefit to paying for many individual diagnostic radiopharmaceutical and nuclear medicine procedure combinations over paying separately for both the item and service, beyond an appearance of bundling. Such an approach would add complexity to ratesetting and would create challenges and cost instability because payments would be based on data from small numbers of claims for certain HCPCS code pairs. As noted above, there are many items and services that we package under the OPPS that are similarly not interchangeable with other related items and services. Therefore, we are not accepting the APC Panel's recommendation to explore developing composite APCs for diagnostic radiopharmaceuticals and nuclear medicine procedures.
We understand that, by packaging payment for a range of products such as diagnostic radiopharmaceuticals, payment for the associated nuclear medicine procedure may be more or less than the hospital's cost for these services in a given case. As stated in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66639) and the CY 2009 OPPS/ASC final rule with comment period (73 FR 68546), we note that a fundamental characteristic of a prospective payment system is that payment is to be set at an average for the service which, by definition, means that some services are paid more or less than the average.
We discussed in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66640) and the CY 2009 OPPS/ASC final rule with comment period (73 FR 68546) the issue of variability in radiopharmaceutical costs or other packaged costs creating potential 2 times violations. We note that 2 times violations are specific to the total cost of the primary service, nuclear medicine Start Printed Page 60387 scans in this case, including packaged costs. We have performed our standard review of the APCs using updated CY 2008 claims data for this final rule with comment period and, as a result, have not identified any 2 times violations in the APCs containing nuclear medicine procedures, when calculated as described above. (For more information on the 2 times rule, we refer readers to sections III.B.2. and III.B.3. of this final rule with comment period.)
Comment: Several commenters expressed concern that CMS was relying on edits in the claims processing system in order to identify those claims that would be used for CY 2010 ratesetting purposes. These commenters suggested that CMS continue to require a diagnostic radiopharmaceutical in order to use a nuclear medicine claim for ratesetting purposes for at least another 2 years in order to ensure that the claims editing process is working properly and that all hospital costs are reflected in the median costs of nuclear medicine procedures.
One commenter noted that CMS' methodology for setting payment rates for nuclear medicine services may be flawed. This commenter contended that CMS should not solely rely on the claims processing edits in order to determine which claims are to be used for ratesetting purposes. The commenter suggested that, even though CMS is using claims that have passed the nuclear medicine-to-radiolabeled product edits, CMS' ratesetting methodology may exclude the cost of diagnostic radiopharmaceuticals when calculating median costs for associated nuclear medicine procedures. Specifically, the commenter stated that the program logic that creates “pseudo” single procedure claims may separate a nuclear medicine scan and the associated diagnostic radiopharmaceutical when the diagnostic radiopharmaceutical appears on a different day and, therefore, CMS would not package the cost of the diagnostic radiopharmaceutical when setting the median cost for the nuclear medicine procedure. The commenter added that CMS' ratesetting methodology for “pseudo” single procedure claims relies on the date of service to identify associated packaged costs. Therefore, the commenter requested that CMS use only single and “pseudo” single nuclear medicine procedure claims that also contain a diagnostic radiopharmaceutical in order to set payment rates for nuclear medicine procedures. More specifically, several commenters requested that CMS not reassign CPT code 78803 (Radiopharmaceutical localization of tumor or distribution of radiopharmaceutical agent(s); tomographic (SPECT)) to APC 0414 (Level II Tumor/Infection Imaging) as proposed, but instead assign CPT code 78803 to APC 0408 (Level III Tumor/Infection Imaging). One commenter believed that the use of “pseudo” single procedure claims to calculate payment rates may have neglected to include the cost of the radiopharmaceutical or other scans that may have been performed on other dates of service and reported on other claims.
Response: As we indicated in the CY 2008 OPPS/ASC final rule with comment period (72 FR 42669), we are aware that several diagnostic radiopharmaceuticals may be used for multiple day studies; that is, a particular diagnostic radiopharmaceutical may be administered on one day and a related diagnostic nuclear medicine procedure may be performed on a subsequent day. While we understand that multiple-day episodes for diagnostic radiopharmaceuticals and the related diagnostic nuclear medicine procedures occur, we found the occurrence of nuclear medicine scans on a different date of service to be a small proportion of all diagnostic nuclear medicine imaging procedures appearing with the radiopharmaceutical. Specifically, our analysis at that time indicated that, roughly, 15 diagnostic radiopharmaceuticals have a half-life longer than one day such that they could support diagnostic nuclear medicine scans on different days. Excluding the 5 percent of diagnostic radiopharmaceutical claims that had no matching diagnostic nuclear medicine scan for the same beneficiary, we found that a diagnostic nuclear medicine scan was reported on the same day as a coded diagnostic radiopharmaceutical 90 percent or more of the time for 10 of these 15 diagnostic radiopharmaceuticals. Further, we found that between 80 and 90 percent of single bills for each of the remaining 5 diagnostic radiopharmaceuticals had a diagnostic nuclear medicine scan on the same day.
Moreover, as the commenter noted, the potential separation of a diagnostic radiopharmaceutical and the associated nuclear medicine procedure would only be relevant to the “pseudo” single procedure claims. In the “natural” single bills we use for ratesetting, we package costs across dates of service. Overall, in examining the CY 2008 claims data available for this final rule with comment period, we observed that “natural” single claims constituted a majority of all single procedure claims used to calculate median costs for APCs with nuclear medicine procedures. Further, we acknowledge that we expect to lose packaged costs on a small proportion of claims when we create “pseudo” single procedure claims by splitting claims based on dates of service. This is an inevitable consequence of the “pseudo” single procedure claim creation process. We believe that the tradeoff is a minor one given the significant benefit of additional claims data, and the vast majority of commenters generally supported our “pseudo” single procedure claim methodology. Finally, we note that the nuclear medicine procedure-to-radiolabeled product I/OCE claims processing edits ( http://www.cms.hhs.gov/HospitalOutpatientPPS/02_device_procedure.asp) to which the commenters referred include therapeutic radiopharmaceuticals and brachytherapy sources. Claims that pass these claims processing edits and enter into the ratesetting methodology without a diagnostic radiopharmaceutical reported on the claim are factored into ratesetting for nuclear medicine procedures as we do not expect that every nuclear medicine procedure would be billed with a diagnostic radiopharmaceutical, although we do expect each to be billed with a radiolabeled product. We note that the only time that we would not expect a nuclear medicine procedure to be billed with a radiolabeled product on an outpatient claim would be in the rare circumstance where a therapeutic radiopharmaceutical is provided in an inpatient setting and a nuclear medicine procedure associated with this radiopharmaceutical is subsequently furnished in the HOPD. In this specific circumstance, we would expect that hospitals would bill HCPCS code C9898 (Radiolabeled product provided during a hospital inpatient stay) in place of the radiolabeled product. Nuclear medicine scans are sometimes performed after the application of brachytherapy sources or the provision of a therapeutic radiopharmaceutical and in these cases the administration of an additional source of radioactivity (a diagnostic radiopharmaceutical) may not be required. While brachytherapy sources and therapeutic radiopharmaceuticals would be paid separately under the OPPS, we believe it is appropriate for us to include the costs of the scans that include a brachytherapy source or therapeutic radiopharmaceutical (or where a therapeutic radiopharmaceutical used for the scan was furnished to an inpatient) but lack a diagnostic radiopharmaceutical in Start Printed Page 60388 calculating the median cost of the nuclear medicine procedure because these claims represent the hospital costs for the scans furnished under these circumstances. We previously discussed this issue in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68647 through 68648).
We believe that the single and “pseudo” single procedure claims resulting from our standard ratesetting methodology accurately capture the cost of providing nuclear medicine scans under a variety of clinical scenarios for several reasons discussed above and summarized again here. First, previous analyses demonstrated that a significant percentage of nuclear medicine procedures are reported on the same day as diagnostic radiopharmaceuticals with an extended half-life and, in these cases, our ratesetting methodology would capture these diagnostic radiopharmaceutical costs. We acknowledge that diagnostic radiopharmaceuticals with an extended half-life may be administered on a different day than the performance of the accompanying nuclear medicine scan. However, administration of the diagnostic radiopharmaceutical on a different day does not mean that these costs are not captured in our APC median costs for nuclear medicine procedures. The majority of the single procedure claims that we use to estimate APC median cost for APCs with nuclear medicine scans are “natural” single procedure claims that package all identified packaged costs (including diagnostic radiopharmaceuticals) into the nuclear medicine procedures, irrespective of the dates of service. While our standard ratesetting methodology also relies on “pseudo” single procedure claims that, by definition, represent only a single service date and potentially eliminate the cost of a packaged diagnostic radiopharmaceutical with an extended half-life billed on a different date of service than the nuclear medicine scan, the potential to ignore packaged costs on other dates of service is true for all procedures for which we use “pseudo” single procedure claims in ratesetting. This small loss of packaging is a tradeoff in adopting our methodology for breaking down multiple procedure claims through the bypass process, as discussed in section II.A.1.b. of this final rule with comment period. Finally, not all claims for nuclear medicine procedures should include a diagnostic radiopharmaceutical because they may include another type of radiolabeled product (such as a brachytherapy source or therapeutic radiopharmaceutical), and these additional radiolabeled products are not packaged. In short, we believe that, overall, the single procedure claims for nuclear medicine scans, both “natural” and “pseudo” single procedure claims, together appropriately represent the full cost of providing various nuclear medicine procedures and result in accurate APC median costs. Therefore, our standard OPPS ratesetting methodology of using median costs calculated from claims data according to our standard methodology from those claims that passed the I/OCE claims processing edits adequately captures the costs of diagnostic radiopharmaceuticals associated with diagnostic nuclear medicine procedures that are not provided on the same date of service.
Specifically with regard to our proposed reassignment of CPT code 78803, with a CPT code-specific median cost of approximately $561, to APC 0414, with an APC median cost of approximately $506, we note that we have almost 3,000 single claims upon with the median cost of CPT code 78803 is based. This CPT code-specific median cost is significantly lower than the median cost of APC 0408 of approximately $954, the APC assignment requested by the commenters and the highest level APC in the tumor/infection imaging series. Therefore, we believe the most appropriate CY 2010 APC assignment for CPT code 78803 is APC 0414, as we proposed for CY 2010. As stated above, we believe that our standard ratesetting methodology adequately incorporates the packaged diagnostic radiopharmaceutical costs associated with nuclear medicine procedures, including the procedure described by CPT code 78803.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to package the costs of all diagnostic radiopharmaceuticals into payment for the associated nuclear medicine procedures utilizing our standard OPPS ratesetting methodology that is applied to claims that passed the nuclear medicine procedure-to-radiolabeled product I/OCE claims processing edits in CY 2008. We also are finalizing our CY 2010 proposal, without modification, to reassign CPT code 78003 to APC 0414, with an APC median cost of approximately $506.
Comment: A number of commenters cited concerns regarding the proposed APC assignments and proposed payment rates for a number of nuclear medicine procedures. These commenters believed that the proposed APC assignments of certain nuclear medicine procedures led to clinically diverse procedures being grouped together for payment purposes.
Specifically, one commenter requested that: (1) CPT code 78645 (Cerebrospinal fluid flow, imaging (not including introduction of material); shunt evaluation) be reassigned from APC 0403 (Level I Nervous System Imaging) to APC 0402 (Level II Nervous System Imaging); (2) CPT code 78608 (Brain imaging, positron emission tomography (PET); metabolic evaluation) be reassigned from APC 0308 (Non-Myocardial Positron Emission Tomography (PET) Imaging) to a more appropriate APC; and (3) CPT codes 78000 (Thyroid uptake; single determination) and 78001 (Thyroid uptake; multiple determinations) be reassigned from APC 0389 (Level I Non-imaging Nuclear Medicine) to APC 0392 (Level II Non-Imaging Nuclear Medicine).
Response: We have performed our annual review of all the procedures and APC groupings for this final rule with comment period based on updated CY 2008 claims data. The CPT code-specific median cost of CPT code 78645 is approximately $246 based on 434 single claims, which is reasonably close to the median cost of APC 0403 of approximately $195, where we proposed to assign the service. The commenter recommended assignment of CPT code 78645 to APC 0402, in the same nervous system imaging series, with a significantly higher APC median cost of approximately $573. Based on this review of the costs and clinical characteristics of other services assigned to these nervous system imaging APCs, we continue to believe CPT code 78645 is most appropriately assigned to APC 0403 as we proposed.
There is a single APC for nonmyocardial PET scans, APC 0308, with an APC median cost of approximately $1,028. The median costs of all CPT codes assigned to that APC, including CPT codes for positron emission tomography (PET) scans and PET/computed tomography (CT) scans and CPT code 78608 for a metabolic evaluation of the brain using PET range from approximately $849 to $1,093, demonstrating very significant resource similarity across all of these procedures. Therefore, we do not agree with the commenter that the proposed configuration of APC 0308 should be modified because all of these nonmyocardial services that use PET technology demonstrate very similar costs and share clinical similarity as well. Start Printed Page 60389
With regard to the thyroid scans described by CPT codes 78000 and 78001, these procedures have CPT code-specific median costs of approximately $91 and $121 based on 1,167 and 982 single claims, respectively. The CPT code-specific median costs of these two procedures are very close to the median cost of APC 0389 of approximately $112, where we proposed to assign them for CY 2010. CPT codes 78000 and 78001 are the only services assigned to this APC with significant volume, and the APC median cost is mostly a reflection of the costs of procedures reported with two codes. In contrast, the median cost of APC 0392, their recommended placement according to the commenter, is approximately $179, substantially greater than the median costs of the two thyroid studies. Furthermore, if we were to reassign CPT codes 78000 and 78001 to APC 0392 as the commenter suggested, the median cost of APC 0392 would decrease to reflect the costs of these two procedures because, based on number of single claims for CPT codes 78000 and 78001, their costs would significantly affect the median cost of the APC. Therefore, we do not believe any changes to the proposed APC assignments of CPT codes 78000 or 78001 are justified.
After consideration of the public comments we received, we are finalizing our CY 2010 proposals, without modification, to assign CPT code 78645 to APC 0403, CPT code 79608 to APC 0308, CPT code 78000 to APC 0389, and CPT code 78001 to APC 0389. The approximate APC median costs of these APCs are as follows: APC 0403 at $195; APC 0308 at $1,028; and APC 0389 at $112.
Comment: A few commenters requested that CMS not reassign CPT code 78807 (Radiopharmaceutical localization of inflammatory process; tomographic (SPECT)) to APC 0406 (Level I Tumor/Infection Imaging) as proposed. These commenters noted that CPT code 78807 is more clinically similar to CPT codes 78805 (Radiopharmaceutical localization of inflammatory process; limited area) and 78806 (Radiopharmaceutical localization of inflammatory process; whole body) that are assigned to APC 0414. Therefore, the commenters requested that CMS continue to assign CPT code 78807 to APC 0414 for CY 2010.
Response: We proposed to assign CPT code 78807, with a CPT code-specific median cost of approximately $371 based on 251 single claims, to APC 0406, with an APC median cost of approximately $287. The significant individual services included in APC 0406 have a range of median costs, from approximately $232 to approximately $371. APC 0406 includes a number of tumor or infection imaging nuclear medicine procedures. Comparatively, APC 0414, where the commenters requested that we assign CPT code 78807, has an APC median cost of approximately $506 and includes significant services with CPT code-specific median costs from approximately $382 to approximately $561. CPT codes 78805 and 78806 are both assigned to APC 0414 and have CPT code-specific median costs of approximately $477 and $538, respectively, significantly higher than the median cost of CPT code 78807. Therefore, we do not believe that there is a reason to assign CPT code 78807 to APC 0414, which principally includes services with significantly higher median costs than CPT code 78807. We note that CPT code 78807 is a SPECT scan to localize an inflammatory process, while the other two codes do not describe services that use SPECT technology. Therefore, we do not believe that CPT code 78807 is sufficiently similar to CPT codes 78805 and 78806 from clinical or resource perspectives to warrant assignment to the mid-level tumor/infection imaging APC along with the other two services.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to assign CPT code 78807 to APC 0406, with an APC median cost of approximately $287.
Comment: Several commenters requested that CMS: (1) Not reassign CPT code 78610 (Brain imaging, vascular flow only) to APC 0403 as proposed but instead assign CPT code 78610 to APC 0402; (2) not reassign CPT code 78601 (Brain imaging, less than 4 static views; with vascular flow) to APC 0402 as proposed but instead assign CPT code 78601 to APC 0403; and (3) not reassign CPT code 78003 (Radiopharmaceutical localization of tumor or distribution of radiopharmaceutical agent(s); tomographic (SPECT)) to APC 0389 as proposed but instead assign CPT code 78003 to APC 0392.
Response: We proposed to assign CPT code 78610, with a CPT-specific median cost of approximately $211, to APC 0403, with an APC median cost of approximately $195. The significant services included in APC 0403 have a range of median costs, from approximately $156 to approximately $246. Comparatively, APC 0402, where the commenters requested that we assign CPT code 78610, has an APC median cost of approximately $573 and includes significant services with CPT code-specific median costs from approximately $540 to approximately $587. We do not believe that reassignment of CPT code 78610 to APC 0402 would be appropriate, given the procedure's relatively low median cost, although we recognize that we have few claims for the procedures. We continue to believe that payment for the resources required to provide CPT code 78610 is appropriately reflected through the procedure's assignment to APC 0403.
We proposed to assign CPT code 78601, with a CPT code-specific median cost of approximately $436, to APC 0402 with an APC median cost of approximately $573. The significant services included in APC 0402 have a range of median costs from approximately $540 to approximately $587. Comparatively, APC 0403, where the commenters requested that we assign CPT code 78601, has an APC median cost of approximately $195 and includes significant services with CPT code-specific median costs ranging from approximately $156 to approximately $246. Although we have few claims for CPT code 78601, we continue to believe it is most appropriately assigned to APC 0402 for CY 2010.
We proposed to assign CPT code 78003, with a CPT code-specific median cost of approximately $82, to APC 0389 with an APC median cost of approximately $112. There are two services included in APC 0389 that have a significant volume, CPT codes 78000 and 78001. These two CPT codes both have higher CPT code-specific median costs than CPT code 78003, approximately $91 and $121, respectively. Comparatively, APC 0392, where the commenters requested that we assign CPT code 78003, has an APC median cost of approximately $179. Based on its median cost, we continue to believe that the resources required for CPT code 78003 are appropriately reflected through its assignment to APC 0389.
Comment: A few commenters expressed their appreciation that the CY 2010 OPPS/ASC proposed rule included a proposed increase in payment for PET services compared to CY 2009 payment rates. These commenters also noted their concerns that hospital claims data for PET services are not predictable and that volatile data over the last several years may limit access to PET services. Some commenters urged CMS to use external data when setting payment rates for these services, while others suggested that CMS continue to monitor data to ensure that payment for these services is sufficient to cover the hospital costs for these resources. Start Printed Page 60390
Response: As we stated in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68547), while we utilized external data in the early years of the OPPS for ratesetting for a few services, we now rely on the cost data from claims as the system has matured and we have gained additional experience in ratesetting for HOPD services. The foundation of a system of relative weights like the OPPS is the relativity of the costs of all services to one another, as derived from a standardized system that uses standardized inputs and a consistent methodology. Further, the OPPS is a prospective payment system that relies on hospital charges and cost report data from the hospitals that furnish the services in order to determine relative costs. Therefore, we believe that our prospective payment rates, calculated based on the costs of those providers furnishing the procedures in CY 2008, provide appropriate payment to the providers who will furnish the services in CY 2010. We continue to believe that this standard ratesetting methodology accurately provides payment for PET services provided to hospital outpatients.
In summary, after consideration of the public comments we received, we are finalizing our CY 2010 proposals, without modification, for the configuration of nuclear medicine APCs. The final CY 2010 median costs for these APCs, as proposed, are calculated according to the standard OPPS ratesetting methodology as applied to claims for nuclear medicine procedures that passed the CY 2008 nuclear medicine procedure-to-radiolabeled product I/OCE claims processing edits. These edits ensure that the claims that are taken through our standard ratesetting process, as described in section II.A.2.b. of this final rule with comment period, that incorporates the creation of “natural” single and “pseudo” single claims, include the radiolabeled product necessary for the performance of the associated nuclear medicine procedure.
(6) Hyperbaric Oxygen Therapy
Since the implementation of the OPPS in August 2000, the OPPS has recognized HCPCS code C1300 (Hyperbaric oxygen under pressure, full body chamber, per 30 minute interval) for hyperbaric oxygen therapy (HBOT) provided in the hospital outpatient setting. In the CY 2005 OPPS final rule with comment period (69 FR 65758 through 65759), we finalized a “per unit” median cost calculation for APC 0659 (Hyperbaric Oxygen) using only claims with multiple units or multiple occurrences of HCPCS code C1300 because delivery of a typical HBOT service requires more than 30 minutes. We observed that claims with only a single occurrence of the code were anomalies, either because they reflected terminated sessions or because they were incorrectly coded with a single unit. In the same rule, we also established that HBOT would not generally be furnished with additional services that might be packaged under the standard OPPS APC median cost methodology. This enabled us to use claims with multiple units or multiple occurrences. Finally, we also used each hospital's overall CCR to estimate costs for HCPCS code C1300 from billed charges rather than the CCR for the respiratory therapy or other departmental cost centers. The public comments on the CY 2005 OPPS proposed rule effectively demonstrated that hospitals report the costs and charges for HBOT in a wide variety of cost centers. Since CY 2005, we have used this methodology to estimate the median cost for HBOT. The median costs of HBOT using this methodology have been relatively stable for the last 4 years. In the CY 2010 OPPS/ASC proposed rule (74 FR 35277), we proposed to continue using the same methodology to estimate a “per unit” median cost for HCPCS code C1300 for CY 2010 of approximately $108, using 279,139 claims with multiple units or multiple occurrences.
We did not receive any public comments on our proposal to continue to use our established ratesetting methodology for calculating the median cost of APC 0659 for payment of HBOT. Therefore, we are finalizing, without modification, our CY 2010 proposal to continue to use our established ratesetting methodology for calculating the median cost of APC 0659 for payment of HBOT, with a final CY 2010 median cost of approximately $106.
(7) Payment for Ancillary Outpatient Services When Patient Expires (-CA Modifier)
In the November 1, 2002 final rule with comment period (67 FR 66798), we discussed the creation of the new HCPCS CA modifier to address situations where a procedure on the OPPS inpatient list must be performed to resuscitate or stabilize a patient (whose status is that of an outpatient) with an emergent, life-threatening condition, and the patient dies before being admitted as an inpatient. In Transmittal A–02–129, issued on January 3, 2003, we instructed hospitals on the use of this modifier. For a complete description of the history of the policy and the development of the payment methodology for these services, we refer readers to the CY 2007 OPPS/ASC final rule with comment period (71 FR 68157 through 68158).
In the CY 2010 OPPS/ASC proposed rule (74 FR 35277 through 35278), we proposed to continue to use our established ratesetting methodology for calculating the median cost of APC 0375 (Ancillary Outpatient Services When Patient Expires) and to continue to make one payment under APC 0375 for the services that meet the specific conditions for using modifier -CA. We proposed to calculate the relative payment weight for APC 0375 by using all claims reporting a status indicator “C” procedure appended with the -CA modifier, using estimated costs from claims data for line-items with a HCPCS code assigned status indicator “G,” “H,” “K,” “N,” “Q1,” “Q2,” “Q3,” “R,” “S,” “T,” “U,” “V,” and “X” and charges for packaged revenue codes without a HCPCS code. We continue to believe that this methodology results in the most appropriate aggregate median cost for the ancillary services provided in these unusual clinical situations.
We believe that hospitals are reporting the -CA modifier according to the policy initially established in CY 2003. We note that the claims frequency for APC 0375 has been relatively stable over the past few years. Although the median cost for APC 0375 has increased, the median in the CY 2008 data used for development of rates for CY 2010 was only slightly higher than that for CY 2009. Variation in the median cost for APC 0375 is expected because of the small number of claims and because the specific cases are grouped by the presence of the -CA modifier appended to an inpatient procedure and not according to the standard APC criteria of clinical and resource homogeneity. Cost variation for APC 0375 from year to year is anticipated and acceptable as long as hospitals continue judicious reporting of the -CA modifier. Table 8 of the proposed rule (74 FR 35278) showed the number of claims and the proposed median costs for APC 0375 for CYs 2007, 2008, and 2009. For CY 2010, we proposed a median cost for APC 0375 of approximately $5,784.
We did not receive any public comments regarding this proposal. Therefore, we are finalizing our CY 2010 proposal, without modification, to continue to use our established ratesetting methodology for calculating the median cost of APC 0375, which has a final CY 2010 APC median cost of approximately $5,911. Start Printed Page 60391
Table 11 below shows the number of claims and the final median cost for APC 0375 from CY 2007 to CY 2010.
| Prospective payment year | Number of claims | APC median cost |
|---|---|---|
| CY 2007 | 260 | $3,549 |
| CY 2008 | 183 | 4,945 |
| CY 2009 | 168 | 5,545 |
| CY 2010 | 182 | 5,911 |
e. Calculation of Composite APC Criteria-Based Median Costs
As discussed in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66613), we believe it is important that the OPPS enhance incentives for hospitals to provide only necessary, high quality care and to provide that care as efficiently as possible. For CY 2008, we developed composite APCs to provide a single payment for groups of services that are typically performed together during a single clinical encounter and that result in the provision of a complete service. Combining payment for multiple independent services into a single OPPS payment in this way enables hospitals to manage their resources with maximum flexibility by monitoring and adjusting the volume and efficiency of services themselves. An additional advantage to the composite APC model is that we can use data from correctly coded multiple procedure claims to calculate payment rates for the specified combinations of services, rather than relying upon single procedure claims which may be low in volume and/or incorrectly coded. Under the OPPS, we currently have composite APC policies for extended assessment and management services, low dose rate (LDR) prostate brachytherapy, cardiac electrophysiologic evaluation and ablation services, mental health services, and multiple imaging services. We refer readers to the CY 2008 OPPS/ASC final rule with comment period for a full discussion of the development of the composite APC methodology (72 FR 66611 through 66614 and 66650 through 66652).
While we continue to consider the development and implementation of larger payment bundles, such as composite APCs (a long-term policy objective for the OPPS), and continue to explore other areas where this payment model may be utilized, in the CY 2010 OPPS/ASC proposed rule, we did not propose any new composite APCs for CY 2010 so that we may monitor the effects of the existing composite APCs on utilization and payment. In response to our CY 2009 proposal to apply a composite payment methodology to multiple imaging procedures provided on the same date of service, several public commenters stated that we should proceed cautiously as we expand service bundling. They commented that we should not implement additional composite methodologies until adequate data are available to evaluate the composite policies' effectiveness and impact on beneficiary access to care (73 FR 68561 through 68562).
In response to the concerns of the public commenters and the APC Panel, in the CY 2010 OPPS proposed rule (74 FR 35278 through 35279) we reviewed the CY 2008 claims data for claims processed through September 30, 2008, for the services in the following composite APCs: APC 8000 (Cardiac Electrophysiologic Evaluation and Ablation Composite); APC 8001 (Low Dose Rate Prostate Brachytherapy Composite); APC 8002 (Level I Extended Assessment and Evaluation Composite); and APC 8003 (Level II Extended Assessment and Evaluation Composite). Our analyses did not consider inflation, changes in beneficiary population, or other comparable variables that can affect changes in aggregate payment from year to year. We found that the average payment for the package of services in both APC 8000 and APC 8001 increased from CY 2007, when payments were made for all individual services, to CY 2008 under the composite payment methodology. We also noted that the proposed median costs for these composite APCs for CY 2010 were higher than the median costs upon which the CY 2009 payments were based. We believe that, in part, this is because we used more claims data for common clinical scenarios to calculate the median costs of these APCs than we were able to use prior to the implementation of the composite payment methodology.
With regard to APCs 8002 and 8003, we compared payment for all visits appearing with observation services in CY 2007 with payments for all visits appearing with observation services in CY 2008 and found that total payment for visits and observation services increased from approximately $197 million to $270 million for claims processed through September 30 in each year. We attribute this increase in payments, in part, to the introduction of a composite payment for visits and observation through the extended assessment and management composite methodology that occurred for CY 2008 and that did not incorporate the International Classification of Diseases, Ninth Edition, Clinical Modification (ICD–9–CM) diagnosis criteria previously necessary for separate payment of observation.
At its February 2009 meeting, the APC Panel recommended that CMS evaluate the implications of creating composite APCs for cardiac resynchronization therapy (CRT) services with a defibrillator or pacemaker and report its findings to the APC Panel. The APC Panel also recommended at its August 2009 meeting that CMS reconsider creating a new composite APC or group of composite APCs for CRT procedures. While we did not propose any new composite APCs for CY 2010, we are accepting both of these APC Panel recommendations. We will reconsider creating composite APCs for CRT services and evaluate the implications of such a potential policy change, and report our findings to the APC Panel at a future meeting. We also will consider bringing other potential composite APCs to the APC Panel for further discussion.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35279), we proposed to continue for CY 2010 our established composite APC policies for extended assessment and management, LDR prostate brachytherapy, cardiac electrophysiologic evaluation and ablation, mental health services, and multiple imaging services, as discussed Start Printed Page 60392 in sections II.A.2.e.(1), II.A.2.e.(2), II.A.2.e.(3), II.A.2.e.(4), and II.A.2.e.(5), respectively, of this final rule with comment period.
Comment: Several commenters supported the development and implementation of the composite APC methodology, remarking that it is consistent with the principles of a prospective payment system and provides more appropriate payment rates through the use of multiple procedure claims for certain services. Many of these commenters also supported CMS' decision to monitor the existing composite APCs' effects on beneficiary access, utilization, and payment for at least another year before implementing additional composite APCs.
Other commenters, however, expressed disappointment that CMS did not propose additional composite APCs for CY 2010 in order to improve OPPS payment accuracy and include more correctly coded, multiple procedure claims in ratesetting. Some commenters recommended the development of composite APCs for nuclear medicine tumor or infection imaging services that encompass multiple days and multiple procedures, with separate payment for the associated diagnostic radiopharmaceuticals.
In addition, many commenters supported the development of composite APCs for CRT with defibrillator (CRT–D) or pacemaker (CRT–P) implantation. They indicated that the procedures involved in the implantation of CRT–D and CRT–P are separately payable services that, if coded correctly, are always represented by the submission of two CPT codes. According to the commenters, the number of single procedure CRT claims available for CY 2010 ratesetting is very low compared to the total number of claims submitted for CRT–D and CRT–P procedures. They argued that the establishment of a composite APC methodology for CRT–D and CRT–P would greatly increase the number of claims used in ratesetting, thereby lessening the year-to-year fluctuations in payment rates for CRT. The commenters also stated that the APC Panel advised CMS to use its discretion in forming one or a group of composite APCs for CRT without the need to report back to the APC Panel. They urged CMS to take this advice and move forward with the composite APC methodology for CRT–D and CRT–P for CY 2010.
Response: We appreciate the commenters' support of the composite APC methodology. As stated in the CY 2010 OPPS/ASC proposed rule (74 FR 35279), we will continue to review the claims data for the impact of all of the composite APCs on payments to hospitals and on services to beneficiaries and will take such data into consideration before proposing or implementing new composite APCs. We recognize the concerns expressed with respect to our CY 2009 proposal by the public commenters that moving ahead too quickly with any nonstandard OPPS payment methodology (even one such as composite APCs that may improve the accuracy of the OPPS payment rates by utilizing more complete claims for common clinical scenarios in ratesetting) could have unintended consequences and requires close monitoring. Because the multiple imaging composite APCs were implemented for the first time in CY 2009, we will not have data available for such monitoring until early CY 2010. Therefore, we continue to believe that it is in the best interest of hospitals and the continuing refinement of the OPPS that we not implement any new composite APC policies for at least one year.
As previously stated, we are accepting the recommendation made by the APC Panel at its August 2009 meeting that we reconsider creating a new composite APC or group of composite APCs for CRT–D and CRT–P procedures. We will evaluate the implications of such a potential policy change and report our findings to the APC Panel at a future meeting. We note that, while the APC Panel did recommend we reconsider creating a new composite APC or group of composite APCs for CRT–D and CRT–P, the Panel did not specify that we should move forward with the composite APC methodology for CRT–D and CRT–P for CY 2010 without first reporting back to the APC Panel, as some commenters indicated. We do not believe it would be appropriate to implement new composite APCs for CRT–D and CRT–P procedures for CY 2010 because neither we nor the public have had the opportunity to evaluate fully all of the implications of such a potential policy change, which may require complex claims processing logic or new claims processing edits and may have significant, unanticipated effects on the payment rates of other services. We also note that the total volume of claims that would qualify for a CRT–P composite APC in particular would be very low; in the past, we have explored composite APCs only for combinations of services that are commonly performed together (73 FR 68551). Because of the complex issues for these procedures with significant device costs, we believe that it is particularly important that the APC Panel and the public, through the annual rulemaking cycle, have the opportunity to comment on the development of composite APCs for CRT–D and CRT–P procedures.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to continue our established composite APC policies for extended assessment and management, LDR prostate brachytherapy, cardiac electrophysiologic evaluation and ablation, mental health services, and multiple imaging services, as discussed in sections II.A.2.e.(1), II.A.2.e.(2), II.A.2.e.(3), II.A.2.e.(4), and II.A.2.e.(5), respectively, of this final rule with comment period.
(1) Extended Assessment and Management Composite APCs (APCs 8002 and 8003)
In the CY 2010 OPPS/SC proposed rule (74 FR 35279 through 35280), we proposed to continue to include composite APC 8002 (Level I Extended Assessment and Management Composite) and composite APC 8003 (Level II Extended Assessment and Management Composite) in the OPPS. For CY 2008, we created these two composite APCs to provide payment to hospitals in certain circumstances when extended assessment and management of a patient occur (an extended visit). In most circumstances, observation services are supportive and ancillary to the other services provided to a patient. In the circumstances when observation care is provided in conjunction with a high level visit or direct referral and is an integral part of a patient's extended encounter of care, payment is made for the entire care encounter through one of two composite APCs as appropriate.
As defined for the CY 2008 OPPS, composite APC 8002 describes an encounter for care provided to a patient that includes a high level (Level 5) clinic visit or direct referral for observation services in conjunction with observation services of substantial duration (72 FR 66648 through 66649). Composite APC 8003 describes an encounter for care provided to a patient that includes a high level (Level 4 or 5) Type A emergency department visit, a high level (Level 5) Type B emergency department visit, or critical care services in conjunction with observation services of substantial duration. HCPCS code G0378 (Observation services, per hour) is assigned status indicator “N,” signifying that its payment is always packaged. As noted in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66648 through 66649), the Integrated Outpatient Code Editor Start Printed Page 60393 (I/OCE) evaluates every claim received to determine if payment through a composite APC is appropriate. If payment through a composite APC is inappropriate, the I/OCE, in conjunction with the OPPS Pricer, determines the appropriate status indicator, APC, and payment for every code on a claim. The specific criteria that must be met for the two extended assessment and management composite APCs to be paid are provided below in the description of the claims that were selected for the calculation of the proposed CY 2010 median costs for these composite APCs. We did not propose to change these criteria for the CY 2010 OPPS.
When we created composite APCs 8002 and 8003 for CY 2008, we retained as general reporting requirements for all observation services those criteria related to physician order and evaluation, documentation, and observation beginning and ending time as listed in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66812). These are more general requirements that encourage hospitals to provide medically reasonable and necessary care and help to ensure the proper reporting of observation services on correctly coded hospital claims that reflect the full charges associated with all hospital resources utilized to provide the reported services. We did not propose to change these reporting requirements for the CY 2010 OPPS. However, as discussed below, the APC Panel at its February 2009 meeting requested that CMS issue guidance clarifying the correct method for reporting the starting time for observation services. The APC Panel noted that the descriptions of the start time for observation services located in the Medicare Claims Processing Manual (Pub. 100–4), Chapter 4, sections 290.2.2 through 290.5, cause confusion for hospitals. We accepted this recommendation and issued clarifying guidance in the Claims Processing Manual through Transmittal 1745, Change Request 6492, issued May 22, 2009 and implemented July 6, 2009.
As noted in detail in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66802 through 66805 and 66814), we saw a normal and stable distribution of clinic and emergency department visit levels in the OPPS claims data through CY 2006 available at that time. We stated that we did not expect to see an increase in the proportion of visit claims for high level visits as a result of the new composite APCs adopted for CY 2008. Similarly, we stated that we expected that hospitals would not purposely change their visit guidelines or otherwise upcode clinic and emergency department visits reported with observation care solely for the purpose of composite payment. As stated in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66648), we expect to carefully monitor any changes in billing practices on a service-specific and hospital-specific level to determine whether there is reason to request that Quality Improvement Organizations (QIOs) review the quality of care furnished, or to request that Benefit Integrity contractors or other contractors review the claims against the medical record.
When we compared total payments for all visits appearing with observation services in CY 2007 to payments in CY 2008, using claims processed through September 30 in CY 2007 and CY 2008, we observed a 37 percent increase in total payments. We believe this increase is, in part, attributable to the expansion of payment under the extended assessment and management composites to all ICD–9–CM diagnoses. To confirm this, we calculated the percentage of visit HCPCS codes billed with HCPCS code G0378 (Observation services, per hour) between CY 2007 and CY 2008 and compared the percentage associated with visit codes included in the extended assessment and management composites in each year. If hospitals had inappropriately changed their visit reporting behavior to maximize payment through the new composite APCs, we would expect to see significant changes in the percentage of visit HCPCS codes included in the composite APCs billed with observation services relative to all other visit HCPCS codes billed with observation services between CY 2007 and CY 2008. We did not observe a sizable increase in the proportion of visit HCPCS codes included in the composite APCs relative to the proportion of all other visit HCPCS codes billed with observation services. For example, the percentage of claims billed with CPT code 99285 (Emergency department visit for the evaluation and management of a patient (Level 5)) and HCPCS code G0378 was 51 percent in the CY 2007 data and 54 percent in the CY 2008 data. Similarly, the percentage of claims billed with CPT code 99284 (Emergency department visit for the evaluation and management of a patient (Level 4)) and HCPCS code G0378 decreased only slightly from 28 percent in the CY 2007 data to 27 percent in the CY 2008 data. We concluded that, although the volume of visits billed with HCPCS code G0378 increased between CY 2007 and CY 2008, the overall pattern of billing visit levels did not change significantly. We stated that we will continue to carefully monitor any changes in billing practices on a service-specific and hospital-specific level.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35280), we proposed to continue for CY 2010 the extended assessment and management composite APC payment methodology for APCs 8002 and 8003. As stated earlier, we also proposed to continue the general reporting requirements for observation services reported with HCPCS code G0378. We continue to believe that the composite APCs 8002 and 8003 and related policies provide the most appropriate means of paying for these services. We proposed to calculate the median costs for APCs 8002 and 8003 using all single and “pseudo” single procedure claims for CY 2008 that meet the criteria for payment of each composite APC.
Specifically, to calculate the proposed median costs for composite APCs 8002 and 8003, we selected single and “pseudo” single claims that met each of the following criteria:
1. Did not contain a HCPCS code to which we have assigned status indicator “T” that is reported with a date of service 1 day earlier than the date of service associated with HCPCS code G0378. (By selecting these claims from single and “pseudo” single claims, we had already assured that they would not contain a code for a service with status indicator “T” on the same date of service.);
2. Contained 8 or more units of HCPCS code G0378; and
3. Contained one of the following codes:
- In the case of composite APC 8002, HCPCS code G0379 (Direct referral of patient for hospital observation care) on the same date of service as G0378; or CPT code 99205 (Office or other outpatient visit for the evaluation and management of a new patient (Level 5)); or CPT code 99215 (Office or other outpatient visit for the evaluation and management of an established patient (Level 5)) provided on the same date of service or one day before the date of service for HCPCS code G0378. We refer readers to section XII.E. of the CY 2010 OPPS/ASC proposed rule (74 FR 35370 through 35371) and section XII.E. of this final rule with comment period for a full discussion of our proposed revision of the code descriptor for HCPCS code G0379 and the final policy for CY 2010.
• In the case of composite APC 8003, CPT code 99284 (Emergency department visit for the evaluation and management of a patient (Level 4)); CPT code 99285 (Emergency department visit for the Start Printed Page 60394 evaluation and management of a patient (Level 5)); CPT code 99291 (Critical care, evaluation and management of the critically ill or critically injured patient; first 30–74 minutes); or HCPCS code G0384 (Level 5 Hospital Emergency Department Visit Provided in a Type B Emergency Department) provided on the same date of service or one day before the date of service for HCPCS code G0378. (As discussed in detail in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68684), we finalized our proposal to add HCPCS code G0384 to the eligibility criteria for composite APC 8003 for CY 2009.)
We applied the standard packaging and trimming rules to the claims before calculating the proposed CY 2010 median costs. The proposed CY 2010 median cost resulting from this process for composite APC 8002 was approximately $384, which was calculated from 14,981 single and “pseudo” single bills that met the required criteria. The proposed CY 2010 median cost for composite APC 8003 was approximately $709, which was calculated from 154,843 single and “pseudo” single bills that met the required criteria. This is the same methodology we used to calculate the medians for composite APCs 8002 and 8003 for the CY 2008 OPPS (72 FR 66649).
As discussed further in section IX. of the CY 2010 OPPS/ASC proposed rule (74 FR 35350) and this final rule with comment period, and consistent with our CY 2008 and CY 2009 final policies, when calculating the median costs for the clinic, Type A emergency department visit, Type B emergency department visit, and critical care APCs (0604 through 0617 and 0626 through 0630), we utilize our methodology that excludes those claims for visits that are eligible for payment through the two extended assessment and management composite APCs, that is APC 8002 or APC 8003. We believe that this approach results in the most accurate cost estimates for APCs 0604 through 0617 and 0626 through 0630 for CY 2010.
At the August 2009 meeting of the APC Panel, the APC Panel recommended that CMS provide the Visits and Observation Subcommittee with an analysis of calendar year 2009 claims data for clinic, ED (Type A and B), and extended assessment and management composite APCs at the next meeting of the APC Panel. The APC Panel also recommended that CMS provide the Visits and Observation Subcommittee with continued analyses of observation services, as previously provided to the APC Panel, including data on frequency, length of stay, and common diagnoses, as well as recovery audit contractor (RAC) data on these subjects if available. Furthermore, the APC Panel recommended that CMS provide the Visits and Observation Subcommittee with analyses of the most common diagnoses and services associated with Type A and Type B ED visits at the next meeting of the APC Panel, including analysis by hospital-specific characteristics. Finally, the APC Panel recommended that the work of the Visits and Observation Subcommittee continue. We accept all of these recommendations and will present the available requested data at the winter 2010 meeting of the APC Panel.
In summary, in the CY 2010 OPPS/ASC proposed rule (74 FR 35279 through 35280), we proposed to continue to include for CY 2010 composite APC 8002 (Level I Extended Assessment and Management Composite) and composite APC 8003 (Level II Extended Assessment and Management Composite) in the OPPS. We proposed to continue the extended assessment and management composite APC payment methodology and criteria that we finalized for CY 2009. We also proposed to calculate the median costs for APCs 8002 and 8003 using all single and “pseudo” single procedure claims from CY 2008 that meet the criteria for payment of each composite APC. We did not propose to change the reporting requirements for observation services for the CY 2010 OPPS. However, in CY 2009 we did issue further clarifying guidance in the Medicare Claims Processing Manual related to observation start time.
Comment: Several commenters expressed appreciation for CMS' issuance of clarifying guidance for reporting the beginning and ending times of observation services.
Response: We appreciate these comments and note again that the guidance was issued in the Claims Processing Manual through Transmittal 1745, Change Request 6492, issued May 22, 2009, and implemented July 6, 2009.
Comment: Several commenters requested clarification of the reporting of observation services in relation to maternity care paid under another payer's policies and in relation to changes in patient status from inpatient to outpatient using Condition Code 44. One commenter pointed out that references to “observation status” versus “inpatient admission” are potentially confusing for beneficiaries and physicians.
Response: Each of these comments/questions is outside of the scope of the proposals in the CY 2010 OPPS/ASC proposed rule. However, we will consider the possibility of addressing these concerns through other available mechanisms, as appropriate. We note that we have continued to emphasize that observation care is a hospital outpatient service, ordered by a physician and reported with a HCPCS code, like any other outpatient service. It is not a patient status for Medicare purposes.
After consideration of the public comments we received, we are finalizing, without modification, our CY 2010 proposal to continue to include composite APC 8002 and composite APC 8003 in the OPPS and to continue the extended assessment and management composite APC payment methodology and criteria that we finalized for CY 2009. We also are calculating the median costs for APCs 8002 and 8003 using all single and “pseudo” single procedure claims from CY 2008 that meet the criteria for payment of each composite APC. The final CY 2010 median cost resulting from this methodology for composite APC 8002 is approximately $378, which was calculated from 17,074 single and “pseudo” single bills that met the required criteria. The final CY 2010 median cost for composite APC 8003 is approximately $699, which was calculated from 176,226 single and “pseudo” single bills that met the required criteria.
(2) Low Dose Rate (LDR) Prostate Brachytherapy Composite APC (APC 8001)
LDR prostate brachytherapy is a treatment for prostate cancer in which hollow needles or catheters are inserted into the prostate, followed by permanent implantation of radioactive sources into the prostate through the needles/catheters. At least two CPT codes are used to report the composite treatment service because there are separate codes that describe placement of the needles/catheters and the application of the brachytherapy sources: CPT code 55875 (Transperineal placement of needles or catheters into prostate for interstitial radioelement application, with or without cystoscopy) and CPT code 77778 (Interstitial radiation source application; complex). Generally, the component services represented by both codes are provided in the same operative session in the same hospital on the same date of service to the Medicare beneficiary being treated with LDR brachytherapy for prostate cancer. As discussed in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66653), OPPS payment rates for CPT code 77778, in Start Printed Page 60395 particular, had fluctuated over the years. We were frequently informed by the public that reliance on single procedure claims to set the median costs for these services resulted in use of only incorrectly coded claims for LDR prostate brachytherapy because a correctly coded claim should include, for the same date of service, CPT codes for both needle/catheter placement and application of radiation sources, as well as separately coded imaging and radiation therapy planning services (that is, a multiple procedure claim).
In order to base payment on claims for the most common clinical scenario, and to further our goal of providing payment under the OPPS for a larger bundle of component services provided in a single hospital encounter, beginning in CY 2008, we provide a single payment for LDR prostate brachytherapy when the composite service, reported as CPT codes 55875 and 77778, is furnished in a single hospital encounter. We base the payment for composite APC 8001 (LDR Prostate Brachytherapy Composite) on the median cost derived from claims for the same date of service that contain both CPT codes 55875 and 77778 and that do not contain other separately paid codes that are not on the bypass list. In uncommon occurrences in which the services are billed individually, hospitals continue to receive separate payments for the individual services. We refer readers to the CY 2008 OPPS/ASC final rule with comment period (72 FR 66652 through 66655) for a full history of OPPS payment for LDR prostate brachytherapy and a detailed description of how we developed the LDR prostate brachytherapy composite APC.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35281), we proposed for CY 2010 to continue paying for LDR prostate brachytherapy services using the composite APC methodology proposed and implemented for CY 2008 and CY 2009. That is, we proposed to use CY 2008 claims on which both CPT codes 55875 and 77778 were billed on the same date of service with no other separately paid procedure codes (other than those on the bypass list) to calculate the payment rate for composite APC 8001. Consistent with our CY 2008 and CY 2009 practice, we proposed not to use the claims that meet these criteria in the calculation of the median costs for APCs 0163 (Level IV Cystourethroscopy and Other Genitourinary Procedures) and 0651 (Complex Interstitial Radiation Source Application), the APCs to which CPT codes 55875 and 77778 are assigned, respectively. The median costs for APCs 0163 and 0651 would continue to be calculated using single and “pseudo” single procedure claims. We continue to believe that this composite APC contributes to our goal of creating hospital incentives for efficiency and cost containment, while providing hospitals with the most flexibility to manage their resources. We also continue to believe that data from claims reporting both services required for LDR prostate brachytherapy provide the most accurate median cost upon which to base the composite APC payment rate.
Using partial year CY 2008 claims data available for the CY 2010 OPPS/ASC proposed rule, we were able to use 669 claims that contained both CPT codes 77778 and 55875 to calculate the median cost upon which the proposed CY 2010 payment for composite APC 8001 was based. The proposed median cost for composite APC 8001 for CY 2010 was approximately $3,106. This was an increase compared to the CY 2009 OPPS/ASC final rule with comment period in which we calculated a final median cost for this composite APC of approximately $2,967 based on a full year of CY 2007 claims data. The CY 2010 proposed median cost for this composite APC was slightly less than $3,268, the sum of the proposed median costs for APCs 0163 and 0651 ($2,453+$815), the APCs to which CPT codes 55875 and 77778 map if one service is billed on a claim without the other. We stated in the CY 2010 OPPS/ASC proposed rule (74 FR 35281) that we believe the proposed CY 2010 median cost for composite APC 8001 of approximately $3,106, calculated from claims we believe to be correctly coded, would result in a reasonable and appropriate payment rate for this service in CY 2010.
Comment: Several commenters requested changes to the bypass list that could potentially affect the number of claims used to calculate the median costs upon which payments for several APCs involving radiation oncology services, including APC 8001, are based. In particular, some commenters requested CMS add CPT code 77470 (Special treatment procedure (eg, total body irradiation, hemibody radiation, per oral, endocavitary or intraoperative cone irradiation)), CPT code 77328 (Brachytherapy isodose plan; complex (multiplane isodose plan, volume implant calculations, over 10 sources/ribbons used, special spatial reconstruction, remote afterloading brachytherapy, over 12 sources), and CPT code 77295 (Therapeutic radiology simulation-aided field setting; 3-dimensional) to the bypass list in order to utilize more single claims in calculating the median costs of APC 8001 and other APCs for radiation oncology services. According to one commenter's analysis, the addition of these CPT codes to the bypass list would result in a 17 percent increase in the median cost for APC 8001.
Response: As discussed in detail in section II.A.1.b. of this final rule with comment period, we are not adding CPT codes 77470, 77328, and 77295 to the list of bypass codes for CY 2010 ratesetting, but we are adding several other CPT codes for radiation oncology services. The addition of these codes to the bypass list results in a modest increase in the number of single claims used to calculate the median cost upon which the final payment rate for CY 2010 for APC 8001 is based, but does not result in a significant increase or decrease in the median cost itself.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to continue paying for LDR prostate brachytherapy services using the composite APC methodology implemented in CY 2008. We were able to use 906 claims that contained both CPT codes 77778 and 55875 to calculate the median cost upon which the CY 2010 final payment for composite APC 8001 is based. The final median cost for composite APC 8001 for CY 2010 is approximately $3,084. We note that this is slightly less than $3,303, the approximate sum of the median costs for APC 0163 and APC 0651 ($2,418 + $885), the APCs to which CPT codes 55875 and 77778 map if one service is billed on a claim without the other. These CPT codes are assigned status indicator “Q3” in Addendum B to this final rule with comment period to identify their status as potentially payable through a composite APC. Their composite APC assignment is identified in Addendum M to this final rule with comment period.
(3) Cardiac Electrophysiologic Evaluation and Ablation Composite APC (APC 8000)
Cardiac electrophysiologic evaluation and ablation services frequently are performed in varying combinations with one another during a single episode-of-care in the hospital outpatient setting. Therefore, correctly coded claims for these services often include multiple codes for component services that are reported with different CPT codes and that, prior to CY 2008, were always paid separately through different APCs (specifically, APC 0085 (Level II Electrophysiologic Evaluation), APC 0086 (Ablate Heart Dysrhythm Focus), Start Printed Page 60396 and APC 0087 (Cardiac Electrophysiologic Recording/Mapping). As a result, there would never be many single bills for cardiac electrophysiologic evaluation and ablation services, and those that are reported as single bills would often represent atypical cases or incorrectly coded claims. As described in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66655 through 66659), the APC Panel and the public expressed persistent concerns regarding the limited and reportedly unrepresentative single bills available for use in calculating the median costs for these services according to our standard OPPS methodology.
Effective January 1, 2008, we established APC 8000 (Cardiac Electrophysiologic Evaluation and Ablation Composite) to pay for a composite service made up of at least one specified electrophysiologic evaluation service and one specified electrophysiologic ablation service. Calculating a composite APC for these services allowed us to utilize many more claims than were available to establish the individual APC median costs for these services, and we also saw this composite APC as an opportunity to advance our stated goal of promoting hospital efficiency through larger payment bundles. In order to calculate the median cost upon which the payment rate for composite APC 8000 is based, we used multiple procedure claims that contained at least one CPT code from group A for evaluation services and at least one CPT code from group B for ablation services reported on the same date of service on an individual claim. Table 9 in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66656) identified the CPT codes that are assigned to groups A and B. For a full discussion of how we identified the group A and group B procedures and established the payment rate for the cardiac electrophysiologic evaluation and ablation composite APC, we refer readers to the CY 2008 OPPS/ASC final rule with comment period (72 FR 66655 through 66659). Where a service in group A is furnished on a date of service that is different from the date of service for a code in group B for the same beneficiary, payments are made under the appropriate single procedure APCs and the composite APC does not apply.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35282), we proposed to continue for CY 2010 to pay for cardiac electrophysiologic evaluation and ablation services using the composite APC methodology proposed and implemented for CY 2008 and CY 2009. Consistent with our CY 2008 and CY 2009 practice, we proposed not to use the claims that meet the composite payment criteria in the calculation of the median costs for APC 0085 and APC 0086, to which the CPT codes in both groups A and B for composite APC 8000 are otherwise assigned. Median costs for APCs 0085 and 0086 would continue to be calculated using single procedure claims. We continue to believe that the composite APC methodology for cardiac electrophysiologic evaluation and ablation services is the most efficient and effective way to use the claims data for the majority of these services and best represents the hospital resources associated with performing the common combinations of these services that are clinically typical. Furthermore, this approach creates incentives for efficiency by providing a single payment for a larger bundle of major procedures when they are performed together, in contrast to continued separate payment for each of the individual procedures.
Using partial year CY 2008 claims data available for the proposed rule, we were able to use 6,975 claims containing a combination of group A and group B codes and calculated a proposed median cost of approximately $10,105 for composite APC 8000. This was an increase compared to the CY 2009 OPPS/ASC final rule with comment period in which we calculated a final median cost for this composite APC of approximately $9,206 based on a full year of CY 2007 claims data. We stated in the CY 2010 OPPS/ASC proposed rule (74 FR 35282) that we believe the proposed median cost of $10,105 calculated from a high volume of correctly coded multiple procedure claims would result in an accurate and appropriate proposed payment for cardiac electrophysiologic evaluation and ablation services when at least one evaluation service is furnished during the same clinical encounter as at least one ablation service. Table 9 of the CY 2010 OPPS/ASC proposed rule (74 FR 35282) listed the groups of procedures upon which we proposed to base composite APC 8000 for CY 2010.
Comment: Several commenters supported CMS' proposal to continue using the composite APCs created in CY 2008, in particular the composite APC for cardiac electrophysiologic evaluation and ablation services. One commenter also supported the modest increase in payment for this APC, stating that it is reflective of the increased costs of providing these important services to patients.
Response: We appreciate commenters' support for the composite payment methodology in general and the composite APC for cardiac electrophysiologic evaluation and ablation in particular.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to continue paying for cardiac electrophysiologic evaluation and ablation services using the composite APC methodology implemented for CY 2008. For this final rule with comment period, we were able to use 7,599 claims from CY 2008 containing a combination of group A and group B codes and calculated a final median cost of approximately $10,026 for composite APC 8000. This is an increase compared to the CY 2009 OPPS/ASC final rule with comment period in which we calculated a final median cost of approximately $9,206 based a full year of CY 2007 claims data. We believe that the final median cost of $10,026 calculated from a high volume of correctly coded multiple procedure claims results in an accurate and appropriate final payment for cardiac electrophysiologic evaluation and ablation services when at least one evaluation service is furnished during the same clinical encounter as at least one ablation service. Table 12 below lists the groups of procedures upon which we are basing composite APC 8000 for CY 2010. These CPT codes are assigned status indicated “Q3” in Addendum B to this final rule with comment period to identify their status as potentially payable through a composite APC. Their composite APC assignment is identified in Addendum M to this final rule with comment period. Start Printed Page 60397
| Codes used in combinations: At least one in Group A and one in Group B | CY 2010 CPT code | Final single code CY 2010 APC | Final CY 2010 SI (composite) |
|---|---|---|---|
| Group A: | |||
| Comprehensive electrophysiologic evaluation with right atrial pacing and recording, right ventricular pacing and recording, His bundle recording, including insertion and repositioning of multiple electrode catheters, without induction or attempted induction of arrhythmia | 93619 | 0085 | Q3 |
| Comprehensive electrophysiologic evaluation including insertion and repositioning of multiple electrode catheters with induction or attempted induction of arrhythmia; with right atrial pacing and recording, right ventricular pacing and recording, His bundle recording | 93620 | 0085 | Q3 |
| Group B: | |||
| Intracardiac catheter ablation of atrioventricular node function, atrioventricular conduction for creation of complete heart block, with or without temporary pacemaker placement | 93650 | 0085 | Q3 |
| Intracardiac catheter ablation of arrhythmogenic focus; for treatment of supraventricular tachycardia by ablation of fast or slow atrioventricular pathways, accessory atrioventricular connections or other atrial foci, singly or in combination | 93651 | 0086 | Q3 |
| Intracardiac catheter ablation of arrhythmogenic focus; for treatment of ventricular tachycardia | 93652 | 0086 | Q3 |
(4) Mental Health Services Composite APC (APC 0034)
In the CY 2010 OPPS/ASC proposed rule (74 FR 35282 through 35283), we proposed to continue our longstanding policy of limiting the aggregate payment for specified less resource-intensive mental health services furnished on the same date to the payment for a day of partial hospitalization, which we consider to be the most resource-intensive of all outpatient mental health treatment for CY 2010. We refer readers to the April 7, 2000 OPPS final rule with comment period (65 FR 18455) for the initial discussion of this longstanding policy. We stated in the CY 2010 OPPS/ASC proposed rule that we continue to believe that the costs associated with administering a partial hospitalization program represent the most resource-intensive of all outpatient mental health treatment. Therefore, we do not believe that we should pay more for a day of individual mental health services under the OPPS than the partial hospitalization per diem payment.
As discussed in the CY 2010 OPPS/ASC proposed rule (74 FR 35356 through 35357), for CY 2010 we proposed to continue using the two tiered payment approach for partial hospitalization services that we implemented in CY 2009: one APC for days with three services (APC 0172) (Level I Partial Hospitalization (3 services)) and one APC for days with four or more services (APC 0173) (Level II Partial Hospitalization (4 or more services)) (74 FR 35282 through 35283). When a CMHC or hospital provides three units of partial hospitalization services and meets all other partial hospitalization payment criteria, we proposed that the CMHC or hospital be paid through APC 0172. When the CMHC or hospital provides 4 or more units of partial hospitalization services and meets all other partial hospitalization payment criteria, we proposed that the CMHC or hospital be paid through APC 0173. We proposed to set the CY 2010 payment rate for mental health services composite APC 0034 (Mental Health Services Composite) at the same rate as we proposed for APC 0173, which is the maximum partial hospitalization per diem payment. We stated in the CY 2010 OPPS/ASC proposed rule that we believe this APC payment rate would provide the most appropriate payment for composite APC 0034, taking into consideration the intensity of the mental health services and the differences in the HCPCS codes for mental health services that could be paid through this composite APC compared with the HCPCS codes that could be paid through partial hospitalization APC 0173. When the aggregate payment for specified mental health services provided by one hospital to a single beneficiary on one date of service based on the payment rates associated with the APCs for the individual services exceeds the maximum per diem partial hospitalization payment, we proposed that those specified mental health services would be assigned to APC 0034. We proposed that APC 0034 would continue to have the same payment rate as APC 0173 and that the hospital would continue to be paid one unit of APC 0034. The I/OCE currently determines, and we proposed for CY 2010 that it would continue to determine, whether to pay these specified mental health services individually or to make a single payment at the same rate as the APC 0173 per diem rate for partial hospitalization for all of the specified mental health services furnished by the hospital on that single date of service.
We also proposed to continue assigning status indicator “Q3” (Codes that May be Paid Through a Composite APC) to the HCPCS codes that are assigned to composite APC 0034 in Addendum M, and to continue assigning status indicator “S” (Significant Procedure, Not Discounted when Multiple), as adopted for CY 2009, to APC 0034 for CY 2010 (74 FR 35283).
Comment: One commenter expressed concern that using claims data from CMHCs and hospitals to calculate the payment rate for APC 0173 would result in reduced access not only for hospital-based partial hospitalization services but also for other less intensive mental health services provided in hospital outpatient departments. The commenter stated that CMS should use hospital data to calculate the payment rates for hospital services.
Response: As discussed in section X. of this final rule with comment period, the final CY 2010 payment rates for APCs 0172 and 0173 are calculated using hospital-only cost data for CY 2010, rather than using both hospital and CMHC cost data. This final policy results in an increase in the median cost for APC 0173 from approximately $200 in CY 2009 to approximately $209. As noted in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66739), we continue to believe that the costs associated with administering a partial hospitalization program Start Printed Page 60398 represent the most resource intensive of all outpatient mental health treatment, and we do not believe that we should pay more for a day of individual mental health services under the OPPS. The mental health payment limitation will rise and fall in the same manner as payment for partial hospitalization services.
After consideration of the public comment we received, we are finalizing our CY 2010 proposal, without modification, to limit the aggregate payment for specified less intensive outpatient mental health services furnished on the same date by a hospital to the payment for a day of partial hospitalization, specifically APC 0173. For CY 2010, we also are finalizing our proposal, without modification, to assign status indicator “Q3” to those HCPCS codes that describe the specified mental health services to which APC 0034 applies in Addendum B to this final rule with comment period. Lastly, we are finalizing our proposal to continue assigning status indicator “S” (Significant Procedure, Not Discounted When Multiple) to APC 0034.
(5) Multiple Imaging Composite APCs (APCs 8004, 8005, 8006, 8007, and 8008)
Prior to CY 2009, hospitals received a full APC payment for each imaging service on a claim, regardless of how many procedures were performed during a single session using the same imaging modality. Based on extensive data analysis, we determined that this practice neither reflected nor promoted the efficiencies hospitals can achieve when performing multiple imaging procedures during a single session (73 FR 41448 through 41450). As a result of our data analysis, and in response to ongoing recommendations from MedPAC to improve payment accuracy for imaging services under the OPPS, we expanded the composite APC model developed in CY 2008 to multiple imaging services. Effective January 1, 2009, we provide a single payment each time a hospital bills more than one imaging procedure within an imaging family on the same date of service. We utilize three imaging families based on imaging modality for purposes of this methodology: ultrasound, computed tomography (CT) and computed tomographic angiography (CTA), and magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA). The HCPCS codes subject to the multiple imaging composite policy, and their respective families, are listed in Table 8 of the CY 2009 OPPS/ASC final rule with comment period (73 FR 68567 through 68569).
While there are three imaging families, there are five multiple imaging composite APCs due to the statutory requirement at section 1833(t)(2)(G) of the Act that we differentiate payment for OPPS imaging services provided with and without contrast. While the ultrasound procedures included in the policy do not involve contrast, both CT/CTA and MRI/MRA scans can be provided either with or without contrast. The five multiple imaging composite APCs established in CY 2009 are: APC 8004 (Ultrasound Composite); APC 8005 (CT and CTA without Contrast Composite); APC 8006 (CT and CTA with Contrast Composite); APC 8007 (MRI and MRA without Contrast Composite); and APC 8008 (MRI and MRA with Contrast Composite). We define the single imaging session for the “with contrast” composite APCs as having at least one or more imaging procedures from the same family performed with contrast on the same date of service. For example, if the hospital performs an MRI without contrast during the same session as at least one other MRI with contrast, the hospital will receive payment for APC 8008, the “with contrast” composite APC.
Hospitals continue to use the same HCPCS codes to report imaging procedures, and the I/OCE determines when combinations of imaging procedures qualify for composite APC payment or map to standard (sole service) APCs for payment. We make a single payment for those imaging procedures that qualify for composite APC payment, as well as any packaged services furnished on the same date of service. The standard (noncomposite) APC assignments continue to apply for single imaging procedures and multiple imaging procedures performed across families. For a full discussion of the development of the multiple imaging composite APC methodology, we refer readers to the CY 2009 OPPS/ASC final rule with comment period (73 FR 68559 through 68569).
As we discussed in the CY 2010 OPPS/ASC proposed rule (74 FR 35283), during the February 2009 meeting of the APC Panel, the APC Panel heard from stakeholders who claimed that a composite payment is not appropriate when multiple imaging procedures are provided on the same date of service but at different times. Some APC Panel members expressed concern that the same efficiencies that may be gained when multiple imaging procedures are performed during the same sitting may not be gained if a significant amount of time passes between the second and subsequent imaging procedures, when the patient may leave not only the scanner, but also the radiology department or hospital. The APC Panel recommended that CMS continue to work with stakeholders to examine different options for APCs for multiple imaging sessions and multiple imaging procedures.
We accepted the APC Panel recommendation that CMS continue to work with stakeholders to examine different options for APCs for multiple imaging sessions and multiple imaging procedures. However, as we stated in the CY 2010 OPPS/ASC proposed rule (74 FR 35283 through 35284), we do not believe it is appropriate to make modifications to the multiple imaging composite policy for CY 2010. We indicated that we continue to believe that composite payment is appropriate even when procedures are provided on the same date of service but at different times, because hospitals do not expend the same facility resources each and every time a patient is seen for a distinct imaging service in a separate imaging session. In most cases, we expect that patients in those circumstances would receive imaging procedures at different times during a single prolonged hospital outpatient encounter, and that the efficiencies that may be gained from providing multiple imaging procedures during a single session are achieved in such ways as not having to register the patient again, or not having to re-establish new intravenous access for an additional study when contrast is required. Furthermore, we stated that even if the same level of efficiencies could not be gained for multiple imaging procedures performed on the same date of service but at different times, we expect that any higher costs associated with these cases would be reflected in the claims data and cost reports we use to calculate the median costs for the multiple imaging composite APCs and, therefore, in their payment rates.
In summary, in the CY 2010 OPPS/ASC proposed rule (74 FR 35284), for CY 2010 we proposed to continue paying for all multiple imaging procedures within an imaging family performed on the same date of service using the multiple imaging composite payment methodology, and we proposed no changes from the final CY 2009 policy. The proposed CY 2010 payment rates for the five multiple imaging composite APCs (APC 8004, APC 8005, APC 8006, APC 8007, and APC 8008) were based on median costs calculated from the partial year CY 2008 claims available for the proposed rule that would have qualified for composite payment under the current policy (that Start Printed Page 60399 is, those claims with more than one procedure within the same family on a single date of service). To calculate the proposed median costs, we used the same methodology that we used to calculate the final CY 2009 median costs for these composite APCs. That is, we removed any HCPCS codes in the OPPS imaging families that overlapped with codes on our bypass list (“overlap bypass codes”) to avoid splitting claims with multiple units or multiple occurrences of codes in an OPPS imaging family into new “pseudo” single claims. The imaging HCPCS codes that we removed from the bypass list for purposes of calculating the proposed multiple imaging composite APC median costs appeared in Table 11 of the CY 2010 OPPS/ASC proposed rule (74 FR 35286). We integrated the identification of imaging composite “single session” claims, that is, claims with multiple imaging procedures within the same family on the same date of service, into the creation of “pseudo” single claims to ensure that claims were split in the “pseudo” single process into accurate reflections of either a composite “single session” imaging service or a standard sole imaging service resource cost. Like all single bills, the new composite “single session” claims were for the same date of service and contained no other separately paid services in order to isolate the session imaging costs. Our last step after processing all claims through the “pseudo” single process was to reassess the remaining multiple procedure claims using the full bypass list and bypass process in order to determine if we could make other “pseudo” single bills. That is, we assessed whether a single separately paid service remained on the claim after removing line-items for the “overlap bypass codes.”
We were able to identify 1.7 million “single session” claims out of an estimated 2.5 million potential composite cases from our ratesetting claims data, or well over half of all eligible claims, to calculate the proposed CY 2010 median costs for the multiple imaging composite APCs. The HCPCS codes subject to the proposed multiple imaging composite policy and their respective families were listed in Table 10 of the proposed rule (74 FR 35284 through 35285).
Comment: Many commenters asserted that a single composite APC payment is not appropriate when multiple imaging services of the same modality are provided on the same date of service but at different times. They argued that the same efficiencies that may be gained when multiple imaging procedures are performed during the same sitting may not be realized if a significant amount of time passes between the first and subsequent imaging procedures, when the patient may have to be repositioned or may have to leave not only the scanner, but also the radiology department or hospital. The commenters stated that, in such cases, facilities must expend equivalent facility resources in each sitting as if the sittings occurred on different dates of service. They noted that not all of these costs are reflected in claims data and, therefore, would not be reflected in the multiple imaging composite APC payment rates. The commenters requested that CMS allow hospitals to report modifier -59 when multiple imaging services of the same modality are provided at different times on the same date of service, and that such cases be excluded from the multiple imaging composite payment methodology. They stated that such an approach is necessary to recognize the provider costs when imaging services must be provided at different sittings due to clinical need or safety requirements. One commenter also asked CMS to work with the AMA to create new CPT codes that describe combined procedures so that providers could use those codes when they provide multiple imaging services in a single session. The commenter argued that utilization of such codes would be easier for providers and would facilitate the capturing of charge data that could be used to create new APCs or payment policies that reflect economies of scale for combined procedures reported through claims data.
Response: We do not agree with the commenters that multiple imaging procedures of the same modality provided on the same date of service but at different times should be exempt from the multiple imaging composite payment methodology. As we indicated in the CY 2010 OPPS/ASC proposed rule (74 FR 35283 through 35284) and the CY 2009 OPPS/ASC final rule with comment period (73 FR 68565), we believe that composite payment is appropriate even when procedures are provided on the same date of service but at different times because hospitals do not expend the same facility resources each and every time a patient is seen for a distinct imaging service in a separate imaging session. In most cases, we expect that patients in these circumstances would receive imaging procedures at different times during a single prolonged hospital outpatient encounter. The efficiencies that may be gained from providing multiple imaging procedures during a single session are achieved in ways other than merely not having to reposition the patient. For example, a patient who has two MRI procedures 3 hours apart during a single hospital outpatient encounter would not have to be registered again, and hospital staff might not have to explain the procedure in detail prior to the second scan. In the case of multiple procedures involving contrast that are provided at different times during a single hospital outpatient encounter, establishment of new intravenous access for the second study would not be necessary. Even if the same level of efficiencies could not be gained for multiple imaging procedures performed on the same date of service but at different times, we expect that any higher costs associated with these cases would be reflected in the claims data and cost reports we use to calculate the median costs for the multiple imaging composite APCs and, therefore, in the payment rates for the multiple imaging composite APCs. We do not believe it is necessary or appropriate for hospitals to report imaging procedures provided on the same date of service but during different sittings any differently than they would report imaging procedures performed consecutively in one sitting with no time in between the imaging services.
We also do not agree with the commenter that it is necessary to create new CPT codes that describe combined services to ease the burden of hospital billing and improve claims data for ratesetting. As we stated in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68565), certain combination CPT codes, specifically those single codes that describe imaging procedures without contrast and then followed by contrast, already allow for hospitals to report commonly performed combinations of imaging procedures in one anatomic area using a single CPT code. Hospitals can continue to use existing codes to report multiple imaging services by reporting multiple HCPCS codes, and for ratesetting, we use the charges reported to us by hospitals on claims for those multiple imaging services to calculate composite APC payment rates. The I/OCE determines whether composite APC payment applies to a claim, so the composite payment policy creates no additional administrative burden for hospitals.
Comment: Several commenters asserted that the multiple imaging composite payment methodology could have a disproportionately negative effect on cancer centers and trauma units, where patients frequently require more than two imaging services during a Start Printed Page 60400 hospital encounter. They argued that the use of a single composite APC payment for an imaging modality regardless of the number of services provided is only appropriate if the underlying claims data used to set the “average” payment rate reflect an average number of services furnished by all providers. According to the commenters, certain providers, such as cancer centers and trauma hospitals, face systematic underpayment of multiple imaging services due to their unique patient population because they routinely provide a greater than average number of imaging services in one sitting or multiple sittings on the same date of service. The commenters stated that, at the same time, all other hospitals experience systematic overpayment.
Response: We do not agree with the commenters that the underlying claims data used to calculate the median costs upon which the payment rates for the multiple imaging composite APCs are inappropriate for payment of all hospitals, or that the multiple imaging composite payment methodology is likely to have a disproportionately negative effect on cancer centers and trauma units. In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68562 through 68563), we explored data from CY 2007 claims in response to similar concerns from commenters and a recommendation by the APC Panel at its August 2008 meeting. An analysis of diagnosis codes present on the CY 2007 multiple imaging “single session” claims did show more variability in the number of scans for cancer patients compared to patients with noncancer diagnoses, consistent with commenters' concerns. We observed that, for several of the more commonly reported cancer diagnoses, more than half of the patients received more than two imaging procedures on the same day, while generally lower proportions of patients with noncancer diagnoses received more than two imaging procedures on a single date of service. We did not observe the same pattern for trauma diagnoses. As we stated in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68562), we do not believe that the higher rate of variability that we observed in the number of scans cancer patients receive was so extreme, however, that the mix of services hospitals provide to patients with diagnoses other than cancer would not balance out higher numbers of scans for cancer patients.
We continue to believe that OPPS hospitals demonstrate sufficient variability in the number of imaging procedures they provide to a single patient on the same day that it is unlikely any particular class of hospital would experience disproportionate financial effects from the multiple imaging composite payment methodology. For CY 2009, the first year of implementation of the multiple imaging composite APC methodology, the modeled impacts of payment changes by class of hospital due to APC recalibration (where the effects of the multiple imaging composite payment methodology and other APC recalibration would be observed), were very modest across classes of hospital, ranging from −2.5 percent to +1.9 percent (73 FR 68799 through 68800).
The goal of the multiple imaging composite payment methodology is to establish incentives for efficiency through larger payment bundles based on the practice patterns of OPPS hospitals as a whole. We acknowledge that there may be a small number of dedicated cancer centers that, relative to other hospitals paid under the OPPS, may provide a higher proportion of imaging services to cancer patients that involve three or more scans. However, as discussed above, our prior analyses do not lead us to believe that any class of hospitals would experience significantly negative effects from the multiple imaging composite payment methodology. We note that we establish national payment policies for the OPPS and, while certain policies may have greater or lesser impact on individual hospitals, on average we believe that the total OPPS payment to a hospital for all of its services is appropriate. Our modeled estimates of changes in total payment for classes of hospitals between CY 2008 and CY 2009 support this conclusion. We do not believe it would be appropriate to establish national policy based on considerations of the service mix of individual hospitals, or to exclude individual hospitals from national policy because of the impact a specific policy may have on one component of a hospital's operations as a result of a particular hospital's service mix. Furthermore, we note that several cancer centers are held permanently harmless under section 1833(t)(7)(D)(ii) of the Act in order to account for the fact that they may be more costly and have different practice patterns than other hospitals paid under the OPPS.
Comment: Some commenters questioned the adequacy of the proposed multiple imaging composite APC payment rates for sessions involving three or more imaging procedures, and expressed general concern that multiple imaging composite payment methodology would limit beneficiary access to imaging services. For example, these commenters asserted that the multiple imaging composite payment methodology could create incentives for hospitals to require patients who need more than two imaging procedures to return for additional sittings on other days if the costs for sessions in which more than two procedures are performed far exceed the multiple imaging composite APC payment rates.
Response: As we stated in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68562), we do not believe that, in aggregate, OPPS payment for multiple imaging services will be inadequate under the multiple imaging composite payment methodology so as to limit beneficiary access, even considering the minority of cases in which hospitals provide more than two imaging procedures on a single date of service. The median costs upon which the payment rates for the multiple imaging composite APCs are based are calculated using CY 2008 claims that would have qualified for composite payment, including those with only two imaging procedures and those with substantially higher numbers of imaging procedures. Payment based on a measure of central tendency is a principle of any prospective payment system. In some individual cases payment exceeds the average cost and in other cases payment is less than the average cost. On balance, however, payment should approximate the relative cost of the average case, recognizing that, as a prospective payment system, the OPPS is a system of averages.
We also do not agree with the commenters that the multiple imaging composite payment methodology will result in hospitals requiring patients who need more than two imaging procedures to return for additional sittings on other days. As we stated in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68562), we do not believe that, in general, hospitals would routinely and for purposes of financial gain put patients at unnecessary risk of harm from radiation or contrast exposure, or inconvenience them or risk lack of timely followup to the point of making them return to the hospital on separate days to receive medically necessary diagnostic studies. However, we again note that we do have the capacity to examine our claims data for patterns of fragmented care. If we were to find a pattern in which a hospital appears to be fragmenting imaging services across multiple days for individual beneficiaries, we could refer it for review by the Quality Improvement Organizations (QIOs) with Start Printed Page 60401 respect to the quality of care furnished, or for review by the Program Safeguard Contractors of claims against the medical record, as appropriate to the circumstances we found.
Comment: Several commenters urged CMS to standardize cost reporting for both advanced imaging procedures and other problematic cost centers before it makes any methodological changes to OPPS payment methodologies, including a composite policy for multiple imaging procedures. One commenter was concerned that observed efficiencies in the multiple imaging composite APC median costs are the result of inaccurate cost report data only and do not reflect true efficiencies from multiple imaging services provided during a single session. According to the commenter, CMS should implement separate cost centers for CT and MRI procedures and the revised revenue code-to-cost center crosswalk, as recommended in the July 2008 report by RTI International (RTI) entitled, “Refining Cost to Charge Ratios for Calculating APC and DRG Relative Payment Weights.” The commenter stated that the creation of the new standard cost centers and the adoption of the revised revenue code-to-cost center crosswalk would provide much more accurate charge and cost data for these imaging modalities, and that the true efficiencies associated with providing multiple imaging procedures in a single session may only be discernable once these data are available. The commenter also remarked that the adoption of these changes would result in significant shifts in the underlying CCRs for all APCs, thereby impacting all relative weights and payment rates across all services over time.
Response: We published information regarding the proposed draft hospital cost report CMS–2552–10 in the Federal Register on July 2, 2009 and the proposed agency information collection activities were open for a 60-day review and comment period (74 FR 31738). The comment period ended August 31, 2009. The proposed cost report can be viewed at: http://www.cms.hhs.gov/PaperworkReductionActof1995/PRAL/itemdetail.asp?filterType=none&filterByDID=-99&sortByDID=2&sortOrder=descending&itemID=CMS1224069&intNumPerPage=10. We will consider all comments received during the comment period in our determination of whether to create new modality-specific standard diagnostic radiology cost centers.
As noted in our response to a comment regarding the recommendations included in RTI's July 2008 report entitled, “Refining Cost to Charge Ratios for Calculating APC and DRG Relative Payment Weights” (73 FR 68526), the current cost report form already includes nonstandard cost centers for CT Scanning and MRI. We also explained that under the principle of departmental apportionment of costs at § 413.53 hospitals are required to report separately the costs and charges for each ancillary department for which charges are customarily billed if the corresponding cost and charge information is accumulated separately in the provider's accounting system. We believe the nonstandard cost center information for CT Scanning and MRI that we currently collect reflects costs and charges for CT Scanning and MRI and we use these data to estimate median costs for ratesetting.
In the meantime, we believe it is fully appropriate to continue the multiple imaging composite payment methodology, which we believe improves the accuracy of OPPS payment rates and promotes efficiency among hospitals. As we stated in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68563), the most recent hospital cost report data are the best and most consistent estimate of relative costs that we have available to us for all hospitals for all hospital services. We will continue to use these data to estimate APC median costs. Should revised cost report data become available for CT and MRI procedures, our composite methodology would automatically incorporate that additional precision into the multiple imaging composite APC median cost estimates.
Comment: One commenter stated that the differential in the CY 2010 proposed payment rates for APC 8007 and APC 8008 appears adequate to account for the substantial differences in costs between magnetic resonance procedures when performed with and without contrast. The commenter asked CMS to evaluate the claims available for the CY 2010 OPPS/ASC final rule with comment period to ensure that payment rates for the two APCs reflect the incremental costs for the contrast agent and contrast administration included in APC 8008.
Response: We agree with the commenter regarding the appropriateness of the proposed differential in payment rates for APC 8007 and APC 8008 for CY 2010. The median costs upon which the CY 2010 final payment rates for APC 8007 and APC 8008 are based ($706 and $986, respectively) also appropriately reflect differences in costs for MRI and MRA imaging sessions with and without the administration of contrast.
Comment: One commenter stated that there was a discrepancy in CMS' estimated volume of APC 8005 single claims for the CY 2010 OPPS/ASC proposed rule. The commenter indicated that CMS' estimated volume of APC 8005 single claims increased by approximately one-third from the CY 2007 claims used in CY 2009 ratesetting to the CY 2008 claims available for the CY 2010 OPPS/ASC proposed rule. The commenter noted that this increase was inconsistent with the commenter's data analysis, which indicated that the total volume of single claims for APC 8005 did not increase significantly over this same time period.
Response: We reviewed the CY 2007 “single session” claims data used in ratesetting for APC 8005 for the CY 2009 OPPS/ASC final rule with comment period, and the CY 2008 “single session” claims data used in ratesetting for APC 8005 for the CY 2010 OPPS/ASC proposed rule. For the CY 2009 final rule, we identified 429,525 “single session” claims out of 809,483 potential composite cases to calculate the median cost for APC 8005. For the CY 2010 OPPS/ASC proposed rule, we identified 423,890 “single session” claims out of 810,469 potential composite cases to calculate the median cost for APC 8005. These published data do not demonstrate an increase of approximately one-third in the volume of “single session” claims from the CY 2007 claims used to calculate the median costs upon which the CY 2009 final payment rates are based compared to the CY 2008 claims used to calculate the median costs upon which the CY 2010 proposed payment rates are based, as the commenter indicated. For this final rule with comment period, we identified 455,191 “single session” claims (an increase of approximately 6 percent compared to CY 2009) out of 882,581 potential composite cases (an increase of approximately 9 percent compared to CY 2009) to calculate the median cost of APC 8005.
Comment: Many commenters requested that CMS thoroughly evaluate the impact of the multiple imaging composite payment methodology and commended CMS for not proposing to expand the multiple imaging composite payment methodology for CY 2010. Commenters asked CMS to review claims data to ensure that hospitals are being adequately paid for providing multiple imaging services, that patients are not being required by hospitals to return to the hospital on multiple days for imaging services, and that certain types or classes of hospitals are not being negatively affected before moving forward with any additional imaging Start Printed Page 60402 composite policies. One commenter noted that while CMS will have data available from CY 2009 to analyze for the winter 2010 APC Panel meeting, the commenter believed that such analyses would be more meaningful if claims data through CY 2012 are use to show impacts and a change in hospital behavior under the composite payment policy. Commenters also stated that any expansion of the multiple imaging composite payment methodology should be subject to full public comment.
Response: We appreciate the commenters' support of our proposal not to implement any significant changes to the composite APC methodology for CY 2010 so that we may monitor the effects of the existing composite APCs on utilization and payment. We also appreciate the commenters' thoughtful suggestions for data analysis that can be performed toward that end once CY 2009 claims data become available and in the longer term. We will take commenters' suggestions into consideration as we review the CY 2009 claims data for the impact of the multiple imaging composite APCs on payments to hospitals and on services to beneficiaries.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to continue paying for all multiple imaging procedures within an imaging family performed on the same date of service using the multiple imaging composite payment methodology. The CY 2010 payment rates for the five multiple imaging composite APCs (APC 8004, APC 8005, APC 8006, APC 8007, and APC 8008) are based on median costs calculated from the CY 2008 claims that would have qualified for composite payment under the current policy (that is, those claims with more than one procedure within the same family on a single date of service). Using the same ratesetting methodology described in the CY 2010 OPPS/ASC proposed rule (74 FR 35284), we were able to identify 1.8 million “single session” claims out of an estimated 2.7 million potential composite cases from our ratesetting claims data, or well over half of all eligible claims, to calculate the final CY 2010 median costs for the multiple imaging composite APCs.
Table 13 below lists the HCPCS codes subject to the final multiple imaging composite policy and their respective families for CY 2010. We note that we have updated Table 13 to reflect HCPCS coding changes for CY 2010. Specifically, we added CPT code 74261 (Computed tomographic (CT) colonography, diagnostic, including image postprocessing; without contrast material) and CPT code 74262 (Computed tomographic (CT) colonography, diagnostic, including image postprocessing, with contrast materials(s) including non-contrast images, if performed) to the CT and CTA family, and removed CPT code 0067T (Computed tomographic (CT) colonography (ie, virtual colonoscopy); diagnostic), which was replaced by these CPT codes. The HCPCS codes listed in Table 13 are assigned status indicated “Q3” in Addendum B to this final rule with comment period to identify their status as potentially payable through a composite APC. Their composite APC assignment is identified in Addendum M to this final rule with comment period. Table 14 below lists the imaging services subject to the composite methodology that overlap with HCPCS codes on the CY 2010 bypass list.
Start Printed Page 60403 Start Printed Page 60404 Start Printed Page 60405 Start Printed Page 60406 Start Printed Page 604073. Calculation of OPPS Scaled Payment Weights
Using the APC median costs discussed in sections II.A.1. and II.A.2. of this final rule with comment period, we calculated the final relative payment weights for each APC for CY 2010 shown in Addenda A and B to this final rule with comment period. In years prior to CY 2007, we standardized all the relative payment weights to APC 0601 (Mid Level Clinic Visit) because mid-level clinic visits were among the most frequently performed services in the hospital outpatient setting. We assigned APC 0601 a relative payment weight of 1.00 and divided the median cost for each APC by the median cost for APC 0601 to derive the relative payment weight for each APC.
Beginning with the CY 2007 OPPS (71 FR 67990), we standardized all of the relative payment weights to APC 0606 (Level 3 Clinic Visits) because we deleted APC 0601 as part of the reconfiguration of the clinic visit APCs. We selected APC 0606 as the base because APC 0606 was the mid-level clinic visit APC (that is, Level 3 of five levels). Therefore, for CY 2010, to maintain consistency in using a median Start Printed Page 60408 for calculating unscaled weights representing the median cost of some of the most frequently provided services, we proposed to continue to use the median cost of the mid-level clinic visit APC, APC 0606, to calculate unscaled weights. Following our standard methodology, but using the proposed CY 2010 median cost for APC 0606, for CY 2010 we assigned APC 0606 a relative payment weight of 1.00 and divided the median cost of each APC by the proposed median cost for APC 0606 to derive the proposed unscaled relative payment weight for each APC. The choice of the APC on which to base the proposed relative weights for all other APCs did not affect the payments made under the OPPS because we scale the weights for budget neutrality.
Section 1833(t)(9)(B) of the Act requires that APC reclassification and recalibration changes, wage index changes, and other adjustments be made in a budget neutral manner. Budget neutrality ensures that the estimated aggregate weight under the OPPS for CY 2010 is neither greater than nor less than the estimated aggregate weight that would have been made without the changes. To comply with this requirement concerning the APC changes, we proposed to compare the estimated aggregate weight using the CY 2009 scaled relative weights to the estimated aggregate weight using the CY 2010 unscaled relative weights. For CY 2009, we multiply the CY 2009 scaled APC relative weight applicable to a service paid under the OPPS by the volume of that service from CY 2008 claims to calculate the total weight for each service. We then add together the total weight for each of these services in order to calculate an estimated aggregate weight for the year. For CY 2010, we perform the same process using the CY 2010 unscaled weights rather than scaled weights. We then calculate the weight scaler by dividing the CY 2009 estimated aggregate weight by the CY 2010 estimated aggregate weight. The service mix is the same in the current and prospective years because we use the same set of claims for service volume in calculating the aggregate weight for each year. For a detailed discussion of the weight scaler calculation, we refer readers to the OPPS claims accounting document available on the CMS Web site at: http://www.cms.hhs.gov/HospitalOutpatientPPS/. We included payments to CMHCs in our comparison of estimated unscaled weight in CY 2010 to estimated total weight in CY 2009 using CY 2008 claims data and holding all other things constant. Based on this comparison, we adjusted the unscaled relative weights for purposes of budget neutrality. In our proposal for CY 2010, the proposed CY 2010 unscaled relative payment weights were adjusted by multiplying them by a proposed weight scaler of 1.2863 to ensure budget neutrality of the proposed CY 2010 relative weights.
Section 1833(t)(14)(H) of the Act, as added by section 621(a)(1) of Public Law 108–173, states that, “Additional expenditures resulting from this paragraph shall not be taken into account in establishing the conversion factor, weighting and other adjustment factors for 2004 and 2005 under paragraph (9) but shall be taken into account for subsequent years.” Section 1833(t)(14) of the Act provides the payment rates for certain “specified covered outpatient drugs.” Therefore, the cost of those specified covered outpatient drugs (as discussed in section V.B.3. of the proposed rule (74 FR 35324 through 35333) and this final rule with comment period) was included in the proposed budget neutrality calculations for the CY 2010 OPPS.
We did not receive any public comments on the proposed methodology for calculating scaled weights from the median costs for the CY 2010 OPPS. Therefore, we are finalizing our proposed methodology, without modification, including updating of the budget neutrality scaler for this final rule with comment period. Under this methodology, the final unscaled payment weights were adjusted by a weight scaler of 1.3222 for this final rule with comment period. The final scaled relative payment weights listed in Addenda A and B to this final rule with comment period incorporate the recalibration adjustments discussed in sections II.A.1. and II.A.2. of this final rule with comment period.
4. Changes to Packaged Services
a. Background
The OPPS, like other prospective payment systems, relies on the concept of averaging, where the payment may be more or less than the estimated cost of providing a service or bundle of services for a particular patient, but with the exception of outlier cases, the payment is adequate to ensure access to appropriate care. Packaging and bundling payment for multiple interrelated services into a single payment create incentives for providers to furnish services in the most efficient way by enabling hospitals to manage their resources with maximum flexibility, thereby encouraging long-term cost containment. For example, where there are a variety of supplies that could be used to furnish a service, some of which are more expensive than others, packaging encourages hospitals to use the least expensive item that meets the patient's needs, rather than to routinely use a more expensive item. Packaging also encourages hospitals to negotiate carefully with manufacturers and suppliers to reduce the purchase price of items and services or to explore alternative group purchasing arrangements, thereby encouraging the most economical health care. Similarly, packaging encourages hospitals to establish protocols that ensure that necessary services are furnished, while carefully scrutinizing the services ordered by practitioners to maximize the efficient use of hospital resources. Finally, packaging payments into larger payment bundles promotes the stability of payment for services over time. Packaging and bundling also may reduce the importance of refining service-specific payment because there is more opportunity for hospitals to average payment across higher cost cases requiring many ancillary services and lower cost cases requiring fewer ancillary services.
Decisions about packaging and bundling payment involve a balance between ensuring that payment is adequate to enable the hospital to provide quality care and establishing incentives for efficiency through larger units of payment. In the CY 2008 OPPS/ASC final rule with comment period (72 FR 66610 through 66659), we adopted the packaging of payment for items and services in the seven categories listed below into the payment for the primary diagnostic or therapeutic modality to which we believe these items and services are typically ancillary and supportive. The seven categories are guidance services, image processing services, intraoperative services, imaging supervision and interpretation services, diagnostic radiopharmaceuticals, contrast media, and observation services. We specifically chose these categories of HCPCS codes for packaging because we believe that the items and services described by the codes in these categories are the HCPCS codes that are typically ancillary and supportive to a primary diagnostic or therapeutic modality and, in those cases, are an integral part of the primary service they support.
We assign status indicator “N” to those HCPCS codes that we believe are always integral to the performance of the primary modality; therefore, we always package their costs into the costs Start Printed Page 60409 of the separately paid primary services with which they are billed. Services assigned status indicator “N” are unconditionally packaged.
We assign status indicator “Q1” (“STVX-Packaged Codes”), “Q2” (“T-Packaged Codes”), or “Q3” (Codes that may be paid through a composite APC) to each conditionally packaged HCPCS code. An “STVX-packaged code” describes a HCPCS code whose payment is packaged when one or more separately paid primary services with the status indicator of “S,” “T,” “V,” or “X” are furnished in the hospital outpatient encounter. A “T-packaged code” describes a code whose payment is packaged when one or more separately paid surgical procedures with the status indicator of “T” are provided during the hospital encounter. “STVX-packaged codes” and “T-packaged codes” are paid separately in those uncommon cases when they do not meet their respective criteria for packaged payment. “STVX-packaged codes” and “T-packaged HCPCS codes” are conditionally packaged. We refer readers to section XIII.A.1. of this final rule with comment period for a complete listing of status indicators.
We use the term “dependent service” to refer to the HCPCS codes that represent services that are typically ancillary and supportive to a primary diagnostic or therapeutic modality. We use the term “independent service” to refer to the HCPCS codes that represent the primary therapeutic or diagnostic modality into which we package payment for the dependent service. We note that, in future years as we consider the development of larger payment groups that more broadly reflect services provided in an encounter or episode-of-care, it is possible that we might propose to bundle payment for a service that we now refer to as “independent.”
In addition, in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66650 through 66659), we finalized additional packaging for the CY 2008 OPPS, which included the establishment of new composite APCs for CY 2008, specifically APC 8000 (Cardiac Electrophysiologic Evaluation and Ablation Composite), APC 8001 (LDR Prostate Brachytherapy Composite), APC 8002 (Level I Extended Assessment & Management Composite), and APC 8003 (Level II Extended Assessment & Management Composite). In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68559 through 68569), we expanded the composite APC model to one new clinical area, multiple imaging services. We created five multiple imaging composite APCs for payment in CY 2009 that incorporate statutory requirements to differentiate between imaging services provided with contrast and without contrast as required by section 1833(t)(2)(G) of the Act. The multiple imaging composite APCs are: APC 8004 (Ultrasound Composite); APC 8005 (CT and CTA without Contrast Composite); APC 8006 (CT and CTA with Contrast Composite); APC 8007 (MRI and MRA without Contrast Composite); and APC 8008 (MRI and MRA with Contrast Composite). We discuss composite APCs in more detail in section II.A.2.e. of this final rule with comment period.
Hospitals include charges for packaged services on their claims, and the estimated costs associated with those packaged services are then added to the costs of separately payable procedures on the same claims in establishing payment rates for the separately payable services. We encourage hospitals to report all HCPCS codes that describe packaged services that were provided, unless the CPT Editorial Panel or CMS provide other guidance. If a HCPCS code is not reported when a packaged service is provided, it can be challenging to track utilization patterns and resource costs.
b. Packaging Issues
(1) Packaged Services Addressed by the February 2009 APC Panel Recommendations
The Packaging Subcommittee of the APC Panel was established to review packaged HCPCS codes. In deciding whether to package a service or pay for a code separately, we have historically considered a variety of factors, including whether the service is normally provided separately or in conjunction with other services; how likely it is for the costs of the packaged code to be appropriately mapped to the separately payable codes with which it was performed; and whether the expected cost of the service is relatively low. As discussed in section II.A.4.a. of this final rule with comment period regarding our packaging approach for CY 2008, we established packaging criteria that apply to seven categories of codes whose payments are packaged.
During the September 2007 APC Panel meeting, the APC Panel requested that CMS evaluate the impact of expanded packaging on beneficiaries. During the March 2008 APC Panel meeting, the APC Panel requested that CMS report to the Panel at the first Panel meeting in CY 2009 regarding the impact of packaging on net payments for patient care. In response to these requests, we shared data with the APC Panel at the February 2009 APC Panel meeting that compared the frequency of specific categories of services billed under the OPPS in CY 2007, before the expanded packaging went into effect, to the frequency of those same categories of services in CY 2008, their first year of packaged payment. In each category, the HCPCS codes that we compared are the ones that we identified in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66659 through 66664) as fitting into one of the seven packaging categories listed in section II.A.4.a. of this final rule with comment period. The data shared with the APC Panel at the February 2009 APC Panel meeting compared CY 2007 claims processed through September 30, 2007 to CY 2008 claims processed through September 30, 2008. We did not make any adjustments for inflation, changes in Medicare population, or other variables that potentially influenced billing between CY 2007 and CY 2008. These data represent about 60 percent of the full year data. A summary of these data analyses is provided below.
Analysis of the diagnostic radiopharmaceuticals category showed that the frequency of the reporting of diagnostic radiopharmaceuticals increased by 1 percent between the first 9 months of CY 2007 and the first 9 months of CY 2008. In CY 2007, some diagnostic radiopharmaceuticals were packaged and others were separately payable, depending on whether their per day mean costs fell above or below the $55 drug packaging threshold for CY 2007. All diagnostic radiopharmaceuticals were uniformly packaged in CY 2008. Two percent more hospitals reported one or more diagnostic radiopharmaceuticals during CY 2008 as compared to CY 2007. Effective for CY 2008, we first required reporting of a radiolabeled product (including diagnostic radiopharmaceuticals) when billing a nuclear medicine procedure, and we believe that the increases in frequency and the number of reporting hospitals reflect hospitals meeting this reporting requirement.
We also found that nuclear medicine procedures (into which diagnostic radiopharmaceuticals were packaged) and associated diagnostic radiopharmaceuticals were billed approximately 3 million times during the first 9 months of both CY 2007 and CY 2008. Further analysis revealed that we paid hospitals over $637 million for nuclear medicine procedures and diagnostic radiopharmaceuticals during the first 9 months of CY 2007, when diagnostic radiopharmaceuticals were separately payable, and over $619 Start Printed Page 60410 million for nuclear medicine procedures and diagnostic radiopharmaceuticals during the first 9 months of CY 2008, when payment for diagnostic radiopharmaceuticals was packaged. This represented a 3 percent decrease in aggregate payment between the first 9 months of CY 2007 and the first 9 months of CY 2008.
Using the same data, we calculated an average payment per service or item billed (including nuclear medicine procedures and packaged or separately payable diagnostic radiopharmaceuticals) of $203 in CY 2007 and $198 in CY 2008 for nuclear medicine procedures. This represented a decrease of 2 percent in average payment per item or service billed between CY 2007 and CY 2008. It is unclear how much of the decrease in estimated aggregate or average per service or item billed payment may be due to packaging payment for diagnostic radiopharmaceuticals (and other services that were newly packaged for CY 2008) and how much may be due to the usual annual APC recalibration and typical fluctuations in service frequency. However, we believe that all of these factors likely contributed to the slight decrease in aggregate payment in CY 2008, as compared to CY 2007. Overall, the observed changes between CY 2007 and CY 2008 were very small and indicated that there has been very little change in frequency or aggregate payment in this clinical area between CY 2007 and CY 2008.
We similarly analyzed 9 months of CY 2007 and CY 2008 data related to all services that were packaged during CY 2008 because they were categorized as guidance services. Analysis of the guidance category (which includes image-guided radiation therapy services) showed that the frequency of guidance services increased by 2 percent between the first 9 months of CY 2007 and the first 9 months of CY 2008. One percent fewer hospitals reported one or more guidance services during CY 2007 as compared to CY 2008.
We further analyzed 9 months of CY 2007 and CY 2008 claims data for radiation oncology services that would be accompanied by radiation oncology guidance. We found that radiation oncology services (including radiation oncology guidance services) were billed approximately 4 million times in CY 2007 and 3.9 million times in CY 2008, representing a decrease in frequency of approximately 5 percent between CY 2007 and CY 2008. These numbers represented each instance where a radiation oncology service or a radiation oncology guidance service was billed. Our analysis indicated that hospitals were paid over $818 million for radiation oncology services and radiation oncology guidance services under the OPPS during the first 9 months of CY 2007, when radiation oncology guidance services were separately payable. During the first 9 months of CY 2008, when payments for radiation oncology guidance were packaged, hospitals were paid over $740 million for radiation oncology services under the OPPS. This $740 million included packaged payment for radiation oncology guidance services and represented a 10 percent decrease in aggregate payment from CY 2007 to CY 2008. Using the first 9 months of data for both CY 2007 and CY 2008, we calculated an average payment per radiation oncology service or item billed of $201 in CY 2007 and $190 in CY 2008, representing a decrease of 5 percent from CY 2007 to CY 2008. It is unclear how much of the decrease in aggregate payment and the decrease in average payment per service provided may be due to packaging payment for radiation oncology guidance services (and other services that were newly packaged for CY 2008) and how much may be due to the usual annual APC recalibration and typical fluctuations in service frequency. This analysis is discussed in further detail below, under “Recommendation 1” in this section of this final rule with comment period. In that analysis, we demonstrated that the volume of some packaged radiation oncology guidance services increased during the period, leading us to conclude that, irrespective of the decline in the frequency of radiation oncology services in general, hospitals did not appear to be changing their practice patterns specifically in response to packaged payment for radiation oncology guidance services.
We similarly analyzed 9 months of CY 2007 and CY 2008 data related to all services that were packaged during CY 2008 because they were categorized as intraoperative services. Analysis of the intraoperative category (which includes intravascular ultrasound (IVUS), intracardiac echocardiography (ICE), and coronary fractional flow reserve (FFR)) showed minimal changes in the frequency and the number of reporting hospitals between CY 2007 and CY 2008.
We found that cardiac catheterization and other percutaneous vascular procedures that would typically be accompanied by IVUS, ICE and FFR (including IVUS, ICE, and FFR) were billed approximately 375,000 times in CY 2007 and approximately 400,000 times in CY 2008, representing an increase of 8 percent in the number of services and items billed between CY 2007 and CY 2008. Further analysis revealed that the OPPS paid hospitals over $912 million for cardiac catheterizations, other related services, and IVUS, ICE, and FFR in CY 2007, when IVUS, ICE, and FFR were separately payable. In the first 9 months of CY 2008, the OPPS paid hospitals approximately $1.1 billion for cardiac catheterization and other percutaneous vascular procedures and IVUS, ICE, and FFR, when payments for IVUS, ICE, and FFR were packaged. This represented a 25 percent increase in payment from CY 2007 to CY 2008. Using the 9 months of data for both CY 2007 and CY 2008, we calculated an average payment per service or item provided of $2,430 in CY 2007 and $2,800 in CY 2008 for cardiac catheterization and other related services. This represented an increase of 15 percent in average payment per item or service from CY 2007 to CY 2008.
We could not determine how much of the 25 percent increase in aggregate payment for these services may be due to the packaging of payment for IVUS, ICE, and FFR (and other services that were newly packaged for CY 2008) and how much may be due to the usual annual APC recalibration and typical fluctuations in service frequency. However, we believe that all of these factors contributed to the increase in payment between these 2 years.
The three remaining packaging categories (excluding observation services, which are further discussed in section II.A.2.e.(1) of this final rule with comment period), contrast agents, image processing services, and imaging supervision and interpretation services, showed minimal changes in frequency between CY 2007 and CY 2008, ranging from a 2 percent increase to a 1 percent decrease in frequency. Similarly, when examining the number of hospitals reporting these services, the data showed similar numbers of hospitals reporting these services in CY 2007, when these services were separately payable, and CY 2008, when they were packaged. Specifically, the percentage change in the number of reporting hospitals for these categories between CY 2007 and CY 2008 ranged from 0 percent to a decrease of 1 percent.
In summary, these preliminary data indicated that hospitals in aggregate did not appear to have significantly changed their service reporting patterns as a result of the expanded packaging adopted for the OPPS beginning in CY 2008.
The APC Panel's Packaging Subcommittee reviewed the packaging status of several CPT codes and reported Start Printed Page 60411 its findings to the APC Panel at its February 2009 meeting. The full report of the February 18 and 19, 2009 APC Panel meeting can be found on the CMS Web site at: http://www.cms.hhs.gov/FACA/05_AdvisoryPanelonAmbulatoryPaymentClassificationGroups.asp. The APC Panel accepted the report of the Packaging Subcommittee, heard several presentations related to packaged services, discussed the deliberations of the Packaging Subcommittee, and recommended that—
1. CMS pay separately for radiation therapy guidance services performed in the treatment room for 2 years and then reevaluate packaging on the basis of claims data. (Recommendation 1)
2. CMS continue to analyze the impact of increased packaging on beneficiaries and provide more detailed versions of the analyses presented at the February 2009 meeting of services initially packaged in CY 2008 at the next Panel meeting. In addition, the Panel requested that, in the more detailed analyses of radiation oncology services that would be accompanied by radiation oncology guidance, CMS stratify the data according to the type of radiation oncology service, specifically, intensity modulated radiation therapy, stereotactic radiosurgery, brachytherapy, and conventional radiation therapy. (Recommendation 2)
3. CMS continue to analyze the impact on beneficiaries of increased packaging of diagnostic radiopharmaceuticals and provide more detailed analyses at the next Panel meeting. In addition, the Panel requested that, in the more detailed analyses of packaging of diagnostic radiopharmaceuticals by type of nuclear medicine scan, CMS break down the data according to the specific CPT codes billed with the diagnostic radiopharmaceuticals. (Recommendation 3)
4. CPT code 36592 (Collection of blood specimen using established central or peripheral catheter, venous, not otherwise specified) remain assigned to APC 0624 (Phlebotomy and Minor Vascular Access Device Procedures) for CY 2010. (Recommendation 4)
5. The Packaging Subcommittee continue its work until the next APC Panel meeting. (Recommendation 5)
In the proposed rule, we addressed each of these recommendations in turn in the discussion that follows.
Recommendation 1
In the CY 2010 OPPS/ASC proposed rule (74 FR 35289), we did not propose to pay separately for radiation therapy guidance services provided in the treatment room for CY 2010, which would have been consistent with the APC Panel's recommendation. Instead, we proposed to maintain the packaged status of radiation therapy guidance services performed in the treatment room for CY 2010.
As discussed below in this section, during the February 2009 APC Panel meeting, we presented data that estimated that aggregate payment for radiation oncology services, including the payment for radiation oncology guidance services, decreased by approximately 10 percent between the first 9 months of CY 2007 (before the expanded packaging went into effect) and the first 9 months of CY 2008 (after the expanded packaging went into effect). This decline may be attributable to many factors, including lower payment rates for common radiation oncology services in CY 2008 specifically and generally reduced volume for separately paid radiation oncology services. The APC Panel expressed concern that this aggregate payment decrease could inhibit patient access to technologically advanced and clinically valuable radiation oncology guidance services whose payment became packaged effective January 1, 2008.
While we presented data to the APC Panel comparing payment between CY 2007 and CY 2008 in response to past APC Panel recommendations, we note that we made changes to the bypass list for CY 2009 to ensure that we more fully captured all packaged costs on each claim, which resulted in significantly increased payment rates for many of these radiation oncology services for CY 2009, as compared to the CY 2008 payment rates for these services.
Specifically, as discussed in detail in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68575), in response to public comments received, several radiation oncology CPT codes had been included on the bypass list for the CY 2008 OPPS, although they failed to meet the empirical criteria for inclusion on the bypass list. For CY 2009, we removed from the bypass list those radiation oncology codes that did not meet the empirical criteria. As a result of these changes to the bypass list, the CY 2009 median costs for several common radiation oncology APCs increased by more than 9 percent as compared to the CY 2008 median costs, while the median costs for some of the other lower volume radiation oncology APCs, most notably the brachytherapy source application APCs, declined. For example, as noted in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68575), these changes to the bypass list resulted in payment for the common combination of intensity modulated radiation therapy (IMRT) and image-guided radiation therapy (IGRT) increasing from $348 in CY 2008 to $411 in CY 2009. Notably, the CY 2007 total payment rate for this combination of services, before the expanded packaging went into effect, was $403.
We do not yet have CY 2009 claims data reflecting utilization based on the payment rates in effect for CY 2009. However, we do not expect that an overall per-service payment comparison between CY 2007 and CY 2009 would likely demonstrate a significant decrease in payment for radiation oncology services because we have adopted a significant increase in the CY 2009 payment rates for the most common radiation oncology services. In addition, we note that CY 2010 proposed rule data indicated that the CY 2010 APC median costs applicable to most radiation oncology services experienced increases of approximately 2 to 15 percent when compared to their CY 2009 final rule median costs. Although a small number of other lower volume radiation oncology APCs, most notably the brachytherapy and stereotactic radiosurgery APCs, experienced declines in median costs, we do not expect that an overall per-service payment comparison between CY 2007 and CY 2010 would likely demonstrate a significant decrease in payment for radiation oncology services over this time period.
While we understand that the CY 2007 to CY 2008 aggregate payment comparison provided to the APC Panel during the February 2009 meeting may have contributed to the APC Panel's particular concern about payment for radiation oncology services for CY 2010, we do not believe that packaging payment for radiation oncology guidance services has primarily caused this decline. In addition, we do not believe that beneficiaries' access to these services has been limited as a result of packaging payment for radiation oncology guidance services. In the data presented to the APC Panel at the February 2009 meeting, the number of all packaged guidance services provided during the first 9 months of CY 2008 represented a 2 percent increase from the number of guidance services provided during the first 9 months of CY 2007. Further, although the CY 2008 volume of the radiation oncology guidance codes that we newly packaged for CY 2008 varied, with some of the services experiencing increases in volume and others experiencing decreases in volume, in aggregate, the reporting of radiation oncology Start Printed Page 60412 guidance services increased by 4 percent in the first 9 months of claims for CY 2008, as compared to the first 9 months of CY 2007, and the number of hospitals reporting these services also increased. This further supports our belief that, irrespective of the decline in the frequency of radiation oncology services in general, hospitals do not appear to be changing their practice patterns specifically in response to packaged payment for radiation oncology guidance services.
Therefore, in the CY 2010 OPPS/ASC proposed rule (74 FR 35289), we did not propose to pay separately for radiation therapy guidance services performed in the treatment room for 2 years as the APC Panel recommended. Instead, for CY 2010, we proposed to maintain the packaged status of all radiation therapy guidance services, including those radiation therapy guidance services performed in the treatment room.
A summary of the public comments and our response on the CY 2010 proposal to package payment for radiation therapy guidance services are included in section II.A.4.b.(2) of this final rule with comment period.
Recommendation 2
In the CY 2010 OPPS/ASC proposed rule (74 FR 35290), we stated that we are accepting the APC Panel recommendation to continue to analyze the impact of increased packaging on beneficiaries and to share more data with the APC Panel. We noted that we would carefully consider which additional data would be most informative for the APC Panel and would discuss these data with the APC Panel at the next CY 2009 APC Panel meeting and/or the first CY 2010 APC Panel meeting. We did not share additional packaging data with the APC Panel at the most recent August 2009 meeting because we believe the APC Panel's discussions would benefit from analyses of an additional year of claims data after CY 2008. Therefore, we plan to incorporate analysis of CY 2009 claims into the information we will bring to the APC Panel for its review at the winter 2010 meeting. Similarly, in the proposed rule, we noted that we would determine what additional detailed data related to radiation oncology services would be helpful to the APC Panel and would share these data at the next CY 2009 APC Panel meeting and/or the first CY 2010 APC Panel meeting. We did not share additional data related to radiation oncology services with the APC Panel at the most recent August 2009 meeting because we believe the APC Panel's discussions would benefit from analyses of an additional year of claims data after CY 2008. Therefore, we plan to incorporate analysis of CY 2009 claims into the information we will bring to the APC Panel for its review at the winter 2010 meeting.
A summary of the public comments and our response regarding the impact of the CY 2010 packaging proposal are included in section II.A.4.b.(2) of this final rule with comment period.
Recommendation 3
In the CY 2010 OPPS/ASC proposed rule (74 FR 35290), we stated that we are accepting the APC Panel's recommendation that CMS continue to analyze the impact on beneficiaries of increased packaging of diagnostic radiopharmaceuticals and provide more detailed analyses at the next APC Panel meeting. In these analyses of diagnostic radiopharmaceuticals by type of nuclear medicine scan, the APC Panel further recommended that CMS analyze the data according to the specific CPT codes billed with the diagnostic radiopharmaceuticals. This APC Panel recommendation is discussed in detail in section II.A.2.d.(5) of this final rule with comment period. In the proposed rule, we noted that we are accepting the APC Panel's recommendation and would provide additional data to the APC Panel at an upcoming meeting. We did not share additional data related to diagnostic radiopharmaceuticals and nuclear medicine scans with the APC Panel at the most recent August 2009 meeting because we believe the APC Panel's discussions would benefit from analyses of an additional year of claims data after CY 2008. Therefore, we plan to incorporate analysis of CY 2009 claims into the information we will bring to the APC Panel for its review at the winter 2010 meeting.
A summary of the public comments and our response on the CY 2010 proposal to package payment for diagnostic radiopharmaceuticals into payment for the associated nuclear medicine procedures are included in sections II.A.2.d.(5) and V.B.2.d. of this final rule with comment period.
Recommendation 4
In the CY 2010 OPPS/ASC proposed rule (74 FR 35290), we proposed to continue for CY 2010 to treat CPT code 36592 (Collection of blood specimen using established central or peripheral catheter, venous, not otherwise specified) as an “STVX packaged code” and to assign it to APC 0624 (Phlebotomy and Minor Vascular Access Device Procedures), the same APC to which CPT code 36591 (Collection of blood specimen from a completely implantable venous access device) is currently assigned as the APC Panel recommended. CPT code 36592 became effective January 1, 2008 and was assigned interim status indicator “N” in the CY 2008 OPPS/ASC final rule with comment period. For CY 2009, in response to public comments, we proposed to treat CPT code 36592 as a conditionally packaged code, with assignment to APC 0624. In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68576), we discussed the public comments we received regarding our proposed treatment of CPT code 36592. Several of these commenters supported our proposal to treat CPT code 36592 as a conditionally packaged code with assignment to APC 0624. We stated in the CY 2009 OPPS/ASC final rule with comment period that when cost data for CPT code 36592 became available for the CY 2010 OPPS annual update, we would reevaluate whether assignment to APC 0624 continued to be appropriate.
Based on our analysis of claims data, our clinical understanding of the service, and our discussion with the APC Panel Packaging Subcommittee, in the CY 2010 OPPS/ASC proposed rule (74 FR 35290), we proposed to maintain the assignment of CPT code 36592 to APC 0624 for CY 2010, consistent with the APC Panel recommendation, and we proposed to continue to treat CPT code 36592 as an “STVX packaged code” and assign it to APC 0624. We noted that we expect hospitals to follow the CPT guidance related to CPT codes 36591 and 36592 regarding when these services should be appropriately reported.
We received no public comments on the CY 2010 proposal to maintain the assignment of CPT code 36592 to APC 0624 and treat it as an “STVX packaged code,” so we are finalizing our proposal, without modification.
Recommendation 5
In response to the APC Panel's recommendation for the Packaging Subcommittee to remain active until the next APC Panel meeting, in the CY 2010 OPPS/ASC proposed rule (74 FR 35290) we noted that we have accepted this recommendation and the APC Panel Packaging Subcommittee remains active. We stated that additional issues and new data concerning the packaging status of codes would be shared for its consideration as information becomes available. We continue to encourage submission of common clinical scenarios involving currently packaged HCPCS codes to the Packaging Subcommittee for its ongoing review. We also encourage recommendations of Start Printed Page 60413 specific services or procedures whose payment would be most appropriately packaged under the OPPS. Additional detailed suggestions for the Packaging Subcommittee should be submitted by e-mail to APCPanel@cms.hhs.gov with Packaging Subcommittee in the subject line.
The Packaging Subcommitee has remained active; the Subcommittee's last meeting to discuss packaging issues was the August 2009 meeting.
(2) Packaged Services Addressed by the August 2009 APC Panel Recommendations
The APC Panel met again on August 5 and 6, 2009 to hear public presentations on the proposals set forth in the CY 2010 OPPS/ASC proposed rule. The APC Panel's Packaging Subcommittee reviewed the packaging status of several CPT codes and reported its findings to the APC Panel. The full report of the August 5 and 6, 2009 APC Panel meeting can be found on the CMS Web site at: http://www.cms.hhs.gov/FACA/05_AdvisoryPanelonAmbulatoryPaymentClassificationGroups.asp. The APC Panel accepted the report of the Packaging Subcommittee, heard several presentations related to packaged services, discussed the deliberations of the Packaging Subcommittee, and recommended that—
1. CMS submit to the Packaging Subcommittee, for its ongoing review, common clinical scenarios involving currently packaged HCPCS codes and recommendations of specific services or procedures for which payment would be most appropriately packaged under the OPPS. (Recommendation 6)
2. When CMS changes the dollar amount of the drug packaging threshold and determines that some drugs within a single therapeutic class fall on either side of the packaging threshold, CMS consider packaging all of the drugs within that class on the basis of feedback from providers, the APC Panel, and stakeholders. (Recommendation 7)
3. CMS continue to study the impact of increased packaging on beneficiaries. (Recommendation 8)
4. The work of the APC Packaging Subcommittee continue. (Recommendation 9)
With respect to these August 2009 APC Panel recommendations, we are accepting recommendations 6, 8, and 9. We are continuing the work of the APC Panel Packaging Subcommitee, and we appreciate the Packaging Subcommitee's expertise and experience regarding packaging under the OPPS and the valuable advice the Subcommittee continues to provide to us. We will continue to bring to the Subcommittee's attention clinical scenarios identified by us or the public regarding services that are currently packaged or are candidates for future packaging under the OPPS. As discussed above, we also will continue to study the impact of increased packaging on Medicare beneficiaries, as the APC Panel has previously recommended to us. We did not share additional packaging data with the APC Panel at the most recent August 2009 meeting because we believe the APC Panel's discussions would benefit from analyses of an additional year of claims data after CY 2008. Therefore, we plan to incorporate analysis of CY 2009 claims into the information we will bring to the APC Panel for its review at the winter 2010 meeting. Finally, our response to recommendation 7 regarding the packaging of payment for all drugs in the same therapeutic class is discussed in section V.B.2.c. of this final rule with public comment.
Comment: Many commenters expressed a wide range of views on the existing policies for packaging payment for categories of services that CMS proposed to continue for CY 2010. One commenter claimed that while packaging provides an incentive for providers to deliver services in the most efficient, cost-effective manner possible, payment bundles that are too small do not enhance efficiencies, while payment bundles that are too large may carry excessive copayments for patients who need only a small proportion of services in the bundle. Another commenter suggested that CMS' packaging policy is likely to lead to less efficient use of resources, limited access to innovative treatment options, and greater instability in payments because, unlike the incentives from packaging under the IPPS, under the OPPS, the hospital would receive greater payment by bringing the outpatient back for a second visit or admitting the patient for inpatient care than by utilizing a more costly approach to providing an outpatient service that would be paid the same, regardless of the approach. The commenter also stated that when an APC's payment rate is significantly less than the cost of a technology, hospitals have a strong disincentive to use that technology, even if it could reduce the costs of care at a later date and provide better care to the patient.
Several commenters asserted that the implications of OPPS packaging policies are unknown due to a lack of transparency in the OPPS ratesetting process and methodology used to determine payment for packaged services, potentially leading to inappropriate payment and underutilization of image-guidance services. The commenters believed that packaging payment for image-guidance leads to hospitals discouraging physicians from using guidance services and that, therefore, CMS should not package payment for image-guidance services. Several commenters urged CMS to consider establishing a 2 to 3 year data collection period during which separate payment would be made for new technology or new applications of existing technology. The commenters further suggested that the data could then be used to evaluate the impact of packaging on clinical utilization and payment and could also be used to determine whether to package or maintain separate payment for the services in the future. Another commenter recommended that CMS adopt a threshold policy that would be similar to the existing policy used to identify packaged drugs, under which separate payment would be made for all services with a median cost in excess of a nominal threshold amount.
Response: We continue to believe that packaging creates incentives for hospitals and their physician partners to work together to establish appropriate protocols that eliminate unnecessary services where they exist and institutionalize approaches to providing necessary services more efficiently. With respect to new services or new applications of existing technology, we believe that packaging payment for ancillary and dependent services creates appropriate incentives for hospitals to seriously consider whether a new service or a new technology offers a benefit that is sufficient to justify the cost of the new service or technology. Where this review results in reductions in services that are only marginally beneficial or hospitals' choices not to utilize certain technologies, we believe that this could improve, rather than harm, the quality of care for Medicare beneficiaries because every service furnished in a hospital carries some level of risk to the patient. Moreover, we believe that hospitals strive to provide the best care they can to the patients they serve so that when new technologies are proven to improve the quality of care, their utilization will increase appropriately, whether the payment for them is packaged or not.
However, we are aware that there are financial pressures on hospitals that might motivate some providers to split services among different hospital encounters in such a way as to maximize payments. While we do not expect that hospitals would routinely change the way they furnish services or the way they bill for services in order Start Printed Page 60414 to maximize payment, we recognize that it would be possible and we consider that possibility as we annually review hospital claims data. We will to continue examine claims data for patterns of fragmented care, and if we find a pattern in which a hospital appears to be dividing care across multiple days, we will refer it for investigation to the QIO or to the program safeguard contractor, as appropriate to the circumstances we find.
In section II.A.1. of this final rule with comment period, we discuss the established methodology we use to incorporate the costs of packaged services into payment for the associated independent procedures. In response to those commenters with concerns about transparency of the ratesetting process that incorporates packaged costs, in general, we package the costs of services into the payment for the major separately paid procedure on the same claim on which the packaged service appears. Hence, it is the practice of hospitals with regard to reporting and charging for packaged services that determines the separately paid service into which the cost of a packaged service is incorporated and the amount of packaged cost included the payment for that separately paid procedure.
Regarding the recommendation that we establish a cost threshold that would guide the packaging of services, we do not agree that this approach would result in appropriate packaging of costs for dependent ancillary services. A threshold policy could create incentives for hospitals to increase charges to ensure that payment for certain services was made separately, and the result would be contrary to the creation of incentives for prudent assessment of the costs and benefits of these services. Furthermore, as we stated in the CY 2009 OPPS/ASC final with comment period (73 FR 68572), it is not clear whether one set of packaging principles or one threshold could apply to the wide variety of services under the OPPS. Finally, to adopt a policy that would only package services that are low cost ancillary and supportive services would essentially negate the concept of averaging that is an underlying premise of a prospective payment system because hospitals would not have a particular incentive to provide care more efficiently.
We believe it is important to continue to advance value-based purchasing by Medicare in the hospital outpatient setting by furthering the focus on value of care rather than volume. While we acknowledge the concerns of the commenters and, as discussed below, are committed to considering the impact of packaging payment on Medicare beneficiaries further in the future, we must balance the concerns of the commenters with our goal of continuing to encourage efficient use of hospital resources. As we noted in the CY 2009 OPPS/ASC final rule with comment period in our response to comments on the CY 2009 OPPS/ASC proposed rule (73 FR 68572) and as we note in our responses to public comments on the CY 2010 OPPS/ASC proposed rule, the suggestions and packaging criteria recommended by most commenters are focused almost exclusively on preventing packaging, rather than on determining when packaging would be appropriate. We also welcome suggestions from the public on approaches to packaging that would encourage efficient use of hospital resources.
Comment: Several commenters commended CMS for reviewing and accepting the APC Panel's February 2009 recommendation that CMS continue to analyze the impact of increased packaging on Medicare beneficiaries. The commenters expressed concern about CMS' current packaging policy and urged CMS to conduct a more detailed review of the hospital claims data in order to verify that current OPPS packaging policies and methodologies are accomplishing CMS' goals. A few commenters offered recommendations for additional data analyses for CMS to consider in the ongoing efforts to study the impact of increased packaging under the OPPS. The commenters recommended that CMS compare utilization of currently packaged services billed and paid separately under the OPPS in CY 2007, before the packaging of additional categories of services went into effect, to the frequency of those same services that were packaged in CY 2008 and later, after the packaging of additional categories of services went into effect. The commenters requested that CMS conduct these studies at the CPT code level. The commenters also recommended that CMS conduct a hospital-level review of the data, in addition to an overall review, and compare overall utilization by packaged HCPCS code for CYs 2005 and 2006 to CYs 2007, 2008, and 2009. Another commenter, in support of a provider-level review of the data, asserted that reviewing the data for packaged services at a national aggregate level can easily mask the behavioral changes of classes of hospitals and, therefore, concluded that more detailed analysis is needed to determine the impact of the policy. Several commenters requested that CMS present its analyses in the final rule with comment period and at upcoming APC Panel meetings and consult with relevant stakeholders before proposing any additional packaging changes. The commenters also recommended that CMS make the data underlying payments for packaged services, including utilization rates and median costs, publicly available to enhance the transparency of its decision making so that stakeholders could assess whether the payment rates truly reflect the costs of providing the bundle of services.
Response: We agree that it is important to examine our claims data to assess the impact of packaging to the extent we can do so. During the September 2007 APC Panel meeting, the APC Panel requested that CMS evaluate the impact of expanded packaging on Medicare beneficiaries. At the March 2008 APC Panel meeting, the APC Panel requested that CMS report to the APC Panel at the first meeting in CY 2009 regarding the impact of packaging on net payments for patient care. In response to these requests, we shared the first available CY 2008 claims data with the APC Panel at the February 2009 APC Panel meeting. In that analysis, we compared the frequency of specific categories of services we newly packaged for CY 2008 as they were billed under the OPPS in CY 2007, before expanded packaging went into effect, to the frequency of those same categories of services in CY 2008, their first year of packaged payment. In each category, the HCPCS codes that we compared are the ones that we identified in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66659 through 66664) as fitting into one of the seven packaging categories listed in section II.A.4.a. of this final rule with comment period. The data shared with the APC Panel at the February 2009 APC Panel meeting compared CY 2007 claims processed through September 30, 2007, to CY 2008 claims processed through September 30, 2008, and represented about 60 percent of the full year data. We did not make any adjustments for inflation, changes in Medicare population, or other variables that potentially influenced billing between CY 2007 and CY 2008. A summary of these data analyses was included in the CY 2010 OPPS/ASC proposed rule (74 FR 35287 through 35289) and is reiterated above.
We note that we plan to present subsequent analyses that compare CY 2007 claims processed through September 30, 2007, to CY 2008 claims processed through September 30, 2008, and to CY 2009 claims processed Start Printed Page 60415 through September 30, 2009, to the APC panel at the APC Panel's winter 2010 meeting. We do not anticipate providing analyses using claims for services furnished during CY 2005 or CY 2006 because the packaging of the seven categories of services was effective for services furnished on and after January 1, 2008, and, therefore, we view CY 2007, the year immediately preceding the year that the packaging expansion went into effect, to be the base year for our comparisons. In addition, we do not anticipate providing the analyses at a provider-specific level or at a HCPCS code level. It is not clear to us how we would be able to use an analysis at the provider-specific level or the HCPCS code level or what value such an analysis would have in the context of national packaging policies for the OPPS.
We note that we make available a considerable amount of data for public analysis each year through the supporting data files that are posted on the CMS Web site in association with the display of the proposed and final rules. In addition, we make available the public use files of claims and a detailed narrative description of our data process for the annual OPPS/ASC proposed and final rules that the public can use to perform any desired analyses. Therefore, commenters are able to examine and analyze these data to develop specific information to support their requests for changes to payments under the OPPS, whether with regard to separate payment for a packaged service or other issues. We understand that the OPPS is a complex payment system and that it may be difficult to determine the quantitative amount of packaged cost included in the median cost for every independent service. However, based on the complex and detailed public comments on prior proposed rules that we have received, some commenters have performed meaningful analyses at a detailed and service-specific level based on the public claims data available.
With regard to the commenters' request that we not expand OPPS packaging until after we have produced data on the impact of packaging policy changes and consulted with stakeholders, we note that we establish all significant OPPS payment policies, including the packaging status of each HCPCS code, through the annual rulemaking process. Integral to this process is a detailed explanation of the claims data on which we base our proposals and the availability of the claims from which we develop those data for the use of the public to perform any level of analysis they choose. Moreover, the OPPS/ASC annual rulemaking process provides a 60-day public comment period, as well as public presentations and discussion of the proposals at the summer APC Panel meeting. We also reply to all public comments that are within the scope of the OPPS/ASC proposed rule when we issue the OPPS/ASC final rule with comment period. In addition, we regularly meet with parties throughout the year who want to share their views on topics of interest to them. All of these activities and discussions provide significant information and opportunities for the public to influence and inform policy changes that we may be considering.
Comment: Some commenters expressed concern about the impact that packaging payment for services described by separate HCPCS codes could have on the submission of claims data by hospitals for those packaged codes and, therefore, with the validity of conclusions that could be drawn from impact analyses performed by CMS. One commenter questioned CMS' assumption that the OPPS packaging policies would allow continued collection of the data necessary to set appropriate, stable payment rates in the future. The commenter believed that greater packaging may eliminate hospitals' incentive to code for items and services for which separate payment is not made. The commenter further argued that CMS' past experiences with packaging payment for ancillary items and services indicate that hospitals do not report HCPCS codes for items and services that do not directly affect hospital payment. Similarly, the commenter explained that, under the IPPS, hospitals report only the data required to assign a case to the highest paying appropriate diagnosis-related group (DRG), even though other data might affect payment in the long term. The commenter saw no reason to believe that the current OPPS packaging approach would have a different outcome unless CMS gives clear instructions that hospitals should continue coding for all items and services used in the care of patients and provides an incentive to report packaged items and services.
Several commenters argued that the costs of new services are not reflected in the historical claims data CMS uses to set payment rates. The commenters believed that if CMS were to package payment for a new imaging service under the same criteria proposed for many existing imaging services, not only would CMS have no basis for determining how much the new service costs in its first 2 years of availability, but also CMS would provide no incentive to hospitals to report codes and charges for the new service for use in future OPPS ratesetting. The commenters further asserted that the resulting incomplete data would lead to inappropriate payments for independent services that, in turn, would limit access to care and would discourage continued innovation to improve patient care.
Response: We do not believe that there will be a significant change in what hospitals report and charge for the outpatient services they furnish to Medicare beneficiaries and other patients as a result of our current packaging methodology. Medicare cost reporting standards specify that hospitals must impose the same charges for Medicare patients as for other patients. We are often told by hospitals that many private payers pay based on a percentage of charges and that, in accordance with Medicare cost reporting rules and generally accepted accounting principles, hospital chargemasters do not differentiate between the charges to Medicare patients and other patients. Therefore, we have no reason to believe that hospitals will stop reporting HCPCS codes and charges for packaged services they provide to Medicare beneficiaries. As we stated in the CY 2009 OPPS/ASC final rule with comment period (74 FR 68575), we strongly encourage hospitals to report a charge for each packaged service they furnish, either by billing the packaged HCPCS code and a charge for that service if separate reporting is consistent with CPT and CMS instructions, by increasing the charge for the separately paid associated service to include the charge for the packaged service, or by reporting the charge for the packaged service with an appropriate revenue code but without a HCPCS code. Any of these means of charging for the packaged service will result in the cost of the packaged service being incorporated into the cost we estimate for the separately paid service. If a HCPCS code is not reported when a packaged service is provided, we acknowledge that it can be challenging to specifically track the utilization patterns and resource cost of the packaged service itself. However, we have no reason to believe that hospitals have not considered the cost of the packaged service in reporting charges for the independent, separately paid service.
We expect that hospitals, as other prudent businesses, have a quality review process that ensures that they accurately and completely report the services they furnish, with appropriate charges for those services to Medicare Start Printed Page 60416 and all other payers. We encourage hospitals to report all HCPCS codes that describe packaged services that were furnished, unless the CPT Editorial Panel or CMS provides other guidance. To the extent that hospitals include separate charges for packaged services on their claims, the estimated costs of those packaged services are then added to the costs of separately paid procedures on the same claims and used in establishing payment rates for the separately paid services.
Comment: One commenter argued that CMS' packaging methodology for guidance services used in radiation oncology procedures is not transparent. Specifically, the commenter claimed that CMS packaged payment for the radiation oncology image-guidance services (shown in Table 15) into the payment for independent radiation therapy services (shown in Table 16) without publishing its packaging methodology. The commenter further stated that the lack of transparency regarding CMS' packaging methodology is of concern to the radiation oncology community, and that it would be helpful if CMS published the information used in the APC Panel's determination of packaging and payment rates.
| CY 2010 CPT code | CY 2010 Long descriptor |
|---|---|
| 77421 | Stereoscopic X ray guidance for localization of target volume for the delivery of radiation therapy. |
| 77014 | Computed tomography guidance for placement of radiation fields. |
| 77417 | Therapeutic radiology port film(s). |
| 76950 | Ultrasonic guidance for aspiration of ova, imaging supervision and interpretation. |
| CY 2010 CPT code | CY 2010 Long descriptor |
|---|---|
| 77402 | Radiation treatment delivery, single treatment area, single port or parallel opposed ports, simple blocks or no blocks; up to 5MeV. |
| 77403 | Radiation treatment delivery, single treatment area, single port or parallel opposed ports, simple blocks or no blocks; 6–10MeV. |
| 77404 | Radiation treatment delivery, single treatment area, single port or parallel opposed ports, simple blocks or no blocks; 11–19 MeV. |
| 77406 | Radiation treatment delivery, single treatment area, single port or parallel opposed ports, simple blocks or no blocks; 20 MeV or greater. |
| 77407 | Radiation treatment delivery, 2 separate treatment areas, 3 or more ports on a single treatment area, use of multiple blocks; up to 5 MeV. |
| 77408 | Radiation treatment delivery, 2 separate treatment areas, 3 or more ports on a single treatment area, use of multiple blocks; 6–10 MeV. |
| 77409 | Radiation treatment delivery, 2 separate treatment areas, 3 or more ports on a single treatment area, use of multiple blocks; 11–19 MeV. |
| 77411 | Radiation treatment delivery, 2 separate treatment areas, 3 or more ports on a single treatment area, use of multiple blocks; 20 MeV or greater. |
| 77412 | Radiation treatment delivery, 3 or more separate treatment areas, custom blocking, tangential ports, wedges, rotational beam, compensators, electron beam; up to 5 MeV. |
| 77413 | Radiation treatment delivery, 3 or more separate treatment areas, custom blocking, tangential ports, wedges, rotational beam, compensators, electron beam; 6–10 MeV. |
| 77414 | Radiation treatment delivery, 3 or more separate treatment areas, custom blocking, tangential ports, wedges, rotational beam, compensators, electron beam; 11–19 MeV. |
| 77416 | Radiation treatment delivery, 3 or more separate treatment areas, custom blocking, tangential ports, wedges, rotational beam, compensators, electron beam; 20 MeV or greater. |
| 77417 | Therapeutic radiology port film(s). |
| 77418 | Intensity modulated treatment delivery, single or multiple fields/arcs, via narrow spatially and temporally modulated beams, binary, dynamic MLC, per treatment session. |
Response: Although the APC Panel provides valuable advice with regard to the establishment of OPPS payment policies and payment rates, the APC Panel does not, as the commenter suggested, determine what services are packaged under the OPPS or establish OPPS payment rates. We adopt the OPPS payment policies regarding packaging and other issues and establish payment rates through the annual rulemaking cycle.
In general, payment for a packaged HCPCS code is included in the payment for the independent service with which it is associated, to the extent that the cost of the packaged service is reflected on the single procedure claims that are used to calculate the median cost for the independent, separately paid service. We intend to further examine the packaging of image-guidance for radiation therapy in the analyses of the impact of packaging that we plan to discuss with the APC Panel at the winter 2010 meeting. However, as we describe earlier in this section, we make available a considerable amount of data for public analysis each year, provide the claims we use to calculate median costs, and provide a detailed narrative description of our data process that the public can use to analyze any topic of interest to them.
Comment: One commenter supported CMS' goal of increased efficiency in hospital outpatient care. However, the commenter was concerned that packaging payment for services too soon could create access problems for technologies that would otherwise improve patient outcomes and reduce costs. The commenter urged CMS to reinstate separate payment in CY 2010 for ICE, FFR, and IVUS until a thorough analysis has been performed on the impact of packaging payment for these services, including the rate of change in their utilization over time and market penetration. Start Printed Page 60417
Response: As discussed earlier in this section, in response to the request from the APC Panel that CMS evaluate the impact of expanded packaging on Medicare beneficiaries, we analyzed 9 months of CY 2007 and CY 2008 data related to all services that were packaged during CY 2008. Analysis of the intraoperative category (which includes IVUS, ICE, and FFR) showed minimal changes in the frequency and the number of hospitals reporting these packaged services between CY 2007 and CY 2008. The IVUS, ICE, and FFR services studied specifically included CPT codes 37250 (Intravascular ultrasound (non-coronary vessel) during diagnostic evaluation and/or therapeutic intervention; initial vessel); 37251 (Intravascular ultrasound (non-coronary vessel) during diagnostic evaluation and/or therapeutic intervention; each additional vessel); 92978 (Intravascular ultrasound (coronary vessel or graft) during diagnostic evaluation and/or therapeutic intervention including imaging supervision, interpretation and report; initial vessel); 92979 (Intravascular ultrasound (coronary vessel or graft) during diagnostic evaluation and/or therapeutic intervention including imaging supervision, interpretation and report; each additional vessel); 93662 (Intracardiac echocardiography during therapeutic/diagnostic intervention, including imaging supervision and interpretation); 93571 (Intravascular Doppler velocity and/or pressure derived coronary flow reserve measurement (coronary vessel or graft) during coronary angiography including pharmacologically induced stress, initial vessel); and 93572 (Intravascular Doppler velocity and/or pressure derived coronary flow reserve measurement (coronary vessel or graft) during coronary angiography including pharmacologically induced stress, each additional vessel).
As discussed previously, in February 2009 we presented an analysis to the APC Panel that showed an increase of 8 percent in the number of services billed and an increase in aggregate payment of 25 percent in CY 2008, when IVUS, ICE and FFR were packaged, in comparison to CY 2007 when IVUS, ICE and FFR were paid separately. Additionally, we intend to continue our analysis of the impact of greater packaging on Medicare beneficiaries and to present additional data to the APC Panel at the winter 2010 meeting.
We note that IVUS, ICE, and FFR services are existing, established technologies and that hospitals have provided some of these services in the HOPD since the implementation of the OPPS in CY 2000. IVUS, FFR, and ICE are all dependent services that are always provided in association with independent services. Given the increase in the number of services furnished and the associated payment between CY 2007 and CY 2008, we have seen no evidence from our claims data that beneficiary access to care is being harmed by packaging payment for IVUS, ICE, and FFR services. We believe that packaging creates appropriate incentives for hospitals and their physician partners to carefully consider the technologies that are used in the care of patients, in order to ensure that technologies are selected for use in each case based on their expected benefit to a particular Medicare beneficiary.
After consideration of the public comments we received, we are finalizing our CY 2010 proposals, without modification, to packaged payment for the seven categories of services, including guidance services, image processing services, intraoperative services, imaging supervision and interpretation services, diagnostic radiopharmaceuticals, contrast media, and observation services. We refer readers to section V.B.2.d. of this final rule with comment period for further discussion of our final policy to package payment for contrast agents and diagnostic radiopharmaceuticals. We refer readers to section II.A.2.e.(1) for further discussion of our final policy to pay for observation services through extended assessment and management composite APCs under certain circumstances. We plan to discuss with the APC Panel additional analyses of the impact of packaging these categories of services at the winter 2010 APC Panel meeting.
(3) Other Service-Specific Packaging Issues
The APC Panel also recommended that CMS reassign CPT code 76098 (Radiological examination, surgical specimen) from APC 0317 (Level II Miscellaneous Radiology Procedures) to APC 0260 (Level I Plain Film), and to place CPT code 76098 on the bypass list. Based on our analysis of the CY 2010 claims containing CPT 76098 and clinical review of the services being furnished, in the CY 2010 OPPS/ASC proposed rule (74 FR 35241), we proposed to treat CPT code 76098 as a “T- packaged” code for CY 2010 with continued assignment to APC 0317. As discussed above, a “T-packaged code,” identified with status indicator “Q2,” describes a code whose payment is packaged when one or more separately paid surgical procedures with a status indicator of “T” are provided during the hospital encounter. The assignment of status indicator “Q2” to CPT code 76098 would result in more claims data being available to set the median costs for the surgical procedures with which CPT code 76098 is most commonly billed (for example, CPT code 19101 (Biopsy of breast, percutaneous, needle core, not using image guidance; open incisional)), while continuing to provide appropriate separate payment that reflects the costs of the service, including its packaged costs, when it is not billed with a surgical procedure. Further discussion related to the proposal is included in section II.A.1.b. of this final rule with comment period.
Comment: One commenter requested that CPT code 76098 remain separately payable instead of conditionally packaged. The commenter acknowledged that radiological examination of a surgical specimen is performed in conjunction with a surgical procedure most of the time but asserted that when the service is conditionally packaged, surgical procedure payment would not cover the cost of the radiological examination of a surgical specimen.
Response: We continue to believe that when CPT code 76098 is furnished on the same date of service as a major separately payable procedure, CPT code 76098 is a dependent service that is ancillary and supportive to the independent service with which it is performed and that, therefore, it is most appropriate to package the cost of CPT code 76098 into the payment for the independent, separately paid procedure. The full cost of CPT code 76098 is packaged into the cost of the independent, separately paid procedure to the extent that the hospital's charge for the packaged service, when reduced to cost by the hospital's applicable CCR, results in an accurate reflection of the cost of the packaged service. As we stated in the CY 2009 OPPS/ASC final rule with comment period (74 FR 68575), we strongly encourage hospitals to report a charge for each packaged service they furnish, either by billing the packaged HCPCS code and a charge for that service if separate reporting is consistent with CPT and CMS instructions, by increasing the charge for the separately paid associated service to include the charge for the packaged service, or by reporting the charge for the packaged service with an appropriate revenue code but without a HCPCS code. Any of these means of charging for the packaged service will result in the costs of the packaged service being incorporated into the cost we estimate for the separately paid Start Printed Page 60418 service. We note that further discussion of CPT code 76098 as it relates to the commenters' requests to add this code to the bypass list is included in section II.A.1.b. of this final rule with comment period.
After consideration of the public comment we received, we are finalizing our CY 2010 proposal, without modification, to assign CPT code 76098 status indicator “Q2” to signify that the service is packaged when it is reported with a separately paid procedure that has a status indicator of “T” on the same date of service and separately paid under APC 0317 when it is not reported on the same date of service with a separately paid surgical procedure that has a status indicator of “T.” The final CY 2010 APC median cost of APC 0317 is approximately $374.
Comment: A number of commenters disagreed with CMS' proposal to package electrodiagnostic guidance for chemodenervation procedures. The commenters asserted that paying chemodenervation procedures at the same rate, regardless of the use of electrodiagnostic guidance, may discourage providers from using guidance to place a needle filled with a potentially fatal substance like botulinum toxin. They urged CMS to consider providing a separate payment for electrodiagnostic needle guidance to ensure that quality of care is not compromised.
Response: While the commenters did not identify specific chemodenervation guidance CPT codes, we note that the costs of chemodenervation guidance services, specifically CPT codes 95873 (Electrical stimulation for guidance in conjunction with chemodenervation (List separately in addition to code for primary procedure)) and 95874 (Needle electromyography for guidance in conjunction with chemodenervation (list separately in addition to code for primary procedure)) are reflected in the median costs of the independent, separately paid chemodenervation procedures as a function of the frequency that chemodenervation services are reported with a particular guidance CPT code. We recognize that, in some cases, supportive and ancillary dependent services are furnished at a high frequency with independent services, and in other cases, they are furnished with independent services at a low frequency. Nonetheless, we believe that packaging should reflect the reality of how these services are furnished. Moreover, we believe that hospitals make prudent and appropriate patient care decisions with regard to when they furnish packaged services.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to unconditionally package payment for chemodenervation guidance services described by CPT codes 95873 and 95874. These CPT codes are, therefore, assigned status indicator “N” in Addendum B to this final rule with comment period.
Comment: One commenter objected to the assignment of status indicator “N” to a number of guidance procedures and requested that CMS conditionally packaged these services so that they could be paid separately if they are the only services furnished in a hospital encounter. The commenter believed that it is not appropriate that the hospital receives no payment when these services are furnished in preparation for a surgical procedure that is canceled after the services have been furnished but before the patient is taken to the operating room. The procedures of concern to the commenter include CPT codes 19290 (Preoperative placement of needle localization wire, breast;); 19291 (Preoperative placement of needle localization wire, breast; each additional lesion (List separately in addition to code for primary procedure)); 19295 (Image guided placement, metallic localization clip, percutaneous, during breast biopsy (List separately in addition to code for primary procedure)); 77031 (Stereotactic localization guidance for breast biopsy or needle placement (e.g., for wire localization or for injection), each lesion, radiological supervision and interpretation)); 77032 (Mammographic guidance for needle placement, breast (e.g., for wire localization or for injection), each lesion, radiological supervision and interpretation); and 76942 (Ultrasonic guidance for needle placement (e.g., biopsy, aspiration, injection, localization device), imaging supervision and interpretation).
Response: We appreciate the commenter's submission of this clinical scenario for our review. The APC Panel Packaging Subcommittee provides substantive advice to us on the packaging of services, either conditionally or unconditionally under the OPPS, and the Subcommittee members bring broad and deep expertise and experience to their review of clinical scenarios. Therefore, we will review these services and the scenario described by the commenter with the APC Panel's Packaging Subcommittee at the winter 2010 APC Panel meeting.
After review of the public comment we received, we are finalizing our CY 2010 proposal, without modification, to unconditionally package payment for CPT codes 19290, 19291, 19295, 77031, 77032, and 76942. These CPT codes are assigned status indicator “N” in Addendum B to this final rule with comment period. We will review the OPPS treatment of these CPT codes with the APC Panel Packaging Subcommittee at the winter 2010 APC Panel meeting.
Comment: One commenter suggested that it is very challenging for hospitals to determine they were paid correctly for services furnished because of CMS' “Q” status indicators and the complexity of determining which HCPCS codes should be separately paid. The commenter asked that CMS make the claims processing system more transparent.
Response: We acknowledge that the OPPS is a complex payment system and that it is difficult to determine the correct payment for a service that is subject to conditional packaging. Addendum D1 to this final rule with comment period describes how services that appear in Addendum B with status indicators “Q1,” “Q2,” and “Q3” are treated in claims processing. In the case of conditionally packaged codes with status indicators “Q1” or “Q2,” where the criteria for separate payment are not met, these codes are treated as packaged services. We assign status indicators “Q1” and “Q2” to conditionally packaged services to indicate that they are usually packaged, except for special circumstances when they are separately payable. Through the I/OCE claims processing logic, the status indicator of a conditionally packaged service reported on a claim is changed either to “N” or the status indicator of the APC to which the code is assigned for separate payment, depending upon the presence or absence of other OPPS services also reported on the claim with the same date of service. Status indicator “Q3” indicates that the code is a member of a composite APC. Addendum M includes the HCPCS codes for all services that are paid either through single code APCs or composite APCs when the criteria for composite APC payment are met. A full discussion of the composite criteria for each composite APC (to which status indicator “Q3” applies) is included in section II.A.2.e. of this final rule with comment period.
In addition to the availability of these resources that describe whether a service is separately payable or packaged (in the case of services with status indicators “Q1” or “Q2”) or a member of a composite APC (in the case of services with status indicator “Q3”), the quarterly I/OCE and the OPPS Pricer that are used by the Fiscal Intermediary Start Printed Page 60419 Standard System (FISS) to process claims paid under the OPPS are both available to the public each calendar quarter. The I/OCE instructions and specifications that are utilized for OPPS and non-OPPS payment for hospital outpatient services are available quarterly for download on the CMS Web site at: http://www.cms.hhs.gov/OutpatientCodeEdit/02_OCEQtrReleaseSpecs.asp#TopOfPage. Providers interested in purchasing the I/OCE software should visit the authorized distributor's Web site at http://www.ntis.gov/products/oceapc.aspx for more information on how to obtain the software. There is no OPPS Pricer application for personal computers at this time. However, providers can download the files that contain the logic, rates, wage indices, and off-set amounts used by the OPPS Pricer program to calculate APC rates, copayments, and deductibles from the CMS Web site at: http://www.cms.hhs.gov/PCPricer/08_OPPS.asp.
B. Conversion Factor Update
Section 1833(t)(3)(C)(ii) of the Act requires us to update the conversion factor used to determine payment rates under the OPPS on an annual basis. Under the authority in section 1833(t)(3)(C)(iv) of the Act, for CY 2010, the update is equal to the hospital inpatient market basket percentage increase applicable to hospital discharges under section 1886(b)(3)(B)(iii) of the Act. The final hospital market basket increase for FY 2010 published in the FY 2010 IPPS/LTCH PPS final rule (74 FR 44002) is 2.1 percent. To set the OPPS conversion factor for CY 2010, we increased the CY 2009 conversion factor of $66.059, as specified in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68584 through 68585), by 2.1 percent. Hospitals that fail to meet the reporting requirements of the Hospital Outpatient Quality Data Reporting Program (HOP QDRP) are subject to a reduction of 2.0 percentage points from the market basket update to the conversion factor. For a complete discussion of the HOP QDRP requirements and the payment reduction for hospitals that fail to meet those requirements, we refer readers to section XVI. of this final rule with comment period.
In accordance with section 1833(t)(9)(B) of the Act, we further adjusted the conversion factor for CY 2010 to ensure that any revisions we made to our updates for a revised wage index and rural adjustment are made on a budget neutral basis. We calculated an overall budget neutrality factor of 0.9997 for wage index changes by comparing total payments from our simulation model using the FY 2010 IPPS final wage index values to those payments using the current (FY 2009) IPPS wage index values. For CY 2010, we did not propose a change to our rural adjustment policy. Therefore, the budget neutrality factor for the rural adjustment is 1.0000.
For this final rule with comment period, we estimated that pass-through spending for both drugs and biologicals and devices for CY 2010 will equal approximately $45.5 million, which represents 0.14 percent of total projected CY 2010 OPPS spending. Therefore, the conversion factor was also adjusted by the difference between the 0.11 percent estimate of pass-through spending set aside for CY 2009 and the 0.14 percent estimate of CY 2010 pass-through spending. Finally, estimated payments for outliers remain at 1.0 percent of total OPPS payments for CY 2010.
The market basket increase update factor of 2.1 percent for CY 2010, the required wage index budget neutrality adjustment of approximately 0.9997, and the adjustment of 0.03 percent of projected OPPS spending for the difference in the pass-through spending set aside resulted in a full market basket conversion factor for CY 2010 of $67.406. To calculate the CY 2010 reduced market basket conversion factor for those hospitals that fail to meet the requirements of the HOP QDRP for the full CY 2010 payment update, we made all other adjustments discussed above, but used a reduced market basket increase update factor of 0.1 percent. This resulted in a reduced market basket conversion factor for CY 2010 of $66.086 for those hospitals that fail to meet the HOP QDRP requirements.
We did not receive any public comments on the calculation of the conversion factor. Therefore, we are finalizing our CY 2010 proposal, without modification, to update the OPPS conversion factor by the FY 2010 IPPS market basket increase update factor of 2.1 percent, resulting in a final full conversion factor of $67.406 and in a reduced conversion factor of $66.086 for those hospitals that fail to meet the HOP QDRP reporting requirements for the full CY 2010 payment update.
C. Wage Index Changes
Section 1833(t)(2)(D) of the Act requires the Secretary to determine a wage adjustment factor to adjust, for geographic wage differences, the portion of the OPPS payment rate, which includes the copayment standardized amount, that is attributable to labor and labor-related cost. This adjustment must be made in a budget neutral manner and budget neutrality is discussed in section II.B. of this final rule with comment period.
The OPPS labor-related share is 60 percent of the national OPPS payment. This labor-related share is based on a regression analysis that determined that approximately 60 percent of the costs of services paid under the OPPS were attributable to wage costs. We confirmed that this labor-related share for outpatient services is still appropriate during our regression analysis for the payment adjustment for rural hospitals in the CY 2006 OPPS final rule with comment period (70 FR 68553). Therefore, in the CY 2010 OPPS/ASC proposed rule (74 FR 35291), we did not propose to revise this policy for the CY 2010 OPPS. We refer readers to section II.G. of this final rule with comment period for a description and example of how the wage index for a particular hospital is used to determine the payment for the hospital.
As discussed in section II.A.2.c. of this final rule with comment period, for estimating national median APC costs, we standardize 60 percent of estimated claims costs for geographic area wage variation using the same FY 2010 pre-reclassified wage indices that the IPPS uses to standardize costs. This standardization process removes the effects of differences in area wage levels from the determination of a national unadjusted OPPS payment rate and the copayment amount.
As published in the original OPPS April 7, 2000 final rule with comment period (65 FR 18545), the OPPS has consistently adopted the final IPPS wage indices as the wage indices for adjusting the OPPS standard payment amounts for labor market differences. Thus, the wage index that applies to a particular acute care short-stay hospital under the IPPS also applies to that hospital under the OPPS. As initially explained in the September 8, 1998 OPPS proposed rule, we believed and continue to believe that using the IPPS wage index as the source of an adjustment factor for the OPPS is reasonable and logical, given the inseparable, subordinate status of the HOPD within the hospital overall. In accordance with section 1886(d)(3)(E) of the Act, the IPPS wage index is updated annually. Therefore, in accordance with our established policy, we proposed to use the final FY 2010 version of the IPPS wage indices used to pay IPPS hospitals to adjust the CY 2010 OPPS payment rates and copayment amounts for geographic differences in labor cost for all providers that participate in the Start Printed Page 60420 OPPS, including providers that are not paid under the IPPS (referred to in this section as “non-IPPS” providers).
We note that the final FY 2010 IPPS wage indices continue to reflect a number of adjustments implemented over the past few years, including revised Office of Management and Budget (OMB) standards for defining geographic statistical areas (Core-Based Statistical Areas or CBSAs), reclassification to different geographic areas, rural floor provisions and the accompanying budget neutrality adjustment, an adjustment for out-migration labor patterns, an adjustment for occupational mix, and a policy for allocating hourly wage data among campuses of multicampus hospital systems that cross CBSAs. For the FY 2010 wage indices, these changes include a continuing transition to the new reclassification threshold criteria that were finalized in the FY 2009 IPPS final rule (73 FR 48568 through 48570), updated 2007–2008 occupational mix survey data, and a continuing transition to state-level budget neutrality for the rural and imputed floors. We refer readers to the FY 2010 IPPS/LTCH PPS final rule (74 FR 43823) for a detailed discussion of all final changes to the FY 2010 IPPS wage indices. In addition, we refer readers to the CY 2005 OPPS final rule with comment period (69 FR 65842 through 65844) and subsequent OPPS rules for a detailed discussion of the history of these wage index adjustments as applied under the OPPS.
The IPPS wage indices that we proposed to adopt in the CY 2010 OPPS/ASC proposed rule (74 FR 35291) include all reclassifications that are approved by the Medicare Geographic Classification Review Board (MGCRB) for FY 2010.
As noted in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68585), after issuance of the CY 2009 OPPS/ASC proposed rule, section 124 of Public Law 110–275 further extended geographic reclassifications under section 508 and certain special exception reclassifications until September 30, 2009. We did not make any proposals related to these provisions for the CY 2009 OPPS wage indices in our CY 2009 proposed rule because Public Law 110–275 was enacted after issuance of the CY 2009 OPPS/ASC proposed rule. In accordance with section 124 of Public Law 110–275, for CY 2009, we adopted all section 508 geographic reclassifications through September 30, 2009. Similar to our treatment of section 508 reclassifications extended under Public Law 110–173 as described in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68586), hospitals with section 508 reclassifications revert to their home area wage index, with out-migration adjustment if applicable, or a current MGCRB reclassification, from October 1, 2009 to December 31, 2009. As we did for CY 2008, we also have extended the special exception wage indices for certain hospitals through December 31, 2009, under the OPPS, in order to give these hospitals the special exception wage indices under the OPPS for the same time period as under the IPPS. We refer readers to the Federal Register notice published subsequent to the FY 2009 IPPS final rule for a detailed discussion of the changes to the wage indices as required by section 124 of Public Law 110–275 (73 FR 57888). Because the provisions of section 124 of Public Law 110–275 expire in 2009 and are not applicable to FY 2010, we did not make any proposals related to those provisions for the OPPS wage indices for CY 2010.
For purposes of the OPPS, we proposed to continue our policy in CY 2010 to allow non-IPPS hospitals paid under the OPPS to qualify for the out-migration adjustment if they are located in a section 505 out-migration county. We note that because non-IPPS hospitals cannot reclassify, they are eligible for the out-migration wage adjustment. Table 4J in the FY 2010 IPPS final rule (74 FR 44118 through 44125), as subsequently corrected at 74 FR 51506, identifies counties eligible for the out-migration adjustment and providers receiving the adjustment. As we have done in prior years, we are reprinting Table 4J, as corrected, as Addendum L to this final rule with comment period, with the addition of non-IPPS hospitals that will receive the section 505 out-migration adjustment under the CY 2010 OPPS.
As stated earlier in this section, we continue to believe that using the IPPS wage indices as the source of an adjustment factor for the OPPS is reasonable and logical, given the inseparable, subordinate status of the HOPD within the hospital overall. Therefore, we proposed to use the final FY 2010 IPPS wage indices for calculating the OPPS payments in CY 2010. With the exception of the out-migration wage adjustment table (Addendum L to this final rule with comment period), which includes non-IPPS hospitals paid under the OPPS, we are not reprinting the FY 2010 IPPS final wage indices referenced in this discussion of the wage index. We refer readers to the CMS Web site for the OPPS at: http://www.cms.hhs.gov/HospitalOutpatientPPS/. At this link, readers will find a link to the FY 2010 IPPS final wage index tables.
Comment: Several commenters expressed support for the CMS proposal to extend the IPPS wage indices to the OPPS in CY 2010, consistent with prior year policies under the OPPS.
Response: We appreciate the support expressed by commenters for our proposed CY 2010 wage index policies.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to use the final FY 2010 IPPS wage indices to adjust the OPPS standard payment amounts for labor market differences.
D. Statewide Average Default CCRs
In addition to using CCRs to estimate costs from charges on claims for ratesetting, CMS uses overall hospital-specific CCRs calculated from the hospital's most recent cost report to determine outlier payments, payments for pass-through devices, and monthly interim transitional corridor payments under the OPPS during the PPS year. Medicare contractors cannot calculate a CCR for some hospitals because there is no cost report available. For these hospitals, CMS uses the statewide average default CCRs to determine the payments mentioned above until a hospital's Medicare contractor is able to calculate the hospital's actual CCR from its most recently submitted Medicare cost report. These hospitals include, but are not limited to, hospitals that are new, have not accepted assignment of an existing hospital's provider agreement, and have not yet submitted a cost report. CMS also uses the statewide average default CCRs to determine payments for hospitals that appear to have a biased CCR (that is, the CCR falls outside the predetermined ceiling threshold for a valid CCR) or for hospitals whose most recent cost report reflects an all-inclusive rate status (Medicare Claims Processing Manual, Pub. 100–04, Chapter 4, Section 10.11). In the CY 2010 OPPS/ASC proposed rule (74 FR 35292), we proposed to update the default ratios for CY 2010 using the most recent cost report data. We discuss our policy for using default CCRs, including setting the ceiling threshold for a valid CCR, in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68594 through 68599) in the context of our adoption of an outlier reconciliation policy for cost reports beginning on or after January 1, 2009.
For CY 2010, we used our standard methodology of calculating the statewide average default CCRs using the same hospital overall CCRs that we Start Printed Page 60421 use to adjust charges to costs on claims data for setting the CY 2010 proposed OPPS relative weights. Table 12 that was published in the CY 2010 OPPS/ASC proposed rule (74 FR 35293 through 35294) listed the proposed CY 2010 default urban and rural CCRs by State and compared them to last year's default CCRs. These CCRs are the ratio of total costs to total charges from each hospital's most recently submitted cost report, for those cost centers relevant to outpatient services weighted by Medicare Part B charges. We also adjusted ratios from submitted cost reports to reflect final settled status by applying the differential between settled to submitted overall CCR for the cost centers relevant to outpatient services from the most recent pair of final settled and submitted cost reports. We then weighted each hospital's CCR by the volume of separately paid line-items on hospital claims corresponding to the year of the majority of cost reports used to calculate the overall CCRs. We refer readers to the CY 2008 OPPS/ASC final rule with comment period (72 FR 66680 through 66682) and prior OPPS rules for a more detailed discussion of our established methodology for calculating the statewide average default CCRs, including the hospitals used in our calculations and our trimming criteria.
For this CY 2010 OPPS/ASC final rule with comment period, approximately 44 percent of the submitted cost reports utilized in the default ratio calculations represented data for cost reporting periods ending in CY 2008 and 55 percent were for cost reporting periods ending in CY 2007. For Maryland, we used an overall weighted average CCR for all hospitals in the nation as a substitute for Maryland CCRs. Few hospitals in Maryland are eligible to receive payment under the OPPS, which limits the data available to calculate an accurate and representative CCR. In general, observed changes in the statewide average default CCRs between CY 2009 and CY 2010 are modest and the few significant changes are associated with areas that have a small number of hospitals.
We did not receive any public comments concerning our CY 2010 proposal to apply our standard methodology of calculating the statewide average default CCRs using the same hospital overall CCRs that we use to adjust charges to costs on claims data for setting the CY 2010 proposed OPPS relative weights. Therefore, we are finalizing the statewide average default CCRs as shown in Table 17 below for OPPS services furnished on or after January 1, 2010.
Start Printed Page 60422 Start Printed Page 60423 Start Printed Page 60424E. OPPS Payment to Certain Rural and Other Hospitals
1. Hold Harmless Transitional Payment Changes Made by Public Law 110–275 (MIPPA)
When the OPPS was implemented, every provider was eligible to receive an additional payment adjustment (called either transitional corridor payments or transitional outpatient payment (TOPs)) if the payments it received for covered OPD services under the OPPS were less than the payments it would have received for the same services under the Start Printed Page 60425 prior reasonable cost-based system (referred to as the pre-BBA amount). Section 1833(t)(7) of the Act provides that the transitional corridor payments are temporary payments for most providers and were intended to ease their transition from the prior reasonable cost-based payment system to the OPPS system. There are two exceptions to this provision, cancer hospitals and children's hospitals, and those hospitals receive the transitional corridor payments on a permanent basis. Section 1833(t)(7)(D)(i) of the Act originally provided for transitional corridor payments to rural hospitals with 100 or fewer beds for covered OPD services furnished before January 1, 2004. However, section 411 of Public Law 108–173 amended section 1833(t)(7)(D)(i) of the Act to extend these payments through December 31, 2005, for rural hospitals with 100 or fewer beds. Section 411 also extended the transitional corridor payments to SCHs located in rural areas for services furnished during the period that began with the provider's first cost reporting period beginning on or after January 1, 2004, and ended on December 31, 2005. Accordingly, the authority for making transitional corridor payments under section 1833(t)(7)(D)(i) of the Act, as amended by section 411 of Public Law 108–173, for rural hospitals having 100 or fewer beds and SCHs located in rural areas expired on December 31, 2005.
Section 5105 of Public Law 109–171 reinstituted the TOPs for covered OPD services furnished on or after January 1, 2006, and before January 1, 2009, for rural hospitals having 100 or fewer beds that are not SCHs. When the OPPS payment was less than the provider's pre-BBA amount, the amount of payment was increased by 95 percent of the amount of the difference between the two payment systems for CY 2006, by 90 percent of the amount of that difference for CY 2007, and by 85 percent of the amount of that difference for CY 2008.
For CY 2006, we implemented section 5105 of Public Law 109–171 through Transmittal 877, issued on February 24, 2006. In the Transmittal, we did not specifically address whether TOPs apply to essential access community hospitals (EACHs), which are considered to be SCHs under section 1886(d)(5)(D)(iii)(III) of the Act. Accordingly, under the statute, EACHs are treated as SCHs. In the CY 2007 OPPS/ASC final rule with comment period (71 FR 68010), we stated that EACHs were not eligible for TOPs under Public Law 109–171. However, we stated they were eligible for the adjustment for rural SCHs. In the CY 2007 OPPS/ASC final rule with comment period (71 FR 68010 and 68228), we updated § 419.70(d) of our regulations to reflect the requirements of Public Law 109–171.
In the CY 2009 OPPS/ASC proposed rule (73 FR 41461), we stated that, effective for services provided on or after January 1, 2009, rural hospitals having 100 or fewer beds that are not SCHs would no longer be eligible for TOPs, in accordance with section 5105 of Public Law 109–171. However, subsequent to issuance of the CY 2009 OPPS/ASC proposed rule, section 147 of Public Law 110–275 amended section 1833(t)(7)(D)(i) of the Act by extending the period of TOPs to rural hospitals with 100 beds or fewer for 1 year, for services provided before January 1, 2010. Section 147 of Public Law 110–275 also extended TOPs to SCHs (including EACHs) with 100 or fewer beds for covered OPD services provided on or after January 1, 2009, and before January 1, 2010. In accordance with section 147 of Public Law 110–275, when the OPPS payment is less than the provider's pre-BBA amount, the amount of payment is increased by 85 percent of the amount of the difference between the two payment systems for CY 2009.
For CY 2009, we revised our regulations at §§ 419.70(d)(2) and (d)(4) and added a new paragraph (d)(5) to incorporate the provisions of section 147 of Public Law 110–275. In addition, we made other technical changes to § 419.70(d)(2) to more precisely capture our existing policy and to correct an inaccurate cross-reference. We also made technical corrections to the cross-references in paragraphs (e), (g), and (i) of § 419.70. In the CY 2010 OPPS/ASC proposed rule (74 FR 35295), for CY 2010, we proposed to make a technical correction to the heading of § 419.70(d)(5) to correctly identify the policy as described in the subsequent regulation text. The paragraph heading should indicate that the adjustment applies to small SCHs, rather than to rural SCHs.
Effective for services provided on or after January 1, 2010, rural hospitals and SCHs (including EACHs) having 100 or fewer beds will no longer be eligible for hold harmless TOPs, in accordance with section 147 of Public Law 110–275.
2. Adjustment for Rural SCHs Implemented in CY 2006 Related to Public Law 108–173 (MMA)
In the CY 2006 OPPS final rule with comment period (70 FR 68556), we finalized a payment increase for rural SCHs of 7.1 percent for all services and procedures paid under the OPPS, excluding drugs, biologicals, brachytherapy sources, and devices paid under the pass-through payment policy in accordance with section 1833(t)(13)(B) of the Act, as added by section 411 of Public Law 108–173. Section 411 gave the Secretary the authority to make an adjustment to OPPS payments for rural hospitals, effective January 1, 2006, if justified by a study of the difference in costs by APC between hospitals in rural areas and hospitals in urban areas. Our analysis showed a difference in costs for rural SCHs. Therefore, for the CY 2006 OPPS, we finalized a payment adjustment for rural SCHs of 7.1 percent for all services and procedures paid under the OPPS, excluding separately payable drugs and biologicals, brachytherapy sources, and devices paid under the pass-through payment policy, in accordance with section 1833(t)(13)(B) of the Act.
In CY 2007, we became aware that we did not specifically address whether the adjustment applies to EACHs, which are considered to be SCHs under section 1886(d)(5)(D)(iii)(III) of the Act. Thus, under the statute, EACHs are treated as SCHs. Therefore, in the CY 2007 OPPS/ASC final rule with comment period (71 FR 68010 and 68227), for purposes of receiving this rural adjustment, we revised § 419.43(g) to clarify that EACHs are also eligible to receive the rural SCH adjustment, assuming these entities otherwise meet the rural adjustment criteria. Currently, fewer than 10 hospitals are classified as EACHs and as of CY 1998, under section 4201(c) of Public Law 105–33, a hospital can no longer become newly classified as an EACH.
This adjustment for rural SCHs is budget neutral and applied before calculating outliers and copayment. As stated in the CY 2006 OPPS final rule with comment period (70 FR 68560), we would not reestablish the adjustment amount on an annual basis, but we may review the adjustment in the future and, if appropriate, would revise the adjustment. We provided the same 7.1 percent adjustment to rural SCHs, including EACHs, again in CY 2008 and CY 2009. Further, in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68590), we updated the regulations at § 419.43(g)(4) to specify, in general terms, that items paid at charges adjusted to costs by application of a hospital-specific CCR are excluded from the 7.1 percent payment adjustment.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35295), for the CY 2010 OPPS, we proposed to continue our policy of a budget neutral 7.1 percent payment adjustment for rural SCHs, including EACHs, for all services and procedures paid under the OPPS, Start Printed Page 60426 excluding separately payable drugs and biologicals, devices paid under the pass-through payment policy, and items paid at charges reduced to costs. We intend to reassess the 7.1 percent adjustment in the near future by examining differences between urban and rural hospitals' costs using updated claims, cost reports, and provider information.
Comment: A number of commenters generally supported the proposal to continue the rural SCH (including EACHs) adjustment for CY 2010 OPPS. Several commenters also asked that CMS extend for CY 2010 the TOPs payment policies that were in effect for CY 2009. The commenters recommended that CMS evaluate the differences in cost between urban and rural hospitals over an extended 3-year period using updated claims, cost reports, and provider information. They further suggested that, during the 3-year period in which CMS would be gathering data, CMS pay SCHs and rural hospitals with less than 100 beds that are not SCHs the greater of the TOPs payment in effect for CY 2009 or the OPPS payment for the applicable calendar year plus the 7.1 percent rural adjustment, whichever is greater. The commenters claimed that CMS' reversal of the TOPs allowance after only 1 year of reimplementation for certain rural hospitals was unreasonable and could irreparably harm those rural hospitals absent a safety net mechanism in place.
Response: We agree that it is appropriate to continue the 7.1 percent adjustment for rural SCHs (including EACHs) as we proposed for CY 2010. However, we are not extending the CY 2009 TOPs payment policies for rural hospitals with 100 beds or less and for SCHs (including EACHs) with 100 or fewer beds for CY 2010. Section 1833(t)(7)(D)(i)(II) of the Act provides that, in the case of a hospital located in a rural area with 100 beds or fewer and that is not a sole community hospital, for covered OPD services furnished on or after January 1, 2006 and before January 1, 2010, for which the PPS amount is less than the pre-BBA amount, the amount of payment should be increased by the applicable percentage of the amount of such difference. Section 1833(t)(7)(D)(i)(III) of the Act also extends TOPs to SCHs (including EACHs) with 100 or fewer beds for covered OPD services provided on or after January 1, 2009 and before January 1, 2010, under the specific circumstances outlined in the statute. Therefore, sections 1833(t)(D)(i)(II) and (III) of the Act specifically expire TOPs payment to these categories of hospitals for services furnished on and after January 1, 2010. Accordingly, in CY 2010, neither rural SCHs nor rural hospitals with less than 100 beds will receive payment at whichever is greater, the TOPs payment in place for CY 2009 or payment for CY 2010, which includes the rural adjustment for rural SCHs, because sections 1833(t)(7)(D)(i)(II) and (III) of the Act expire TOPS payments as explained above. As we indicate above, we intend to reassess the 7.1 percent rural adjustment in the near future by examining differences between urban and rural hospitals' costs using updated claims, cost reports, and provider information.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to apply the 7.1 percent payment adjustment to rural SCHs for most services paid under the CY 2010 OPPS, excluding drugs, biologicals, and devices paid under the pass-through payment policy, and items paid at charges adjusted to cost. We also are making a technical correction to the heading of § 419.70(d)(5) to correctly identify the policy described in the regulation text of § 419.70(d)(5). The paragraph heading indicates that the adjustment applies to small SCHs, rather than to rural SCHs.
F. Hospital Outpatient Outlier Payments
1. Background
Currently, the OPPS pays outlier payments on a service-by-service basis. For CY 2009, the outlier threshold is met when the cost of furnishing a service or procedure by a hospital exceeds 1.75 times the APC payment amount and exceeds the APC payment rate plus a $1,800 fixed-dollar threshold. We introduced a fixed-dollar threshold in CY 2005 in addition to the traditional multiple threshold in order to better target outliers to those high cost and complex procedures where a very costly service could present a hospital with significant financial loss. If the cost of a service meets both of these conditions, the multiple threshold and the fixed-dollar threshold, the outlier payment is calculated as 50 percent of the amount by which the cost of furnishing the service exceeds 1.75 times the APC payment rate. Before CY 2009, this outlier payment had historically been considered a final payment by longstanding OPPS policy. We implemented a reconciliation process similar to the IPPS outlier reconciliation process for cost reports with cost reporting periods beginning on or after January 1, 2009 (73 FR 68594 through 68599).
It has been our policy for the past several years to report the actual amount of outlier payments as a percent of total spending in the claims being used to model the proposed OPPS. We previously estimated that CY 2008 outlier payments were approximately 0.73 percent of the total CY 2008 OPPS payments (73 FR 68592). Our current estimate of total outlier payments as a percent of total CY 2008 OPPS payment, using CY 2008 claims processed through June 30, 2009, and the revised OPPS expenditure estimate for the 2009 Trustees Report, is approximately 1.2 percent of the total aggregated OPPS payments. Therefore, for CY 2008, we estimate that we paid approximately 0.2 percent more than the CY 2008 outlier target of 1.0 percent of total aggregated OPPS payments.
As explained in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68594), we set our projected target for aggregate outlier payments at 1.0 percent of the aggregate total payments under the OPPS for CY 2009. The outlier thresholds were set so that estimated CY 2009 aggregate outlier payments would equal 1.0 percent of the total aggregated payments under the OPPS. Using our final rule CY 2008 claims data and CY 2009 payment rates, we currently estimate that the aggregate outlier payments for CY 2009 would be approximately 1.03 percent of the total CY 2009 OPPS payments. The difference between 1.0 percent and 1.03 percent is reflected in the regulatory impact analysis in section XXI.B. of this final rule with comment period. We note that we provide estimated CY 2010 outlier payments for hospitals and CMHCs with claims included in the claims data that we used to model impacts in the Hospital-Specific Impacts—Provider-Specific Data file on the CMS Web site at: http://www.cms.hhs.gov/HospitalOutpatientPPS/.
2. Outlier Calculation
In the CY 2010 OPPS/ASC proposed rule (74 FR 35296), we proposed to continue our policy of estimating outlier payments to be 1.0 percent of the estimated aggregate total payments under the OPPS in CY 2010. We proposed that a portion of that 1.0 percent, specifically 0.02 percent, would be allocated to CMHCs for PHP outlier payments. This is the amount of estimated outlier payments that would result from the proposed CMHC outlier threshold as a proportion of total estimated outlier payments. As discussed in section X.C. of this final rule with comment period, for CMHCs, we proposed that if a CMHC's cost for partial hospitalization services, paid under either APC 0172 (Level I Partial Start Printed Page 60427 Hospitalization (3 services)) or APC 0173 (Level II Partial Hospitalization (4 or more services)), exceeds 3.40 times the payment for APC 0173, the outlier payment would be calculated as 50 percent of the amount by which the cost exceeds 3.40 times the APC 0173 payment rate. For further discussion of CMHC outlier payments, we refer readers to section X.C. of this final rule with comment period.
To ensure that the estimated CY 2010 aggregate outlier payments would equal 1.0 percent of estimated aggregate total payments under the OPPS, we proposed that the hospital outlier threshold be set so that outlier payments would be triggered when the cost of furnishing a service or procedure by a hospital exceeds 1.75 times the APC payment amount and exceeds the APC payment rate plus a $2,225 fixed-dollar threshold. This proposed threshold reflected the methodology discussed below in this section, as well as the proposed APC recalibration for CY 2010.
We calculated the fixed-dollar threshold for the CY 2010 OPPS/ASC proposed rule using largely the same methodology as we did in CY 2009 (73 FR 41462). For purposes of estimating outlier payments for the CY 2010 OPPS/ASC proposed rule, we used the hospital-specific overall ancillary CCRs available in the April 2009 update to the Outpatient Provider-Specific File (OPSF). The OPSF contains provider-specific data, such as the most current CCR, which are maintained by the Medicare contractors and used by the OPPS Pricer to pay claims. The claims that we use to model each OPPS update lag by 2 years. For the CY 2010 OPPS/ASC proposed rule, we used CY 2008 claims to model the CY 2010 OPPS. In order to estimate the CY 2010 hospital outlier payments for the CY 2010 OPPS/ASC proposed rule, we inflated the charges on the CY 2008 claims using the same inflation factor of 1.1511 that we used to estimate the IPPS fixed-dollar outlier threshold for the FY 2010 IPPS/LTCH PPS proposed rule (74 FR 24245). For 1 year, the inflation factor we used was 1.0729. The methodology for determining this charge inflation factor was discussed in the FY 2010 IPPS/LTCH PPS proposed rule (74 FR 24245). As we stated in the CY 2005 OPPS final rule with comment period (69 FR 65845), we believe that the use of this charge inflation factor is appropriate for the OPPS because, with the exception of the routine service cost centers, hospitals use the same cost centers to capture costs and charges across inpatient and outpatient services.
As noted in the CY 2007 OPPS/ASC final rule with comment period (71 FR 68011), we are concerned that we could systematically overestimate the OPPS hospital outlier threshold if we did not apply a CCR inflation adjustment factor. Therefore, we proposed to apply the same CCR inflation adjustment factor that we proposed to apply for the FY 2010 IPPS outlier calculation to the CCRs used to simulate the CY 2010 OPPS outlier payments that determine the fixed-dollar threshold. Specifically, for CY 2010, we proposed to apply an adjustment of 0.9840 to the CCRs that were in the April 2009 OPSF to trend them forward from CY 2009 to CY 2010. The methodology for calculating this adjustment is discussed in the FY 2010 IPPS/LTCH PPS proposed rule (74 FR 24245 through 24247) and the FY 2010 IPPS/LTCH PPS final rule (74 FR 44007 through 44011).
Therefore, to model hospital outlier payments for the CY 2010 OPPS/ASC proposed rule, we applied the overall CCRs from the April 2009 OPSF file after adjustment (using the proposed CCR inflation adjustment factor of 0.9840 to approximate CY 2010 CCRs) to charges on CY 2008 claims that were adjusted (using the proposed charge inflation factor of 1.1511 to approximate CY 2010 charges). We simulated aggregated CY 2010 hospital outlier payments using these costs for several different fixed-dollar thresholds, holding the 1.75 multiple threshold constant and assuming that outlier payment would continue to be made at 50 percent of the amount by which the cost of furnishing the service would exceed 1.75 times the APC payment amount, until the total outlier payments equaled 1.0 percent of aggregated estimated total CY 2010 OPPS payments. We estimated that a proposed fixed-dollar threshold of $2,225, combined with the proposed multiple threshold of 1.75 times the APC payment rate, would allocate 1.0 percent of aggregated total OPPS payments to outlier payments. We proposed to continue to make an outlier payment that equals 50 percent of the amount by which the cost of furnishing the service exceeds 1.75 times the APC payment amount when both the 1.75 multiple threshold and the proposed fixed-dollar $2,225 threshold are met. For CMHCs, if a CMHC's cost for partial hospitalization services, paid under either APC 0172 or APC 0173, exceeds 3.40 times the payment for APC 0173, the outlier payment would be calculated as 50 percent of the amount by which the cost exceeds 3.40 times the APC 0173 payment rate.
Section 1833(t)(17)(A) of the Act, which applies to hospitals as defined under section 1886(d)(1)(B) of the Act, requires that hospitals that fail to report data required for the quality measures selected by the Secretary, in the form and manner required by the Secretary under 1833(t)(17)(B) of the Act, incur a 2.0 percentage point reduction to their OPD fee schedule increase factor, that is, the annual payment update factor. The application of a reduced OPD fee schedule increase factor results in reduced national unadjusted payment rates that will apply to certain outpatient items and services furnished by hospitals that are required to report outpatient quality data and that fail to meet the HOP QDRP requirements. For hospitals that fail to meet the HOP QDRP requirements, we proposed to continue our policy that we implemented in CY 2009 that the hospitals' costs would be compared to the reduced payments for purposes of outlier eligibility and payment calculation. For more information on the HOP QDRP, we refer readers to section XVI. of this final rule with comment period.
Comment: Several commenters supported the proposal to increase the outlier fixed-dollar threshold to maintain a target outlier spending percentage of 1.0 percent. One commenter requested that CMS not overestimate the fixed-dollar outlier threshold by decreasing the CY 2010 proposed threshold proportionally to only account for the amount Medicare paid in excess of the 1 percent target outlier percentage in CY 2009. A few commenters suggested that the target outlier spending percentage be raised. One commenter recommended that the target outlier spending percentage be raised to maintain the $1,800 fixed-dollar threshold that is in effect for CY 2009. Another commenter requested that CMS increase the amount of outlier payment from 50 percent to 80 percent of the difference between the OPPS payment and the estimated provider cost for the service to make OPPS outlier policy more consistent with IPPS outlier policy. One commenter expressed concern that changes in outlier payments disproportionately affected the safety net hospitals. One commenter supported the proposal to use the same assumptions regarding charge inflation and CCR inflation as under the IPPS.
Response: We appreciate the commenters' support regarding the development of the OPPS outlier policy. We are not raising the threshold to recover the 0.03 percent of OPPS payment that we estimate was paid in Start Printed Page 60428 addition to the target outlier percent of 1 percent for CY 2009 because we do not adjust the fixed-dollar threshold in future years for either paying too much or too little in outlier payments in past years. We are not increasing the percent of total OPPS payment that we attribute to outlier payments, either for general purposes or to maintain the $1,800 threshold for CY 2010, because we continue to believe that it is appropriate to maintain the target outlier percentage of 1 percent of total payment under the OPPS and to have a fixed-dollar threshold so that OPPS outlier payments are made only where the hospital would experience a significant loss for supplying a particular service. Similarly, we are not increasing the outlier payment percentage from 50 percent to 80 percent of the difference between the amount by which the cost of furnishing the service exceeds 1.75 times the APC payment rate because we do not believe that hospitals carry the same level of risk when they furnish outpatient hospital services as when they furnish inpatient hospital services. OPPS outlier payments are intended to protect hospitals from excessive losses when providing an extraordinarily costly service, and we believe that the potential for loss when furnishing OPPS services is limited. Payment bundles under the OPPS are small relative to those under the IPPS, and the OPPS pays separately for many services. The OPPS would pay hospitals for many individual services provided to a very costly patient reducing their financial risk. Patients for whom a hospital may incur extraordinary costs for providing individual OPPS services would usually require hospital admission. As described above, outlier payments are designed to protect hospitals from financial risk in providing services to costly patients, and are not designed to affect any specific hospital classes, such as safety net hospitals. With regard to the application of charge inflation factors, we agree that the charge inflation factors that apply to inpatient hospitals services are equally applicable to services provided under the OPPS. Therefore, as specified below, we are applying the charge inflation factors that were used to calculate the outlier fixed-dollar threshold for the IPPS in the calculation of the fixed-dollar threshold for the CY 2010 OPPS.
Comment: Several commenters asked that CMS eliminate outlier payments for CMHCs and use the funds allocated to outlier payments for CMHCs to increase payments for services provided by CMHCs.
Response: Outlier payments to CMHCs are discussed in section X.C. of this final rule with public comment. We respond to this comment as part of that discussion.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal for the outlier calculation, without modification, as outlined below.
3. Final Outlier Calculation
For CY 2010, we are applying the overall CCRs from the July 2009 OPSF file with a CCR adjustment factor of 0.988 to approximate CY 2010 CCRs to charges on the final CY 2008 claims that were adjusted to approximate CY 2010 charges (using the final 2-year charge inflation factor of 1.1418). We simulated aggregated CY 2010 hospital outlier payments using these costs for several different fixed-dollar thresholds, holding the 1.75 multiple threshold constant and assuming that outlier payment would continue to be made at 50 percent of the amount by which the cost of furnishing the service would exceed 1.75 times the APC payment amount, until the total outlier payments equaled 1.0 percent of aggregated estimated total CY 2010 OPPS payments. We estimate that a fixed-dollar threshold of $2,175, combined with the multiple threshold of 1.75 times the APC payment rate, will allocate 1.0 percent of aggregated total OPPS payments to outlier payments.
In summary, for CY 2010, we will continue to make an outlier payment that equals 50 percent of the amount by which the cost of furnishing the service exceeds 1.75 times the APC payment amount when both the 1.75 multiple threshold and the final fixed-dollar $2,175 threshold are met. For CMHCs, if a CMHC's cost for PHP services, paid under either APC 0172 or APC 0173, exceeds 3.40 times the payment for APC 0173, the outlier payment is calculated as 50 percent of the amount by which the cost exceeds 3.40 times the APC 0173 payment rate. We estimate that this threshold will allocate 0.03 percent of outlier payments to CMHCs for PHP outlier payments.
4. Outlier Reconciliation
In the CY 2009 OPPS/ASC final rule with comment period (73 CFR 68599), we adopted as final policy a process to reconcile hospital or CMHC outlier payments at cost report settlement for services furnished during cost reporting periods beginning in CY 2009. OPPS outlier reconciliation ensures accurate outlier payments for those facilities whose CCRs fluctuate significantly relative to the CCRs of other facilities, and who receive a significant amount of outlier payments. OPPS outlier reconciliation thresholds are provided in the Medicare Claims Processing Manual (Pub. 100–4), Chapter 4, Section 10.7.2.1, reevaluated annually, and modified if necessary. When the cost report is settled, reconciliation of outlier payments will be based on the hospital-specific overall ancillary CCR, calculated as the ratio of costs and charges computed from the cost report at the time the cost report coinciding with the service dates is settled. Reconciling outlier payments ensures that the outlier payments made are appropriate and that final outlier payments reflect the most accurate cost data. In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68599), we also finalized a proposal to adjust the amount of final outlier payments determined during reconciliation for the time value of money. The OPPS outlier reconciliation process will require recalculating outlier payments for individual claims in order to accurately determine the net effect of a change in a hospital's or CMHC's overall CCR on the facility's total outlier payments. For cost reporting periods beginning in CY 2009, Medicare contractors will begin to identify cost reports that require outlier reconciliation as a component of cost report settlement. At this time, CMS continues to develop a method for reexamining claims to calculate the change in total outlier payments in order to reconcile outlier payments for these cost reports.
As under the IPPS, we do not adjust the fixed-dollar threshold or amount of total OPPS payment set aside for outlier payments for reconciliation activity. The predictability of the fixed-dollar threshold is an important component of a prospective payment system. We do not adjust the prospectively set outlier threshold for the amount of outlier payment reconciled at cost report settlement because such action would be contrary to the prospective nature of the system. Our outlier threshold calculation assumes that overall ancillary CCRs accurately estimate hospital costs based on the information available to us at the time we set the prospective fixed-dollar outlier threshold. For these reasons, we are not incorporating any assumptions about the effects of reconciliation into our calculation of the OPPS fixed-dollar outlier threshold.
Comment: A number of commenters asked that CMS report the amount of outlier reconciliation activity, including aggregate amounts recovered by provider type and region. They suggested that, if the reconciled amounts are significant, these amounts Start Printed Page 60429 should be factored into the annual fixed-dollar outlier threshold. Several commenters supported the current reconciliation thresholds identified in the CMS manual (Medicare Claims Processing Manual (Pub. 100–04), Chapter 4, Section 10.7.2.1). One commenter asked that CMS apply the outlier reconciliation thresholds established in manual instructions to the claims used for estimating outlier payment and the fixed-dollar threshold to achieve the most accurate estimates possible.
Response: We revised Worksheet E, Part B, of the Medicare hospital cost report form CMS 2552–10 to collect OPPS outlier reconciliation information for cost reports beginning on or after January 1, 2009. This information will be available to the public through the Hospital Cost Report Information System (HCRIS). We do not expect to take outlier reconciliation amounts into account in our projections of future outlier payments. We believe that the reconciliation CCR and outlier payment thresholds implemented in the final rule (73 CFR 68599) are generous and that most hospitals will not be subject to outlier reconciliation upon cost report settlement. Further, it is difficult to predict the specific hospitals that will have CCRs and outlier payments reconciled in any given year. We also note that reconciliation occurs because hospitals' actual CCRs for the cost reporting period are different than the interim CCRs used to calculate outlier payment when a bill is processed. Our fixed-dollar threshold calculation assumes that CCRs accurately estimate hospital costs based on information available to us at the time we set the prospective fixed-dollar outlier threshold. We do not believe that estimating the fixed-dollar threshold to estimate the amount of payment that may be recovered as a result of outlier reconciliation in any given year would necessarily result in a more accurate estimate of outlier payments or a more accurate calculation of the fixed-dollar threshold for outlier payment for the prospective payment year. For these reasons, we will not make any assumptions about the amount of anticipated reconciliation of outlier payments on the outlier threshold calculation.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, for an OPPS outlier reconciliation policy. We are implementing the outlier reconciliation policy for each hospital and CMHC for services furnished during cost reporting periods beginning in CY 2010, and we are including an adjustment for the time value of money.
G. Calculation of an Adjusted Medicare Payment From the National Unadjusted Medicare Payment
The basic methodology for determining prospective payment rates for HOPD services under the OPPS is set forth in existing regulations at 42 CFR part 419, subparts C and D. The payment rate for most services and procedures for which payment is made under the OPPS is the product of the conversion factor calculated in accordance with section II.B. of this final rule with comment period and the relative weight determined under section II.A. of this final rule with comment period. Therefore, the final national unadjusted payment rate for most APCs contained in Addendum A to this final rule with comment period and for most HCPCS codes to which separate payment under the OPPS has been assigned in Addendum B to this final rule with comment period was calculated by multiplying the final CY 2010 scaled weight for the APC by the final CY 2010 conversion factor.
We note that section 1833(t)(17) of the Act, which applies to hospitals as defined under section 1886(d)(1)(B) of the Act, requires that hospitals that fail to submit data required to be submitted on quality measures selected by the Secretary, in the form and manner and at a time specified by the Secretary, receive a 2.0 percentage point reduction to their OPD fee schedule increase factor, that is, the annual payment update factor. The application of a reduced OPD fee schedule increase factor results in reduced national unadjusted payment rates that apply to certain outpatient items and services provided by hospitals that are required to report outpatient quality data and that fail to meet the Hospital Outpatient Quality Data Reporting Program (HOP QDRP) requirements. For further discussion of the payment reduction for hospitals that fail to meet the requirements of the HOP QDRP, we refer readers to section XVI.D. of this final rule with comment period.
We demonstrate in the steps below how to determine the APC payments that would be made in a calendar year under the OPPS to a hospital that fulfills the HOP QDRP requirements and to a hospital that fails to meet the HOP QDRP requirements for a service that has any of the following status indicator assignments: “P,” “Q1,” “Q2,” “Q3,” “R,” “S,” “T,” “U,” “V,” or “X” (as defined in Addendum D1 to this final rule with comment period), in a circumstance in which the multiple procedure discount does not apply, the procedure is not bilateral, and conditionally packaged services (status indicator of “Q1” and “Q2”) qualify for separate payment. We note that blood and blood products with status indicator “R” are not subject to wage adjustment but are subject to reduced payments when a hospital fails to meet the HOP QDRP requirements, as outlined in the steps and examples below.
Individual providers interested in calculating the payment amount that they would receive for a specific service from the national unadjusted payment rates presented in Addenda A and B to this final rule with comment period should follow the formulas presented in the following steps. For purposes of the payment calculations below, we refer to the national unadjusted payment rate for hospitals that meet the requirements of the HOP QDRP as the “full” national unadjusted payment rate. We refer to the national unadjusted payment rate for hospitals that fail to meet the requirements of the HOP QDRP as the “reduced” national unadjusted payment rate. The reduced national unadjusted payment rate is calculated by multiplying the reporting ratio of 0.98 times the “full” national unadjusted payment rate. The national unadjusted payment rate used in the calculations below is either the full national unadjusted payment rate or the reduced national unadjusted payment rate, depending on whether the hospital met its HOP QDRP requirements in order to receive the full CY 2010 OPPS increase factor.
Step 1. Calculate 60 percent (the labor-related portion) of the national unadjusted payment rate. Since the initial implementation of the OPPS, we have used 60 percent to represent our estimate of that portion of costs attributable, on average, to labor. We refer readers to the April 7, 2000 OPPS final rule with comment period (65 FR 18496 through 18497) for a detailed discussion of how we derived this percentage. We confirmed that this labor-related share for hospital outpatient services is still appropriate during our regression analysis for the payment adjustment for rural hospitals in the CY 2006 OPPS final rule with comment period (70 FR 68553).
The formula below is a mathematical representation of Step 1 and identifies the labor-related portion of a specific payment rate for a specific service.
X is the labor-related portion of the national unadjusted payment rate.
X = .60 * (national unadjusted payment rate)
Start Printed Page 60430Step 2. Determine the wage index area in which the hospital is located and identify the wage index level that applies to the specific hospital. The wage index values assigned to each area reflect the geographic statistical areas (which are based upon OMB standards) to which hospitals are assigned for FY 2010 under the IPPS, reclassifications through the MGCRB, section 1886(d)(8)(B) “Lugar” hospitals, reclassifications under section 1886(d)(8)(E) of the Act, as defined in § 412.103 of the regulations and hospitals designated as urban under section 601(g) of Public Law 98–21. We note that the reclassifications of hospitals under section 508 of Public Law 108–173, as extended by section 124 of Public Law 110–275, expired on September 30, 2009, and will not be applicable under the IPPS for FY 2010. Therefore, these reclassifications will not apply to the CY 2010 OPPS. For further discussion of the changes to the FY 2010 IPPS wage indices, as applied to the CY 2010 OPPS, we refer readers to section II.C. of this final rule with comment period. The wage index values include the occupational mix adjustment described in section II.C. of this final rule with comment period that was developed for the FY 2010 IPPS final payment rates published in the Federal Register on August 27, 2009 (74 FR 43827).
Step 3. Adjust the wage index of hospitals located in certain qualifying counties that have a relatively high percentage of hospital employees who reside in the county, but who work in a different county with a higher wage index, in accordance with section 505 of Public Law 108–173. Addendum L to this final rule with comment period contains the qualifying counties and the final wage index increase developed for the FY 2010 IPPS and published as Table 4J in the FY 2010 IPPS final rule (74 FR 44118 through 44125), as corrected in the Federal Register on October 2, 2009 (74 FR 51506) This step is to be followed only if the hospital is not reclassified or redesignated under section 1886(d)(8) or section 1886(d)(10) of the Act.
Step 4. Multiply the applicable wage index determined under Steps 2 and 3 by the amount determined under Step 1 that represents the labor-related portion of the national unadjusted payment rate.
The formula below is a mathematical representation of Step 4 and adjusts the labor-related portion of the national payment rate for the specific service by the wage index.
Xais the labor-related portion of the national unadjusted payment rate (wage adjusted).
Xa= .60 * (national unadjusted payment rate) * applicable wage index.
Step 5. Calculate 40 percent (the nonlabor-related portion) of the national unadjusted payment rate and add that amount to the resulting product of Step 4. The result is the wage index adjusted payment rate for the relevant wage index area.
The formula below is a mathematical representation of Step 5 and calculates the remaining portion of the national payment rate, the amount not attributable to labor, and the adjusted payment for the specific service.
Y is the nonlabor-related portion of the national unadjusted payment rate.
Y = .40 * (national unadjusted payment rate)
Adjusted Medicare Payment = Y +Xa
Step 6. If a provider is a SCH, set forth in the regulations at § 412.92, or an EACH, which is considered to be a SCH under section 1886(d)(5)(D)(iii)(III) of the Act, and located in a rural area, as defined in § 412.64(b), or is treated as being located in a rural area under § 412.103, multiply the wage index adjusted payment rate by 1.071 to calculate the total payment.
The formula below is a mathematical representation of Step 6 and applies the rural adjustment for rural SCHs.
Adjusted Medicare Payment (SCH or EACH) = Adjusted Medicare Payment * 1.071
We have provided examples below of the calculation of both the full and reduced national unadjusted payment rates that would apply to certain outpatient items and services performed by hospitals that meet and that fail to meet the HOP QDRP requirements, using the steps outlined above. For purposes of this example, we use a provider that is located in Wayne, New Jersey that is assigned to CBSA 35644. This provider bills one service that is assigned to APC 0019 (Level I Excision/Biopsy). The CY 2010 full national unadjusted payment rate for APC 0019 is $294.06. The reduced national unadjusted payment rate for a hospital that fails to meet the HOP QDRP requirements is $288.17. This reduced rate is calculated by multiplying the reporting ratio of 0.98 by the full unadjusted payment rate for APC 0019.
The FY 2010 wage index for a provider located in CBSA 35644 in New Jersey is 1.3005. The labor-related portion of the full national unadjusted payment is $229.45 (.60 * $294.06 * 1.3005). The labor-related portion of the reduced national unadjusted payment is $224.85 (.60 * $288.17 * 1.3005). The nonlabor-related portion of the full national unadjusted payment is $117.62 (.40 * $294.06). The nonlabor-related portion of the reduced national unadjusted payment is $115.26 (.40 * $288.17). The sum of the labor-related and nonlabor-related portions of the full national adjusted payment is $347.07 ($229.45 + $117.62). The sum of the reduced national adjusted payment is $340.11 ($224.85 + $115.26).
We did not receive any public comments concerning our proposed methodology for calculating an adjusted payment from the national unadjusted Medicare payment amount for CY 2010. Therefore, we are finalizing our proposed CY 2010 methodology, without modification.
H. Beneficiary Copayments
1. Background
Section 1833(t)(3)(B) of the Act requires the Secretary to set rules for determining the unadjusted copayment amounts to be paid by beneficiaries for covered OPD services. Section 1833(t)(8)(C)(ii) of the Act specifies that the Secretary must reduce the national unadjusted copayment amount for a covered OPD service (or group of such services) furnished in a year in a manner so that the effective copayment rate (determined on a national unadjusted basis) for that service in the year does not exceed a specified percentage. As specified in section 1833(t)(8)(C)(ii)(V) of the Act, for all services paid under the OPPS in CY 2010, and in calendar years thereafter, the percentage is 40 percent of the APC payment rate.
Section 1833(t)(3)(B)(ii) of the Act provides that, for a covered OPD service (or group of such services) furnished in a year, the national unadjusted copayment amount cannot be less than 20 percent of the OPD fee schedule amount. Sections 1834(d)(2)(C)(ii) and (d)(3)(C)(ii) of the Act further require that the copayment for screening flexible sigmoidoscopies and screening colonoscopies be equal to 25 percent of the payment amount. Since the beginning of the OPPS, we have applied the 25-percent copayment to screening flexible sigmoidoscopies and screening colonoscopies.
2. Copayment Policy
In the CY 2010 OPPS/ASC proposed rule (74 FR 35298), for CY 2010, we proposed to determine copayment amounts for new and revised APCs using the same methodology that we implemented beginning in CY 2004. (We refer readers to the November 7, Start Printed Page 60431 2003 OPPS final rule with comment period (68 FR 63458)). In addition, we proposed to use the same standard rounding principles that we have historically used in instances where the application of our standard copayment methodology would result in a copayment amount that is less than 20 percent and cannot be rounded, under standard rounding principles, to 20 percent. (We refer readers to the CY 2008 OPPS/ASC final rule with comment period (72 FR 66687) in which we discuss our rationale for applying these rounding principles.) The national unadjusted copayment amounts for services payable under the OPPS that will be effective January 1, 2010, are shown in Addenda A and B to this final rule with comment period. As discussed in section XVI.D. of this final rule with comment period, as we proposed, we are providing that, for CY 2010, the Medicare beneficiary's minimum unadjusted copayment and national unadjusted copayment for a service to which a reduced national unadjusted payment rate applies will equal the product of the reporting ratio and the national unadjusted copayment, or the product of the reporting ratio and the minimum unadjusted copayment, respectively, for the service.
Comment: One commenter recommended that CMS continue its educational outreach and keep Medicare beneficiaries informed about the benefits of supplemental/secondary insurance in reducing their out-of-pocket costs for orthopedic procedures.
Response: We appreciate the commenter's support for our educational efforts on the availability of supplemental/secondary insurance and refer beneficiaries seeking information about their Medicare benefits and supplemental/secondary insurance coverage to the Web site at: http://www.medicare.gov.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, for determining APC copayment amounts.
3. Calculation of an Adjusted Copayment Amount for an APC Group
Individuals interested in calculating the national copayment liability for a Medicare beneficiary for a given service provided by a hospital that met or failed to meet its HOP QDRP requirements should follow the formulas presented in the following steps.
Step 1. Calculate the beneficiary payment percentage for the APC by dividing the APC's national unadjusted copayment by its payment rate. For example, using APC 0019, $64.51 is 22 percent of the full national unadjusted payment rate of $294.06. For APCs with only a minimum unadjusted copayment in Addendum A and B of this final rule with comment period, identify a beneficiary payment percentage of 20 percent.
The formula below is a mathematical representation of Step 1 and calculates national copayment as a percentage of national payment for a given service.
B is the beneficiary payment percentage.
B = National unadjusted copayment for APC/national unadjusted payment rate for APC
Step 2. Calculate the appropriate wage-adjusted payment rate for the APC for the provider in question, as indicated in Steps 2 through 4 under section II.G. of this final rule with comment period. Calculate the rural adjustment for eligible providers as indicated in Step 6 under section II.G. of this final rule with comment period.
Step 3. Multiply the percentage calculated in Step 1 by the payment rate calculated in Step 2. The result is the wage-adjusted copayment amount for the APC.
The formula below is a mathematical representation of Step 3 and applies the beneficiary percentage to the adjusted payment rate for a service calculated under section II.G. of this final rule with comment period, with and without the rural adjustment, to calculate the adjusted beneficiary copayment for a given service.
Wage-adjusted copayment amount for the APC = Adjusted Medicare Payment * B
Wage-adjusted copayment amount for the APC (SCH or EACH) = (Adjusted Medicare Payment * 1.071) * B
Step 4. For a hospital that failed to meet its HOP QDRP requirements, multiply the copayment calculated in Step 3 by the reporting ratio of 0.98.
The unadjusted copayments for services payable under the OPPS that will be effective January 1, 2010, are shown in Addenda A and B to this final rule with comment period. We note that the national unadjusted payment rates and copayment rates shown in Addenda A and B to this final rule with comment period reflect the full market basket conversion factor increase, as discussed in section XVI.D. of this final rule with comment period.
III. OPPS Ambulatory Payment Classification (APC) Group Policies
A. OPPS Treatment of New CPT and Level II HCPCS Codes
CPT and Level II HCPCS codes are used to report procedures, services, items, and supplies under the hospital OPPS. Specifically, CMS recognizes the following codes on OPPS claims: (1) Category I CPT codes, which describe medical services and procedures; (2) Category III CPT codes, which describe new and emerging technologies, services, and procedures; and (3) Level II HCPCS codes, which are used primarily to identify products, supplies, temporary procedures, and services not described by CPT codes. CPT codes are established by the AMA and the Level II HCPCS codes are established by the CMS HCPCS Workgroup. These codes are updated and changed throughout the year. CPT and HCPCS code changes that affect the OPPS are published both through the annual rulemaking cycle and through the OPPS quarterly update Change Requests (CRs). CMS releases new Level II HCPCS codes to the public or recognizes the release of new CPT codes by the AMA and makes these codes effective (that is, the codes can be reported on Medicare claims) outside of the formal rulemaking process via OPPS quarterly update CRs. This quarterly process offers hospitals access to codes that may more accurately describe items or services furnished and/or provides payment or more accurate payment for these items or services in a timelier manner than if CMS waited for the annual rulemaking process. We solicit comments on these new codes and finalize our proposals related to these codes through our annual rulemaking process.
We note that we sought public comments in the CY 2009 OPPS/ASC final rule with comment period on the new CPT and Level II HCPCS codes that were effective January 1, 2009. We also sought public comments in the CY 2009 OPPS/ASC final rule with comment period on the new Level II HCPCS codes effective October 1, 2008. These new codes with an effective date of October 1, 2008 or January 1, 2009 were flagged with comment indicator “NI” (New code, interim APC assignment; comments will be accepted on the interim APC assignment for the new code) in Addendum B to the CY 2009 OPPS/ASC final rule with comment period to indicate that we were assigning them an interim payment status and an APC and payment rate, if applicable, which were subject to public comment following publication of the CY 2009 OPPS/ASC final rule with comment period. Summaries of public comments on the codes flagged with comment indicator “NI” in the CY 2009 OPPS/ASC final rule with comment period and our responses are included in the sections of this final rule with Start Printed Page 60432 comment period that are relevant to the services described by those codes.
In Table 13 of the CY 2010 OPPS/ASC proposed rule (74 FR 35299), which is reproduced as Table 18 in this final rule with comment period, we summarized our process for updating codes through our OPPS quarterly update CRs, seeking public comment, and finalizing their treatment under the OPPS.
| OPPS quarterly update CR | Type of code | Effective date | Comments sought | When finalized |
|---|---|---|---|---|
| April 1, 2009 | Level II HCPCS Codes | April 1, 2009 | CY 2010 OPPS/ASC proposed rule | CY 2010 OPPS/ASC final rule with comment period. |
| July 1, 2009 | Level II HCPCS Codes | July 1, 2009 | CY 2010 OPPS/ASC proposed rule | CY 2010 OPPS/ASC final rule with comment period. |
| Category I (certain vaccine codes) and Category III CPT Codes | July 1, 2009 | CY 2010 OPPS/ASC proposed rule | CY 2010 OPPS/ASC final rule with comment period. | |
| October 1, 2009 | Level II HCPCS Codes | October 1, 2009 | CY 2010 OPPS/ASC final rule with comment period | CY 2011 OPPS/ASC final rule with comment period. |
| January 1, 2010 | Level II HCPCS Codes | January 1, 2010 | CY 2010 OPPS/ASC final rule with Comment Period | CY 2011 OPPS/ASC final rule with comment period. |
| Category I and Category III CPT Codes | January 1, 2010 | CY 2010 OPPS/ASC final rule with comment period | CY 2011 OPPS/ASC final rule with comment period. |
1. Treatment of New Level II HCPCS Codes and Category I CPT Vaccine Codes and Category III CPT Codes
In the April 1 and July 1 CRs for CY 2009, we made effective a total of 13 new Level II HCPCS codes that were not addressed in the CY 2009 OPPS/ASC final rule with comment period that updated the OPPS and we allowed separate payment for 12 of these new codes. Through the April 1, 2009 CR, we also changed the OPPS status indicator for one existing Level II HCPCS code from the interim status indicator designated in the CY 2009 OPPS/ASC final rule with comment period to a status indicator that allowed separate pass-through payment for this code. In addition to the changes for Level II HCPCS codes, we made effective 5 new Category I vaccine and Category III CPT codes that were not addressed in the CY 2009 OPPS/ASC final rule with comment period that updated the OPPS and we allowed separate payment for 3 of these new codes.
Through the April 2009 OPPS quarterly update CR (Transmittal 1702, Change Request 6416, dated March 13, 2009), we allowed separate payment for a total of 2 additional Level II HCPCS codes, specifically existing HCPCS code C9247 (Iobenguane, I–123, diagnostic, per study dose, up to 10 millicuries) and new HCPCS code C9249 (Injection, certolizumab pegol, 1 mg). HCPCS code C9249, which received separate payment as a result of its pass-through status under the OPPS, was made effective on April 1, 2009. HCPCS code C9247 was released January 1, 2009 through the January 2009 OPPS quarterly update CR (Transmittal 1657, Change Request 6320, dated December 31, 2008). From January 1, 2009 through March 31, 2009, HCPCS code C9247 was packaged under the OPPS and assigned status indicator “N” (Items and Services Packaged into APC Rates). We note that between January 1, 2009 through March 31, 2009, HCPCS code C9247 was recognized as a nonpass-through diagnostic radiopharmaceutical. Because nonpass-through diagnostic radiopharmaceuticals are packaged under the OPPS, there was no separate APC payment for HCPCS code C9247 from January 1, 2009 through March 31, 2009. However, effective April 1, 2009, HCPCS code C9247 was allowed separate pass-through payment and its status indicator was revised from “N” to “G” (Pass-Through Drugs and Biologicals).
In the CY 2010 OPPS/ASC proposed rule, we solicited public comments on the status indicators and APC assignments of HCPCS codes C9247 and C9249, which were listed in Table 14 of that proposed rule (74 FR 35301) and now appear in Table 19 of this final rule with comment period.
We did not receive any public comments on the proposed APC assignments and status indicators for HCPCS codes C9247 and C9249. However, for CY 2010, the HCPCS Workgroup replaced both HCPCS C-codes with permanent HCPCS codes. Specifically, C9247 was replaced with A9582 (Iodine I–123 iobenguane, diagnostic, per study dose, up to 15 millicuries) and C9249 was replaced with J0718 (Injection, certolizumab pegol, 1 mg). Consistent with our general policy of using permanent HCPCS codes if appropriate rather than HCPCS C-codes for the reporting of drugs under the OPPS in order to streamline coding, we are showing the replacement HCPCS codes in Table 19 that will replace the HCPCS C-codes effective January 1, 2010. Both HCPCS C-codes will be deleted December 31, 2009. Because HCPCS code J0718 describes the same drug and the same dosage currently designated by HCPCS code C9249 and this drug will continue on pass-through status in CY 2010, we are assigning HCPCS code J0718 the same status indicator and APC as its predecessor C-code, as shown in Table 19. Although the dosage descriptor of HCPCS code A9582 indicates “per study dose, up to 15 millicuries” and the descriptor of its predecessor C-code designates “per study dose, up to 10 millicuries,” because we believe that the reporting of one unit for a study dose would be the same in almost all cases under either HCPCS code, we are assigning HCPCS code A9582 to the same APC as its predecessor C-code, as shown in Table 19. The recommended dose of I–123 iobenguane is 10 millicuries for adult patients, so we expect that hospitals would report 1 unit of new HCPCS code A9582 for the typical dose in CY 2010, just as they would have reported one unit of HCPCS code C9247 previously for the typical dose. We also note this diagnostic radiopharmaceutical will continue on Start Printed Page 60433 pass-through status in CY 2010; therefore, its CY 2010 status indicator remains as “G.” Because we did not receive any public comments on the new Level II HCPCS codes that were implemented in April 2009, we are adopting as final, without modification, our proposal to assign the Level II HCPCS codes listed in Table 19 to the APCs and status indicators as proposed for CY 2010.
Table 19 below shows the final APC and status indicator assignments for both HCPCS codes A9582 and J0718.
| CY 2010 HCPCS code | CY 2009 HCPCS code | CY 2010 long descriptor | Final CY 2010 status indicator | Final CY 2010 APC |
|---|---|---|---|---|
| A9582 | C9247 | Iodine I–123 iobenguane, diagnostic, per study dose, up to 15 millicuries | G | 9247 |
| J0718 | C9249 | Injection, certolizumab pegol, 1 mg | G | 9249 |
Through the July 2009 OPPS quarterly update CR (Transmittal 107, Change Request 6492, dated May 22, 2009), which included HCPCS codes that were made effective July 1, 2009, we allowed separate payment for a total of 11 new Level II HCPCS codes for pass-through drugs and biologicals and nonpass-through drugs and nonimplantable biologicals. Specifically, we provided separate payment for HCPCS codes C9250 (Human plasma fibrin sealant, vapor-heated, solvent-detergent (Artiss), 2ml); C9251 (Injection, C1 esterase inhibitor (human), 10 units); C9252 (Injection, plerixafor, 1 mg); C9253 (Injection, temozolomide, 1 mg); C9360 (Dermal substitute, native, non-denatured collagen, neonatal bovine origin (SurgiMend Collagen Matrix), per 0.5 square centimeters); C9361 (Collagen matrix nerve wrap (NeuroMend Collagen Nerve Wrap), per 0.5 centimeter length); C9362 (Porous purified collagen matrix bone void filler (Integra Mozaik Osteoconductive Scaffold Strip), per 0.5 cc); C9363 (Skin substitute, Integra Meshed Bilayer Wound Matrix, per square centimeter); C9364 (Porcine implant, Permacol, per square centimeter); Q2023 (Injection, factor viii (antihemophilic factor, recombinant) (Xyntha), per i.u.); and Q4116 (Skin substitute, Alloderm, per square centimeter).
Although HCPCS code Q4115 (Skin substitute, Alloskin, per square centimeter) was made effective July 1, 2009, because ASP pricing information was not available at the time the code was made effective, the HCPCS code was not paid separately and it was assigned status indicator “M” (Items and Services Not Billable to the Fiscal Intermediary/MAC) in the CY 2010 OPPS/ASC proposed rule (74 FR 35300 through 35301). For the October 2009 OPPS quarterly update, the status indicator for HCPCS code Q4115 was revised from “M” to K” (Nonpass-Through Drugs and Biologicals) effective October 1, 2009 because pricing information was available, and this product was paid separately as a new biological HCPCS code based on the ASP methodology, consistent with the final CY 2009 policy and the final CY 2010 policy for payment of new drug and biological HCPCS codes without pass-through status. The change in status indicator assignment was announced through the October 2009 OPPS quarterly update CR (Transmittal 1803, Change Request 6626, dated August 28, 2009).
In the CY 2010 OPPS/ASC proposed rule, we solicited public comments on the status indicators, APC assignments, and payment rates of these codes, which were listed in Table 15 of that proposed rule (74 FR 35301) and now appear in Table 20 of this final rule with comment period. Because of the timing of the proposed rule, the codes implemented in the July 2009 OPPS update were not included in Addendum B of that proposed rule, while those codes based upon the April 2009 OPPS update were included in Addendum B. In the CY 2009 OPPS/ASC proposed rule, we proposed to assign the new HCPCS codes for CY 2010 to the designated APCs listed in Table 15 for each HCPCS code and incorporate them into our final rule with comment period for CY 2010, which is consistent with our annual APC updating policy.
We did not receive any public comments on the proposed APC assignments, payment rates, and status indicators designated for the codes listed in Table 15 of the proposed rule. However, for CY 2010, the HCPCS Workgroup created permanent HCPCS J-codes for 4 of the 11 separately payable drug codes. Consistent with our general policy of using permanent HCPCS codes if appropriate rather than HCPCS C-codes for the reporting of drugs under the OPPS in order to streamline coding, we are showing the HCPCS J-codes in Table 20 of this final rule with comment period that will replace the HCPCS C-codes effective January 1, 2010. HCPCS code C9251 is replaced with J0598 (Injection, C1 esterase inhibitor (human), 10 units); C9252 with J2562 (Injection, plerixafor, 1 mg); C9253 is replaced with J9328 (Injection, temozolomide, 1 mg); and Q2023 is replaced with J7185 (Injection, factor viii (antihemophilic factor, recombinant) (Xyntha), per i.u.). The HCPCS J-codes describe the same drugs and the same dosages as the HCPCS C-codes that will be deleted December 31, 2009. We note that HCPCS C-codes are temporary national HCPCS codes. To avoid duplication, temporary national HCPCS codes, such as “C,” “G,” “K,” and “Q” codes, are generally deleted once permanent national HCPCS codes are created that describe the same item, service, or procedure. Because three of the four new HCPCS J-codes describe the same drugs and the same dosages that are currently designated by HCPCS codes C9251, C9252, and C9253 and all three of these drugs will continue on pass-through status in CY 2010, we are assigning the HCPCS J-codes to the same APCs and status indicators as their predecessor HCPCS C-codes, as shown in Table 20. That is, HCPCS code J0598 is assigned to the same APC and status indicator as HCPCS code C9251 (APC 9251); HCPCS code J2562 is assigned to APC 9252; and HCPCS J9328 is assigned to APC 9253. Also, we note that, effective January 1, 2010, HCPCS code Q2023 will be replaced with HCPCS code J7185, which has the same descriptor and is assigned to the same APC and status indicator as HCPCS code Q2023.
Because we did not receive any public comments on the new Level II HCPCS codes that were implemented in July 2009, we are adopting as final, without modification, our proposal to assign the Level II HCPCS codes listed in Table 20 to the APCs and status indicators as proposed for CY 2010. Start Printed Page 60434
| CY 2010 HCPCS code | CY 2009 HCPCS code | CY 2010 Long descriptor | Final CY 2010 status indicator | Final CY 2010 APC |
|---|---|---|---|---|
| C9250 | C9250 | Human plasma fibrin sealant, vapor-heated, solvent-detergent (Artiss), 2ml | G | 9250 |
| J0598 | C9251 | Injection, C1 esterase inhibitor (human), 10 units | G | 9251 |
| J2562 | C9252 | Injection, plerixafor, 1 mg | G | 9252 |
| J9328 | C9253 | Injection, temozolomide, 1 mg | G | 9253 |
| C9360 | C9360 | Dermal substitute, native, non-denatured collagen, neonatal bovine origin (SurgiMend Collagen Matrix), per 0.5 square centimeters | G | 9360 |
| C9361 | C9361 | Collagen matrix nerve wrap (NeuroMend Collagen Nerve Wrap), per 0.5 centimeter length | G | 9361 |
| C9362 | C9362 | Porous purified collagen matrix bone void filler (Integra Mozaik Osteoconductive Scaffold Strip), per 0.5 cc | G | 9362 |
| C9363 | C9363 | Skin substitute, Integra Meshed Bilayer Wound Matrix, per square centimeter | G | 9363 |
| C9364 | C9364 | Porcine implant, Permacol, per square centimeter | G | 9364 |
| J7185 | Q2023 | Injection, factor viii (antihemophilic factor, recombinant) (Xyntha), per i.u. | K | 1268 |
| Q4115 | Q4115 | Skin substitute, Alloskin, per square centimeter | K | 1287 |
| Q4116 | Q4116 | Skin substitute, Alloderm, per square centimeter | K | 1270 |
In the CY 2010 OPPS/ASC proposed rule (74 FR 35300), we proposed to continue our established policy of recognizing Category I CPT vaccine codes for which FDA approval is imminent and Category III CPT codes that the AMA releases in January of each year for implementation in July through the OPPS quarterly update process. Under the OPPS, Category I vaccine codes and Category III CPT codes that are released on the AMA Web site in January are made effective in July of the same year through the July OPPS quarterly update CR, consistent with the AMA's implementation date for the codes. Through the July 2009 OPPS quarterly update CR, we allowed separate payment for 3 of the 5 new Category I vaccine and Category III CPT Codes effective July 1, 2009. Specifically, as displayed in Table 16 of the CY 2010 OPPS/ASC proposed rule (74 FR 35301) and reproduced in this final rule with comment period as Table 21, we allowed payment for CPT codes 0199T (Physiologic recording of tremor using accelerometer(s) and gyroscope(s), (including frequency and amplitude) including interpretation and report); 0200T (Percutaneous sacral augmentation (sacroplasty), unilateral injection(s), including the use of a balloon or mechanical device (if utilized), one or more needles); and 0201T (Percutaneous sacral augmentation (sacroplasty), bilateral injections, including the use of a balloon or mechanical device (if utilized), two or more needles). We note that CPT code 0202T (Posterior vertebral joint(s) arthroplasty (e.g., facet joint[s] replacement) including facetectomy, laminectomy, foraminotomy and vertebral column fixation, with or without injection of bone cement, including fluoroscopy, single level, lumbar spine) was assigned status indicator “C” (Inpatient Procedures) because we believe that this procedure may only be safely performed on Medicare beneficiaries in the hospital inpatient setting. In addition, CPT code 90670 (Pneumococcal conjugate vaccine, 13 valent, for intramuscular use), a Category I CPT vaccine code, was assigned status indicator “E” (Items, Codes, and Services not paid by Medicare when submitted on outpatient claims (any outpatient bill type)) because the drug has not yet been approved by the FDA for marketing.
Because the July 2009 OPPS quarterly update CR was issued close to the publication of the CY 2010 OPPS/ASC proposed rule, the Category I vaccine and Category III CPT codes implemented through the July 2009 OPPS quarterly update CR were not be included in Addendum B to the proposed rule, but these codes were listed in Table 16 of the proposed rule. Additionally, we proposed to incorporate them into Addendum B to this CY 2010 OPPS/ASC final rule with comment period, which is consistent with our annual OPPS update policy.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35301), we solicited public comments on the proposed status indicators, APC assignments, and payment rates for the new Category I and III CPT codes. We did not receive any public comments on our proposals for CPT codes 0199T, 0200T, 0201T, 0202T, and 90670. Therefore, we are finalizing our CY 2010 proposals for these codes, without modification. The final CY 2010 status indicators and APC assignments for CPT codes 0199T, 0200T, 0201T, 0202T, and 90670 are listed in Table 21 below, as well as in Addendum B to this final rule with comment period.
| CY 2010 HCPCS code | CY 2010 long descriptor | Final CY 2010 status indicator | Final CY 2010 APC |
|---|---|---|---|
| 0199T | Physiologic recording of tremor using accelerometer(s) and gyroscope(s), (including frequency and amplitude) including interpretation and report | S | 0215 |
| 0200T | Percutaneous sacral augmentation (sacroplasty), unilateral injection(s), including the use of a balloon or mechanical device (if utilized), one or more needles | T | 0049 |
| Start Printed Page 60435 | |||
| 0201T | Percutaneous sacral augmentation (sacroplasty), bilateral injections, including the use of a balloon or mechanical device (if utilized), two or more needles | T | 0050 |
| 0202T | Posterior vertebral joint(s) arthroplasty (e.g., facet joint[s] replacement) including facetectomy, laminectomy, foraminotomy and vertebral column fixation, with or without injection of bone cement, including fluoroscopy, single level, lumbar spine | C | Not applicable. |
| 90670 | Pneumococcal conjugate vaccine, 13 valent, for intramuscular use | E | Not applicable. |
2. Process for New Level II HCPCS Codes and Category I and Category III CPT Codes for Which We Are Soliciting Public Comments on the CY 2010 OPPS/ASC Final Rule With Comment Period
As has been our practice in the past, we incorporate those new Category I and III CPT codes and new Level II HCPCS codes that are effective January 1 in the final rule with comment period updating the OPPS for the following calendar year. These codes are released to the public via the CMS HCPCS (for Level II HCPCS codes) and AMA Web sites (for CPT codes), and also through the January OPPS quarterly update CRs. In the past, we also have released new Level II HCPCS codes that are effective October 1 through the October OPPS quarterly update CRs and incorporated these new codes in the final rule with comment period updating the OPPS for the following calendar year. All of these codes are flagged with comment indicator “NI” in Addendum B to the OPPS/ASC final rule with comment period to indicate that we are assigning them an interim payment status which is subject to public comment. Specifically, the status indicator and the APC assignment, and payment rate, if applicable, for all such codes flagged with comment indicator “NI” are open to public comment in this final rule with comment period, and we respond to these comments in the final rule with comment period for the next calendar year's OPPS/ASC update. In the CY 2010 OPPS/ASC proposed rule (74 FR 35302), we proposed to continue this process for CY 2010. Specifically, for CY 2010, we proposed to include in Addendum B to the CY 2010 OPPS/ASC final rule with comment period the new Category I and III CPT codes effective January 1, 2010 (including those Category I vaccine and Category III CPT codes that were released by the AMA in July 2009) that would be incorporated in the January 2010 OPPS quarterly update CR and the new Level II HCPCS codes, effective October 1, 2009 or January 1, 2010, that would be released by CMS in its October 2009 and January 2010 OPPS quarterly update CRs. Excluding those Category I vaccine and Category III CPT codes that were released by the AMA in July 2009 which were subject to comment in the CY 2010 OPPS/ASC proposed rule as described above, these codes would be flagged with comment indicator “NI” in Addendum B to this CY 2010 OPPS/ASC final rule with comment period to indicate that we have assigned them an interim OPPS payment status. We proposed that their status indicators and their APC assignments and payment rates, if applicable, would be open to public comment in the CY 2010 OPPS/ASC final rule with comment period and would be finalized in the CY 2011 OPPS/ASC final rule with comment period.
Comment: One commenter requested that CMS solicit public comments on APC assignments for the newly implemented CPT codes that go into effect January 1 and, when necessary, revise their APC assignments and implement the changes in the next quarterly OPPS update to promote payment accuracy.
Response: For new HCPCS codes with an interim final APC and/or status indicator designation in a final rule, we are only able to finalize their assignments in another OPPS final rule in order to allow for the necessary public notice and comment period and to allow for CMS to respond to such comments. Therefore, we only assign HCPCS codes permanently for the year through the annual regulatory process. Because we are not able to revise APC and/or status indicator assignments for the newly implemented HCPCS codes in CY 2010 that are assigned an interim final status in this CY 2010 OPPS/ASC final rule with comment period outside of the rulemaking process, the next available opportunity to update an APC or status indicator for these codes is in the CY 2011 OPPS update. These HCPCS codes retain their interim final APC and status indicator assignments for all of CY 2010. Therefore, only in the CY 2011 OPPS/ASC final rule with comment period will we be able to finalize the APC and/or status indicator assignments of the new CY 2010 HCPCS codes and respond to all public comments received on their interim designations.
After consideration of the public comment we received, we are finalizing our proposal, without modification, to provide interim final status indicators and APC assignments and payment rates, if applicable, for all CPT codes newly implemented in January 2010 and all HCPCS codes newly implemented in October 2009 or January 2010 in Addendum B to this final rule with comment period. The interim final OPPS treatment of these codes is open to public comment in the CY 2010 OPPS/ASC final rule with comment period and will be finalized in the CY 2011 OPPS/ASC final rule with comment period.
B. OPPS Changes—Variations Within APCs
1. Background
Section 1833(t)(2)(A) of the Act requires the Secretary to develop a classification system for covered outpatient department services. Section 1833(t)(2)(B) of the Act provides that the Secretary may establish groups of covered outpatient department services within this classification system, so that services classified within each group are comparable clinically and with respect to the use of resources (and so that an implantable item is classified to the group that includes the service to which the item relates). In accordance with these provisions, we developed a grouping classification system, referred to as APCs, as set forth in § 419.31 of the regulations. We use Level I and Level II HCPCS codes and descriptors to identify and group the services within each APC. The APCs are organized such that each group is homogeneous both clinically and in terms of resource use. Using this classification system, we have Start Printed Page 60436 established distinct groups of similar services, as well as medical visits. We also have developed separate APC groups for certain medical devices, drugs, biologicals, therapeutic radiopharmaceuticals, and brachytherapy devices.
We have packaged into payment for each procedure or service within an APC group the costs associated with those items or services that are directly related to and supportive of performing the main independent procedures or furnishing the services. Therefore, we do not make separate payment for these packaged items or services. For example, packaged items and services include: (1) Use of an operating, treatment, or procedure room; (2) use of a recovery room; (3) observation services; (4) anesthesia; (5) medical/surgical supplies; (6) pharmaceuticals (other than those for which separate payment may be allowed under the provisions discussed in section V.3. of this final rule with comment period); (7) incidental services such as venipuncture; and (8) guidance services, image processing services, intraoperative services, imaging supervision and interpretation services, diagnostic radiopharmaceuticals, and contrast media. Further discussion of packaged services is included in section II.A.4. of this final rule with comment period.
In CY 2008 (72 FR 66650), we implemented composite APCs to provide a single payment for groups of services that are typically performed together during a single clinical encounter and that result in the provision of a complete service. Under our CY 2010 OPPS policy, we provide composite APC payment for certain extended assessment and management services, low dose rate (LDR) prostate brachytherapy, cardiac electrophysiologic evaluation and ablation, mental health services, and multiple imaging services. Further discussion of composite APCs is included in section II.A.2.e. of this final rule with comment period.
Under the OPPS, we generally pay for hospital outpatient services on a rate-per-service basis, where the service may be reported with one or more HCPCS codes. Payment varies according to the APC group to which the independent service or combination of services is assigned. Each APC weight represents the hospital median cost of the services included in that APC relative to the hospital median cost of the services included in APC 0606 (Level 3 Hospital Clinic Visits). The APC weights are scaled to APC 0606 because it is the middle level clinic visit APC (that is, where the Level 3 clinic visit CPT code of five levels of clinic visits is assigned), and because middle level clinic visits are among the most frequently furnished services in the hospital outpatient setting.
Section 1833(t)(9)(A) of the Act requires the Secretary to review not less often than annually and revise the groups, relative payment weights, and the wage and other adjustments under the OPPS to take into account changes in medical practice, changes in technology, the addition of new services, new cost data, and other relevant information and factors. Section 1833(t)(9)(A) of the Act, as amended by section 201(h) of the BBRA, also requires the Secretary to consult with an outside panel of experts to review (and advise the Secretary concerning) the clinical integrity of the APC groups and the relative payment weights (the APC Panel recommendations for specific services for the CY 2010 OPPS and our responses to them are discussed in the relevant specific sections throughout this final rule with comment period).
Finally, section 1833(t)(2) of the Act provides that, subject to certain exceptions, the items and services within an APC group cannot be considered comparable with respect to the use of resources if the highest median cost (or mean cost as elected by the Secretary) for an item or service in the group is more than 2 times greater than the lowest median cost (or mean cost, if so elected) for an item or service within the same group (referred to as the “2 times rule”). We use the median cost of the item or service in implementing this provision. In performing this analysis, we examine data from the significant services assigned to an APC, specifically those HCPCS codes with a single claim frequency of greater than 1,000 or a frequency of greater than 99 and a percentage of all single claims that is equal to or greater than 2 percent. Because, as a matter of policy, HCPCS codes that are unlisted procedures, not otherwise classified, or not otherwise specified codes are assigned to the lowest level APC that is appropriate to the clinical nature of the service (69 FR 65724 through 65725), we do not consider the costs of these services in assessing APCs for 2 times violations. Section 1833(t)(2) of the Act authorizes the Secretary to make exceptions to the 2 times rule in unusual cases, such as low-volume items and services (but the Secretary may not make such an exception in the case of a drug or biological that has been designated as an orphan drug under section 526 of the Federal Food, Drug, and Cosmetic Act).
2. Application of the 2 Times Rule
In accordance with section 1833(t)(2) of the Act and § 419.31 of the regulations, we annually review the items and services within an APC group to determine, with respect to comparability of the use of resources, if the median cost of the highest cost item or service within an APC group is more than 2 times greater than the median of the lowest cost item or service within that same group. In the CY 2010 OPPS/ASC proposed rule (74 FR 35303), we proposed to make exceptions to this limit on the variation of costs within each APC group in unusual cases, such as low-volume items and services for CY 2010.
During the APC Panel's February 2009 meeting, we presented median cost and utilization data for services furnished during the period of January 1, 2008 through September 30, 2008, because we had concerns or the public had raised concerns regarding their APC assignments, status indicator assignments, or payment rates. In addition to the assignment of specific services to APCs that we discussed with the APC Panel, we also identified APCs with 2 times violations that were not specifically discussed with the APC Panel but for which we proposed changes to their HCPCS codes' APC assignments in Addendum B to the CY 2010 OPPS/ASC proposed rule. In these cases, to eliminate a 2 times violation or to improve clinical and resource homogeneity, we proposed to reassign the codes to APCs that contain services that are similar with regard to both their clinical and resource characteristics. We also proposed to rename existing APCs or create new clinical APCs to complement proposed HCPCS code reassignments. In many cases, the proposed HCPCS code reassignments and associated APC reconfigurations for CY 2010 included in the proposed rule were related to changes in median costs of services that were observed in the CY 2008 claims data newly available for CY 2010 ratesetting. In addition, we proposed changes to the status indicators for some codes that were not specifically and separately discussed in the proposed rule. In these cases, we proposed to change the status indicators for some codes because we believed that another status indicator would more accurately describe their payment status from an OPPS perspective based on the policies that we proposed for CY 2010.
Addendum B to the CY 2010 OPPS/ASC proposed rule identified, with comment indicator “CH,” those HCPCS codes for which we proposed a change to the APC assignment or status Start Printed Page 60437 indicator that were initially assigned in the April 2009 Addendum B update (Transmittal 1702, Change Request 6416, dated March 13, 2009).
Comment: One commenter generally supported CMS' adherence to the 2 times rule to ensure appropriate payment for OPPS services and to provide incentives for patients to be treated in the most suitable clinical setting. In particular, the commenter supported the proposed reassignments of the following CPT codes: CPT code 20103 (Exploration of penetrating wound (separate procedure); extremity) from APC 0136 (Level IV Skin Repair) to APC 0007 (Level II Incision & Drainage); and CPT code 29888 (Arthroscopically aided anterior cruciate ligament repair/augmentation or reconstruction) and CPT code 29889 (Arthroscopically aided posterior cruciate ligament repair/augmentation or reconstruction) from APC 0042 (Level II Arthroscopy) to APC 0052 (Level IV Musculoskeletal Procedures Except Hand and Foot).
Response: We appreciate the commenter's support for the 2 times rule in general and for the proposed APC reassignments of CPT codes 20103, 29888, and 29889 in particular. We agree with the commenter that the 2 times rule is an important mechanism for ensuring appropriate payment for OPPS services. Based on the CY 2008 claims and cost report data available for this final rule with comment period, we also agree with the commenter that we should adopt the proposed reassignments of CPT codes 20103, 29888, and 29889 in order to improve clinical and resource homogeneity.
Therefore, after consideration of the public comment we received, we are finalizing, without modification, our CY 2010 proposals to reassign CPT code 20103 to APC 0007, with a final CY 2010 APC median cost of approximately $843 and CPT codes 29888 and 29889 to APC 0052, with a final CY 2010 APC median cost of approximately $5,921.
3. Exceptions to the 2 Times Rule
As discussed earlier, we may make exceptions to the 2 times limit on the variation of costs within each APC group in unusual cases such as low-volume items and services. We stated in the CY 2010 OPPS/ASC proposed rule (74 FR 35303) that we took into account the APC changes that we proposed for CY 2010 based on the APC Panel recommendations, the other proposed changes to status indicators and APC assignments as identified in Addendum B to the proposed rule, and the use of CY 2008 claims data available for the proposed rule to calculate the median costs of procedures classified in the APCs. We then reviewed all of the APCs to determine which APCs would not satisfy the 2 times rule and to determine which APCs should be proposed as exceptions to the 2 times rule for CY 2010. We used the following criteria to decide whether to propose exceptions to the 2 times rule for affected APCs:
- Resource homogeneity;
- Clinical homogeneity;
- Hospital outpatient setting;
- Frequency of service (volume); and
- Opportunity for upcoding and code fragments.
For a detailed discussion of these criteria, we refer readers to the April 7, 2000 OPPS final rule with comment period (65 FR 18457).
Table 17 of the CY 2010 OPPS/ASC proposed rule (74 FR 35303) listed 14 APCs that we proposed to exempt from the 2 times rule for CY 2010 based on the criteria cited above. For cases in which a recommendation by the APC Panel appeared to result in or allow a violation of the 2 times rule, we generally accepted the APC Panel's recommendation because those recommendations were based on explicit consideration of resource use, clinical homogeneity, hospital specialization, and the quality of the CY 2008 claims data used to determine the APC payment rates that we proposed for CY 2010. The median costs for hospital outpatient services for these and all other APCs that were used in the development of the CY 2010 OPPS/ASC proposed rule and this final rule with comment period can be found on the CMS Web site at: http://www.cms.hhs.gov/HospitalOutpatientPPS/01_overview.asp.
For the CY 2010 OPPS/ASC proposed rule, we based the listed exceptions to the 2 times rule on claims data for dates of service between January 1, 2008, and December 31, 2008, that were processed before January 1, 2009. For this final rule with comment period, we used claims data for dates of service between January 1, 2008, and December 31, 2008, that were processed on or before June 30, 2008, and updated CCRs, if available. Thus, after responding to all of the public comments on the CY 2010 OPPS/ASC proposed rule and making changes to APC assignments based on those comments, we analyzed the CY 2008 claims data used for this final rule with comment period to identify the APCs with 2 times violations. Based on the final CY 2008 claims data, we found that there are 15 APCs with 2 times rule violations, a cumulative increase of 1 APC from the proposed rule. We applied the criteria as described earlier to identify the APCs that are exceptions to the 2 times rule for CY 2010, and identified 4 additional APCs that meet the criteria for exception to the 2 times rule for this final rule with comment period: APC 0057 (Bunion Procedures); APC 0060 (Manipulation Therapy); APC 0341 (Skin Tests); and APC 0409 (Red Blood Cell Tests). These APC exceptions are listed in Table 22 below. We also determined that there are 3 APCs that no longer violate the 2 times rule: APC 0237 (Level II Posterior Segment Eye Procedures); APC 0325 (Group Psychotherapy); and APC 0436 (Level I Drug Administration). We have not included in this count those APCs where a 2 times violation is not a relevant concept, such as APC 0375 (Ancillary Outpatient Services when Patient Expires), with an APC median cost set based on multiple procedure claims, so that we have identified only final APCs, including those with criteria-based median costs, such as device-dependent APCs, with 2 times violations.
Comment: One commenter requested that CMS not exempt any imaging and radiation therapy APCs from the 2 times rule. According to the commenter, violations of the 2 times rule should demonstrate to CMS that a particular APC is incorrectly constructed. The commenter recommended that, rather than exempting these APCs from the 2 times rule, CMS review the configurations of the APCs and make any necessary revisions.
Response: We do not agree with the commenter that we should not exempt any imaging and radiation therapy APCs from the 2 times rule. As stated earlier in this section, we may make exceptions to the 2 times limit on the variation of costs within each APC group in unusual cases such as low-volume items and services. In the CY 2010 OPPS/ASC proposed rule (74 FR 35303), we proposed to exempt one imaging APC and one radiation therapy APC from the 2 times rule, specifically APC 0128 (Echocardiogram with Contrast) and APC 0664 (Level I Proton Beam Radiation Therapy), respectively. As discussed in greater detail in section II.A.2.d.(4) of this final rule with comment period, we believe that the median costs of the echocardiography procedures assigned to APC 0128 do not warrant assignment of any of those procedures to a new clinical APC. APC 0664 includes CPT code 77520 (Proton treatment delivery; simple, without compensation), with a median cost of approximately $396 (based on 243 single claims of 251 total claims), and CPT code 77522 (Proton treatment delivery; simple, with compensation), Start Printed Page 60438 with a median cost of approximately $934 (based on 11,012 single claims of 12,252 total claims). We continue to believe that the resources and clinical characteristics of the low-volume procedure described by CPT code 77520 are sufficiently similar to the procedure described by CPT code 77522 to warrant continued assignment of both CPT codes to APC 0664. Therefore, we are finalizing our proposal to exempt APC 0128 and APC 0664 from the 2 times rule for CY 2010. Consistent with our standard policy, we will continue to review, on an annual basis, the APC assignments for all OPPS services to ensure appropriate placement.
We also received a number of specific public comments regarding some of the procedures assigned to APCs that we proposed to exempt from the 2 times rule for CY 2010. Discussions of those public comments are included elsewhere in this final rule with comment period in the specific sections related to the types of procedures that were the subjects of the comments.
After consideration of the public comments we received and our review of the CY 2008 costs from claims available for this final rule with comment period, we are exempting 15 APCs from the 2 times rule for CY 2010, as described previously in this section. Our final list of 15 APCs exempted from the 2 times rule is displayed in Table 22 below.
| Final CY 2010 APC | Final CY 2010 APC Title |
|---|---|
| 0057 | Bunion Procedures. |
| 0060 | Manipulation Therapy. |
| 0080 | Diagnostic Cardiac Catheterization. |
| 0105 | Repair/Revision/Removal of Pacemakers, AICDs, or Vascular Devices. |
| 0128 | Echocardiogram with Contrast. |
| 0141 | Level I Upper GI Procedures. |
| 0142 | Small Intestine Endoscopy. |
| 0245 | Level I Cataract Procedures without IOL Insert. |
| 0303 | Treatment Device Construction. |
| 0341 | Skin Tests. |
| 0381 | Single Allergy Tests. |
| 0409 | Red Blood Cell Tests. |
| 0432 | Health and Behavior Services. |
| 0604 | Level 1 Hospital Clinic Visits. |
| 0664 | Level I Proton Beam Radiation Therapy. |
C. New Technology APCs
1. Background
In the November 30, 2001 final rule (66 FR 59903), we finalized changes to the time period a service was eligible for payment under a New Technology APC. Beginning in CY 2002, we retain services within New Technology APC groups until we gather sufficient claims data to enable us to assign the service to a clinically appropriate APC. This policy allows us to move a service from a New Technology APC in less than 2 years if sufficient data are available. It also allows us to retain a service in a New Technology APC for more than 2 years if sufficient data upon which to base a decision for reassignment have not been collected.
We note that the cost bands for New Technology APCs range from $0 to $50 in increments of $10, from $50 to $100 in increments of $50, from $100 through $2,000 in increments of $100, and from $2,000 through $10,000 in increments of $500. These cost bands identify the APCs to which new technology procedures and services with estimated service costs that fall within those cost bands are assigned under the OPPS. Payment for each APC is made at the mid-point of the APC's assigned cost band. For example, payment for New Technology APC 1507 (New Technology—Level VII ($500–$600)) is made at $550. Currently, there are 82 New Technology APCs, ranging from the lowest cost band assigned to APC 1491 (New Technology—Level IA ($0–$10)) through the highest cost band assigned to APC 1574 (New Technology—Level XXXVII ($9,500–$10,000). In CY 2004 (68 FR 63416), we last restructured the New Technology APCs to make the cost intervals more consistent across payment levels and refined the cost bands for these APCs to retain two parallel sets of New Technology APCs, one set with a status indicator of “S” (Significant Procedures, Not Discounted when Multiple. Paid under OPPS; separate APC payment) and the other set with a status indicator of “T” (Significant Procedure, Multiple Reduction Applies. Paid under OPPS; separate APC payment). These current New Technology APC configurations allow us to price new technology services more appropriately and consistently.
2. Movement of Procedures From New Technology APCs to Clinical APCs
As we explained in the November 30, 2001 final rule (66 FR 59902), we generally keep a procedure in the New Technology APC to which it is initially assigned until we have collected sufficient data to enable us to move the procedure to a clinically appropriate APC. However, in cases where we find that our original New Technology APC assignment was based on inaccurate or inadequate information (although it was the best information available at the time), or where the New Technology APCs are restructured, we may, based on more recent resource utilization information (including claims data) or the availability of refined New Technology APC cost bands, reassign the procedure or service to a different New Technology APC that most appropriately reflects its cost.
Consistent with our current policy, in the CY 2010 OPPS/ASC proposed rule (74 FR 35304), for CY 2010, we proposed to retain services within New Technology APC groups until we gather sufficient claims data to enable us to assign the service to a clinically appropriate APC. The flexibility associated with this policy allows us to move a service from a New Technology APC in less than 2 years if sufficient data are available. It also allows us to retain a service in a New Technology APC for more than 2 years if sufficient Start Printed Page 60439 hospital claims data upon which to base a decision for reassignment have not been collected.
Table 18 of the proposed rule (74 FR 35304) listed the HCPCS code and its associated status indicator that we proposed to reassign from a New Technology APC to a clinically appropriate APC for CY 2010. Based on the CY 2008 OPPS claims data available for the proposed rule, we believe we had sufficient claims data to propose reassignment of CPT code 0182T to a clinically appropriate APC. Specifically, we proposed to reassign this electronic brachytherapy service from APC 1519 (New Technology—Level IXX ($1,700–$1,800)) to APC 0313 (Brachytherapy), where other brachytherapy services also reside. Based on hospital claims data for CPT code 0182T, its hospital resource costs are similar to those of other services assigned to APC 0313.
The proposed CY 2010 APC reassignment of CPT code 0182T was discussed with the APC Panel at its August 2009 meeting. One public presenter indicated that CPT code 0182T describes both single-fraction and multiple-fraction electronic brachytherapy, and that most of the claims on which CMS based its CY 2010 proposal were from hospitals reporting multi-fraction electronic brachytherapy. The presenter believed that the hospital resources required for these two types of brachytherapy were significantly different from one another. The presenter also stated that, unlike the conventional brachytherapy procedures that are assigned to APC 0313 and for which the associated brachytherapy sources are all paid separately under the OPPS, payment for the brachytherapy source associated with CPT code 0182T is packaged into the procedure payment. The APC Panel noted that the problem of distinguishing single-fraction from multiple-fraction electronic brachytherapy is a coding issue that would not be resolved by additional claims data because the two types of procedures are reported with the same CPT code. After discussion of the median cost of CPT code 0182T observed in claims data and the potential contribution of the brachytherapy source cost to the overall procedure cost, the APC Panel made no recommendation on the CY 2010 APC assignment for CPT code 0182T.
Comment: Several commenters disagreed with the CMS proposal to reassign CPT code 0182T to APC 0313 for CY 2010. Commenters responding regarding both single-fraction and multiple-fraction electronic brachytherapy procedures reported with CPT code 0182T asserted that the proposed payment of approximately $747 does not cover the full costs of providing these services. They indicated that the current payment rate of $1,750 that is associated with APC 1519, where CPT code 0182T is assigned, includes the cost of the electronic brachytherapy source, whereas the payment rate for APC 0313 does not. Several commenters noted that the claims data used in determining the reassignment for CPT code 0182T are very limited and pointed out that the claims data for this service came from only a few hospitals that were early adopters of the multiple-source electronic brachytherapy technology. They argued that data from these hospitals represented pre-commercial clinical site data and that, therefore, these hospitals had received equipment and sources at reduced cost. One commenter suggested that CMS should develop coding that would distinguish between single-fraction and multiple-fraction electronic brachytherapy procedures in order to pay appropriately for each type of service because the current single payment for both technologies provides a financial incentive for the use of multiple-fraction electronic brachytherapy. Most commenters urged CMS to continue to assign CPT code 0182T to APC 1519 for at least another year to enable CMS to gather sufficient claims data from more hospitals in order to appropriately reassign CPT code 0182T to a clinical APC based on the clinical and resource costs of the procedure.
Response: CPT code 0182T was initially assigned to New Technology APC 1519 with a payment rate of $1,750 when the code was implemented in July 2007, and it has been assigned to that same APC through CY 2009. For CY 2010, we proposed to reassign CPT code 0182T from New Technology APC 1519 to APC 0313, which had a proposed payment rate of approximately $747 and a proposed median cost of approximately $753 (and now has a final rule median cost of $770). Analysis of hospital claims data from CY 2007 and CY 2008 revealed that the procedure described by CPT code 0182T is not commonly performed on Medicare patients. For CY 2008, claims data show 223 total claims with a median cost of about $506 for this procedure. For CY 2007, claims data (6 months due to implementation of the code in July 2007) show only 21 total claims with a median cost of about $495. Therefore, we believe that the hospital resources required for CPT code 0182T are consistent with the costs of other services assigned to APC 0313 and payment for CPT code 0182T would be appropriately made through that clinical APC.
We are not creating a new Level II HCPCS code for single-fraction electronic brachytherapy at this time because we believe that the two forms of electronic brachytherapy, whether provided in a single-fraction or multiple-fraction regimen, depending on the technology, are both described by CPT code 0182T, which is appropriately assigned to a single APC. We note that the payment is per-fraction according to the code descriptor for CPT code 0182T, and that would include a single-fraction treatment as well. While we recognize that CPT code 0182T describes both single- and multiple-fraction electronic brachytherapy, we commonly pay for different technologies under the OPPS that are reported in a single CPT code and we expect the hospital claims data to reflect the resources required for all of the different technologies reported under the one code. To the extent that one technology is predominantly used by hospitals, then the costs of that technology will have a greater effect on the procedure's median cost, but we do not believe payment through such groupings inappropriately encourages the use of certain technologies, such as the provision of multiple-fraction electronic brachytherapy in the case of CPT code 0182T. Our standard OPPS ratesetting methodology provides a single payment based on historical hospital costs that reflect utilization patterns of the various technologies, consistent with prospective payment for groups of similar services in order to encourage hospital efficiencies. The hospital cost information for services always reflects the discounts available to hospitals in the claims year, such as the commenters indicate was the case for multiple-fraction electronic brachytherapy in CY 2008, that may not be available in the payment year for those services, and those discounts may vary from year to year for different HOPD services. Nevertheless, we rely on the relativity of median costs as reflected in claims data to be appropriate. Payment based on a measure of central tendency is a principle of any prospective payment system like the OPPS. In some individual cases payment exceeds the average cost and in other cases payment is less than the average cost. On balance, however, payment should approximate the relative cost of the average case, recognizing that, as a prospective payment system, the OPPS is a system of averages. In the case of CPT code 0182T, we believe that its assignment to Start Printed Page 60440 APC 0313 for CY 2010 is fully consistent with our standard ratesetting methodology that provides appropriate incentives for efficiency.
As of January 2010, CPT code 0182T will have been assigned to New Technology APC 1519 for 2 1/2 years. While we have relatively few claims data from CY 2007 and CY 2008 for electronic brachytherapy, a commenter on a prior rule has indicated that this service may only be used to treat a small number of patients (72 FR 66691). To the extent that more hospitals furnish electronic brachytherapy in future years and that hospital costs from commercialization of the technology change, we expect to see those costs reflected in our claims data for those future years, which we will annually review for electronic brachytherapy, just as we do for all OPPS services. Moreover, while we acknowledge that, in the case of conventional brachytherapy procedures where distinct radioactive sources are implanted, the statute requires separate payment of the associated radioactive brachytherapy source so that APC 0313 only pays for the application of those sources, we have no reason to believe that reported hospital costs for CPT code 0182T do not include the cost of the source. As we stated in the CY 2007 OPPS/ASC final rule with comment period (71 FR 68113), we do not consider specific devices, beams of radiation, or equipment that do not constitute separate sources that utilize radioactive isotopes to deliver radiation to be brachytherapy sources for separate payment, as such items do not meet the statutory requirements provided in section 1833(t)(2)(H) of the Act. Electronic brachytherapy, described by CPT code 0182T, utilizes such devices, beams of radiation, or equipment to generate the radiation for the treatment, rather than distinct radioactive sources. Therefore, in CY 2008, hospitals would have included the costs of the devices or equipment that are necessary to generate the radiation in their charges for the electronic brachytherapy procedures, in contrast to the conventional brachytherapy procedures also assigned to APC 0313 where the sources would have been separately reported and paid. Therefore, just as in the case of other OPPS services, our ratesetting methodology relies upon hospitals' consideration of all costs associated with furnishing services, including the costs of those items and services for which payment is packaged, in setting hospital charges for separately payable procedures such as electronic brachytherapy. Although the hospital median costs for other HCPCS codes assigned to APC 0313 do not include the cost of radioactive brachytherapy sources that are separately paid, we believe the hospital cost of CPT code 0182T includes the cost of the devices or equipment used to generate the radiation for the treatment. This difference in packaging source payment between conventional and electronic brachytherapy procedures alone does not lead us to conclude that these procedures do not share sufficient cost and clinical similarity to be assigned to the same clinical APC. The overall hospital costs for conventional and electronic brachytherapy procedures, including the associated packaged costs, that are paid through the procedure codes for electronic and conventional brachytherapy are comparable and the procedures are clinically similar so we believe that their assignment to the same APC is appropriate, regardless of the differences in their packaged costs.
Therefore, we continue to believe that APC 0313 is an appropriate APC for assignment of CPT code 0182T based on our consideration of the clinical characteristics of electronic brachytherapy and hospital costs from claims data. Maintaining CPT code 0182T in APC 0313 for another year would pay at a rate that is three times the cost of this service as reflected in the hospital outpatient claims data, and we do not believe continued payment at $1,750 is appropriate. To the extent that hospitals' costs change over time if the procedure is more broadly furnished, consistent with our current policy to annually assess the appropriateness of the APC assignments for all services under the hospital OPPS, we will continue to monitor our claims data for CPT code 0182T in the future.
After consideration of the public comments we received, we are finalizing our proposal, without modification, to assign CPT code 0182T to APC 0313, which has a final CY 2010 APC median cost of approximately $770. Table 23 below lists the HCPCS code and its associated status indicator for CY 2010.
| CY 2010 HCPCS code | Short descriptor | CY 2009 SI | CY 2009 APC | Final CY 2010 SI | Final CY 2010 APC |
|---|---|---|---|---|---|
| 0182T | Hdr elect brachytherapy | S | 1519 | S | 0313 |
D. OPPS APC-Specific Policies
In this section, we discuss HCPCS codes and their proposed status indicators and APC reassignments for which we provided explicit discussion in section III.D. of the CY 2010 OPPS/ASC proposed rule or for which we received public comments on their proposed CY 2010 OPPS treatment. Certain HCPCS codes are discussed in other sections of this final rule with comment period, as appropriate to the items or services they describe. The final CY 2010 OPPS/ASC treatment of all other HCPCS not explicitly discussed in this final rule with comment period is displayed in Addendum B to this final rule with comment period.
1. Cardiovascular Services
a. Cardiovascular Telemetry (APC 0209)
For CY 2010, we proposed to continue to assign CPT code 93229 (Wearable mobile cardiovascular telemetry with electrocardiographic recording, concurrent computerized real time data analysis and greater than 24 hours of accessible ECG data storage (retrievable with query) with ECG-triggered and patient-selected events transmitted to a remote attended surveillance center for up to 30 days; technical support for connection and patient instructions for use, attended surveillance, analysis and physician prescribed transmission of daily and emergent data reports) to APC 0209 (Level II Extended EEG, Sleep, and Cardiovascular Studies), with a proposed payment rate of approximately $774. Because CPT code 93229 was a new code for CY 2009, in the CY 2009 OPPS/ASC final rule with comment period, we finalized an interim final APC assignment for this code of APC 0209, with a payment rate of approximately $754.
Comment: Some commenters recommended that CMS assign status indicator “A” (Services furnished to a hospital outpatient that are paid under a fee schedule or payment system other Start Printed Page 60441 than OPPS) to CPT code 93229 in order to make this service nonpayable under the OPPS for CY 2010. The commenters argued that there are currently no hospitals that can provide the type of constant monitoring the service described by CPT code 93229 requires. For this reason, according to the commenters, any claims submitted for CPT code 93229 by hospitals are incorrectly coded. The commenters stated that, if CMS chose not to adopt their recommendation and instead chose to continue recognizing CPT code 93229 as payable under the OPPS, CMS should reconsider the proposed assignment of the service to APC 0209. According to the commenters, the service described by CPT code 93229 is not similar clinically or in terms of resource utilization to the other procedures assigned to APC 0209, in particular, the polysomnography procedures described by CPT codes 95810 (Polysomnography; sleep staging with 4 or more additional parameters of sleep, attended by a technologist) and 95811 (Polysomnography; sleep staging with 4 or more additional parameters of sleep, with initiation of continuous positive airway pressure therapy or bilevel ventilation, attended by a technologist), which are the most commonly reported procedures in APC 0209 with the highest number of single claims contributing to the APC's median cost. The commenters urged CMS to assign CPT code 93229 to New Technology APC 1513 (New Technology—Level XIII ($1,100–$1,200)) with a payment rate of $1,150, or New Technology APC 1514 (New Technology—Level XIV ($1,200–$1,300)) with a payment rate of $1,250. The commenters argued that, if any hospitals were to provide the remote cardiac monitoring service described by CPT code 93229, the proposed payment rate for APC 0209 would be less than hospitals' costs for providing this service.
Response: We do not agree with the commenters that we should assign status indicator “A” to CPT code 93229 in order to make the service nonpayable under the OPPS for CY 2010. For each new calendar year, we typically recognize for OPPS payment purposes new HCPCS codes describing services that could be covered by Medicare when provided to hospital outpatients, regardless of whether those services are actually being provided by hospitals at the time the OPPS/ASC final rule with comment period for the upcoming year is issued. We believe that CPT code 93229 describes a diagnostic study that could be provided to Medicare beneficiaries in the hospital outpatient setting and, therefore, could be covered by Medicare. We also do not agree that the service described by CPT code 93229 is not similar clinically and in terms of resource utilization to the other procedures assigned to APC 0209 for CY 2010. For example, similar to the remote cardiac monitoring service described by CPT code 93229, the polysomnography procedures described by CPT codes 95810 and 95811 involve continuous and simultaneous monitoring and recording of various physiological and pathophysiological parameters, with attendance by a technologist.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to assign CPT code 93229 to APC 0209, with a final CY 2010 APC median cost of approximately $764.
b. Implantable Loop Recorder Monitoring (APC 0689)
For CY 2010, we proposed to reassign CPT code 93299 (Interrogation device evaluation(s), (remote) up to 30 days; implantable cardiovascular monitor system or implantable loop recorder system, remote data acquisition(s), receipt of transmissions and technician review, technical support and distribution of results) to APC 0689 (Level II Electronic Analysis of Devices), with a proposed payment rate of approximately $40. In CY 2009, this CPT code was assigned to APC 0209 (Level II Extended EEG, Sleep, and Cardiovascular Studies), with a payment rate of approximately $754.
Comment: One commenter supported the proposed reassignment of CPT code 93299 to APC 0689 for CY 2010. According to the commenter, the procedure described by CPT code 93299 is similar clinically and in terms of resource utilization to other procedures assigned to APC 0689.
Response: We appreciate the commenter's support of our proposal to reassign CPT code 93299 to APC 0689 for CY 2010. We agree that the procedure described by CPT code 93299 is similar clinically and in terms of resource utilization to other procedures assigned to APC 0689.
After consideration of the public comment we received, we are finalizing our CY 2010 proposal, without modification, to reassign CPT code 93299 to APC 0689, which has a final CY 2010 APC median cost of approximately $38.
c. Transluminal Balloon Angioplasty (APC 0279)
For CY 2010, we proposed to reassign CPT code 75978 (Transluminal balloon angioplasty, venous (eg, subclavian stenosis), radiological supervision and interpretation) from APC 0083 (Coronary or Non-Coronary Angioplasty and Percutaneous Valvuloplasty) to APC 0279 (Level II Angiography and Venography), with a proposed payment rate of approximately $2,000.
Comment: Some commenters disagreed with the proposed APC reassignment of CPT code 75978. The commenters noted that CPT code 75978 is a therapeutic interventional procedure that is not similar to the other diagnostic procedures assigned to APC 0279. The commenters requested that CMS continue to assign CPT code 75978 to APC 0083 where they believe the service is most appropriately placed based on considerations of clinical coherence and resource costs.
Response: The proposed CY 2010 median cost for APC 0083 of approximately $3,380 is significantly higher than the proposed CY 2010 median cost of CPT code 75978 of approximately $2,597. Given the difference in median costs, we do not believe that we should continue to assign this procedure to APC 0083. After further analysis and review by CMS medical advisors, we believe that CPT code 75978 would be most appropriately assigned to APC 0093 (Vascular Reconstruction/Fistula Repair without Device) for CY 2010 because it is a therapeutic procedure performed on veins, similar to other therapeutic blood vessel procedures that are currently assigned to APC 0093. Further, the CY 2010 final median cost of CPT code 75978 of approximately $2,597 is very similar to the CY 2010 final median cost of APC 0093 of approximately $2,378.
After consideration of the public comments we received, we are modifying our proposed CY 2010 reassignment of CPT code 75978 from APC 0083 to APC 0279. In this final rule with comment period, for CY 2010, we are reassigning CPT code 75978 from APC 0083 to APC 0093, which has a final CY 2010 APC median cost of approximately $2,378.
2. Gastrointestinal Services
a. Change of Gastrostomy Tube (APC 0676)
For CY 2010, we proposed to reassign CPT code 43760 (Change of gastrostomy tube, percutaneous, without imaging or endoscopic guidance) from APC 0121 (Level I Tube or Catheter Changes or Repositioning) to APC 0676 Start Printed Page 60442 (Thrombolysis and Other Device Revisions), with a proposed CY 2010 payment rate of approximately $160.
Comment: Some commenters disagreed with the proposed APC reassignment and requested that CMS continue to assign CPT code 43760 to APC 0121 because they believe the gastrostomy tube change procedure shares significant clinical and resource characteristics with other procedures assigned to APC 0121.
Response: Prior to CY 2008, OPPS payment for CPT code 43760 captured both the procedure and the imaging or endoscopic guidance, if used, that was associated with percutaneously changing a gastrostomy tube because its descriptor read, “Change of gastrostomy tube,” and the OPPS packages payment for all guidance into payment for the associated procedures. However, effective January 1, 2008, the CPT Editorial Panel revised the code descriptor by adding the words “without imaging or endoscopic guidance” to further clarify that the code should be reported for tube change procedures that do not require imaging or endoscopic guidance. The CPT Editorial Panel further determined that gastrostomy tube placement requiring fluoroscopic or endoscopic guidance should be reported with either CPT code 49450 (Replacement of gastrostomy or cecostomy (or other colonic) tube, percutaneous, under fluoroscopic guidance including contrast injection(s), image documentation and report) or CPT code 43246 (Upper gastrointestinal endoscopy including esophagus, stomach, and either the duodenum and/or jejunum as appropriate; with directed placement of percutaneous gastrostomy tube). Based on the median cost from CY 2008 claims data that reflects the new service reported under CPT code 43760, we believe a reassignment of the CPT code for CY 2010 is necessary.
We disagree with the commenters that CPT code 43760 would be appropriately assigned to APC 0121, which has a median cost of approximately $426 for CY 2010. We note that the median cost for CPT code 43760 from CY 2007, when the code represented services provided with and without guidance, was higher at approximately $216, compared with the CY 2008 median cost of the revised procedure code. Claims data from CY 2008 reveal that we have 21,178 single claims (out of 38,246 total claims) for CPT code 43760, with a lower median cost of approximately $160. The median cost for CPT code 43760 closely aligns with the median cost of approximately $160 for APC 0676. We note that the procedure for gastrostomy tube placement using fluoroscopic guidance, specifically CPT code 49450, is assigned to APC 0121, and the procedure for gastrostomy tube placement using endoscopic guidance, specifically CPT code 43246, is assigned to APC 0141 (Level I Upper GI Procedures). We believe that both of these other procedures are appropriately assigned to APCs 0121 and 0141, respectively, based on considerations of clinical and resource homogeneity. As expected, their CPT code-specific median costs that include the cost of fluoroscopy or endoscopic guidance are significantly higher than the median cost of CPT code 43760, which is provided without guidance.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to reassign CPT code 43760 from APC 0121 to APC 0676, which has a final CY 2010 APC median cost of approximately $160.
b. Laparoscopic Liver Cryoablation (APC 0131)
For CY 2010, we proposed to continue to assign CPT code 47371 (Laparoscopy, surgical, ablation of one or more liver tumor(s); cryosurgical) to APC 0131 (Level II Laparoscopy), with a proposed payment rate of approximately $3,181.
Comment: One commenter requested that CMS reassign CPT code 47371 from APC 0131 to APC 0174 (Level IV Laparoscopy), which had a proposed payment rate of approximately $7,766, to better reflect the actual costs of the procedure. The commenter stated that CPT code 47371 is neither similar in resource costs nor clinical characteristics to the other procedures assigned to APC 0131, and that the four single claims for procedure available for ratesetting are not reflective of the costs of the procedure. In addition, the commenter indicated that most of the procedures assigned to APC 0131 describe abdominal biopsy or repair procedures, in contrast to CPT code 47371, which describes cryosurgical ablation of a liver tumor. The commenter noted that there are other similar laparoscopic liver tumor ablation procedures already assigned to APC 0174.
Response: We agree with the commenter's argument that CPT code 47371 would be more appropriately assigned to APC 0174, where other laparoscopic liver and renal ablation procedures are assigned. Although we have few CY 2008 claims for CPT code 47371, our claims data show a higher median cost of approximately $4,229 for CPT code 47371 based on four single claims out of seven total claims, compared to the APC median cost of approximately $3,128 for APC 0131.
We also note that since CPT code 47371 was made effective in CY 2002, the procedure for the code is rarely performed on Medicare beneficiaries in the HOPD based on analysis of our hospital outpatient claims data. Based upon OPPS claims submitted from CY 2002 through CY 2008, the median cost for this code has varied widely, perhaps due to the small volume of claims annually. Specifically, the historical median cost for CPT code 47371 has ranged from $1,850 based on two single claims to $6,839 based on one single claim. Although this procedure is not commonly performed on Medicare beneficiaries in the HOPD, because we believe CPT code 47371 is similar in clinical characteristics and resource costs to the other procedures currently assigned to APC 0174, we agree with the commenter's recommendation.
After consideration of the public comment we received, we are modifying our CY 2010 proposal and assigning CPT code 47371 to APC 0174, which has a final CY 2010 APC median cost of approximately $7,342.
c. Cholangioscopy (APC 0151)
The CPT Editorial Panel created a new add-on code for cholangioscopy, CPT code 43273 (Endoscopic cannulation of papilla with direct visualization of common bile duct(s) and/or pancreatic ducts (List separately in addition to code(s) for primary procedure), effective January 1, 2009. We assigned CPT code 43273 to APC 0151 (Endoscopic Retrograde Cholangio-Pancreatography (ERCP)) on an interim final basis in the CY 2009 OPPS/ASC final rule with comment period (73 FR 69030), and the CPT code was flagged with comment indicator “NI” to indicate that its OPPS treatment was open to comment on that final rule with comment period. For CY 2010, we proposed to continue the assignment of CPT code 43273 to APC 0151, with a proposed payment rate of approximately $1,527.
At the August 2009 APC Panel meeting, the APC Panel heard a public presentation recommending that CPT code 43273 be reassigned to APC 0152 (Level I Percutaneous Abdominal and Biliary Procedures). However, the APC Panel recommended that CPT code 43273 continue to be assigned to APC 0151.
Comment: One commenter on the CY 2009 OPPS/ASC final rule with comment period and again on the CY 2010 OPPS/ASC proposed rule recommended that CPT code 43273 be reassigned to APC 0152 and that CMS Start Printed Page 60443 rename APCs 0151 and 0152 to accommodate the reassignment. The commenter provided cost information for CPT code 43273 in a New Technology APC application previously filed with CMS, estimating that the cost of the cholangioscopy procedure is approximately $2,958. Because the procedure is always performed with an ERCP procedure, the commenter estimated that the combined cost of ERCP and cholangioscopy is approximately $4,484 by adding the CY 2008 median cost of APC 0151 to the estimated cost of the cholangioscopy procedure. The commenter pointed out that, because all ERCP CPT codes, which are assigned to APC 0151, have a status indicator of “T” (Significant Procedure, Multiple Reduction Applies), under CMS' proposal, CPT code 43273 would be paid at 50 percent of the CY 2010 proposed payment rate for APC 0151, or $757, a rate that is approximately $2,200 less than the reported cost of $2,958 for the cholangioscopy procedure alone. The commenter further estimated the cost of disposable devices alone for cholangioscopy in combination with ERCP to be approximately $2,064. The commenter argued that the proposed CY 2010 payment hospitals would receive for the two procedures of approximately $2,271 would barely cover the device costs of $2,064, and not the additional procedure costs. The commenter maintained that if CMS reassigned CPT code 43273 to APC 0152, payment for the combination of the two procedures would be approximately $2,821, partially closing the gap between OPPS payment and the commenter's estimated combined cost of the two procedures of $4,484.
The commenter explained that cholangioscopy is a complex, resource-intensive procedure requiring additional physician training, which adds 45 minutes to the ERCP procedure, for a total of approximately 112 minutes for the two procedures. The commenter also asserted that the clinical intensity of cholangioscopy is closer to the non-draining percutaneous procedures assigned to APC 0152 than to the ERCP procedures assigned to APC 0151. Further, the commenter explained that the primary clinical difference between non-draining percutaneous procedures and CPT code 43273 is the method of access to the biliary and pancreatic area, noting that a small incision is required for the percutaneous procedures and the use of an additional endoscope for cholangioscopy.
The commenter also recommended that APC 0151 be renamed “Level I Hepatobiliary Procedures” and APC 0152 be renamed “Level II Hepatobiliary Procedures,” and that lower complexity hepatobiliary procedures be assigned to APC 0151 and higher complexity hepatobiliary procedures, including percutaneous procedures and cholangioscopy, be assigned to APC 0152. The commenter believed that renaming and reconfiguring APCs 0151 and 0152 would improve the clinical homogeneity of the APCs and appropriately account for differences in the resource costs of biliary procedures. The commenter argued that CMS has previously renamed existing APCs, created multilevel APCs for specific clinical areas, and configured APCs to incorporate surgical procedures that include a variety of access types.
Response: Because CPT code 43273 was new for CY 2009, we do not yet have cost information for the procedure based upon hospital claims for CY 2010 ratesetting. According to our established policy, a New Technology APC applicant's cost estimate is only one source of information we consider in determining the cost of a new service for purposes of its initial APC assignment under the OPPS (66 FR 59900; 73 FR 68614). We generally assign new CPT codes to an APC based on input from a variety of sources, including, but not limited to, review of the resource costs and clinical similarity of the service to existing procedures; input from CMS medical advisors; information from interested specialty societies; and review of all other information available to us. We note that, while CPT code 43273 is new for CY 2009, cholangioscopy in association with ERCP procedures has been performed, using a variety of technologies, for many years. We expect that its costs have already been incorporated into the OPPS, either packaged into payment for the associated ERCP procedures or under an unlisted CPT procedure code.
We continue to believe that APC 0151 is an appropriate APC assignment for CPT code 43273 for CY 2010, based on consideration of the procedure's clinical and resource characteristics. CPT code 43273, which is an add-on code to ERCP procedures, clinically resembles the ERCP procedures that also are assigned to APC 0151 because they all use an endoscope to examine various components of the hepatobiliary system. While cholangioscopy extends ERCP procedures to visualize the common bile duct(s) and/or pancreatic duct(s) through use of an additional endoscope, many of the other ERCP procedures assigned to APC 0151 also have additional procedures associated with them that require the use of other devices or equipment as well. We do not agree with the commenter that percutaneous biliary procedures that require incisions and, in some cases drainage tubes, are more clinically similar to cholangioscopy than ERCP procedures that also examine the hepatobiliary system with an endoscope.
Furthermore, we understand that there are a variety of technologies that can be used to perform cholangioscopy with variable resource costs. Therefore, we do not believe that the commenter's estimate based on one type of new cholangioscopy technology is necessarily an appropriate representation of the cost of the procedure described by CPT code 43273 to hospitals. We believe that the cost of cholangioscopy is similar to the cost of ERCP procedures that require similar procedure time and devices, and that CPT code 43273 is appropriately assigned to APC 0151 along with these ERCP procedures. Moreover, we believe that applying the multiple procedure discount to payment for cholangioscopy as a result of its status indicator “T” and its routine performance with ERCP procedures that also are assigned status indicator “T” is appropriate because cholangioscopy is performed directly after ERCP and much of the preparatory procedure work is performed during the ERCP.
Finally, because we are not reassigning CPT code 43273 to APC 0152, no renaming of APCs 0151 and 0152 is warranted because we are maintaining the endoscopic and percutaneous biliary procedures in separate APCs.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to maintain the assignment of CPT code 43273 to APC 0151, which has a final CY 2010 APC median cost of approximately $1,510.
d. Laparoscopic Hernia Repair (APC 0131)
For CY 2010, we proposed to reassign the following six laparoscopic hernia repair CPT codes that were new for CY 2009 from APC 0130 (Level I Laparoscopy), with a proposed payment rate of approximately $2,538, to APC 0131 (Level II Laparoscopy), with a proposed payment rate of approximately $3,181: CPT code 49652 (Laparoscopy, surgical, repair, ventral, umbilical, spigelian or epigastric hernia (includes mesh insertion, when performed); reducible); CPT code 49653 (Laparoscopy, surgical, repair, ventral, umbilical, spigelian or epigastric hernia (includes mesh insertion, when performed); incarcerated or Start Printed Page 60444 strangulated); CPT code 49654 (Laparoscopy, surgical, repair, incisional hernia (includes mesh insertion, when performed); reducible); CPT code 49655 (Laparoscopy, surgical, repair, incisional hernia (includes mesh insertion, when performed); incarcerated or strangulated); CPT code 49656 (Laparoscopy, surgical, repair, recurrent incisional hernia (includes mesh insertion, when performed); reducible); and CPT code 49657 (Laparoscopy, surgical, repair, recurrent incisional hernia (includes mesh insertion, when performed); incarcerated or strangulated).
Comment: One commenter indicated that the resource costs associated with the six laparoscopic hernia repair CPT codes are significantly greater than the proposed payment rate of approximately $3,181 for APC 0131 and suggested that the procedures in APC 0132 (Level III Laparoscopy) are more similar to the six laparoscopic hernia repair codes because they have similar resource costs. The commenter requested that CMS review the clinical characteristics and resource costs associated with the six CPT codes and consider reassigning these codes to APC 0132, which had a CY 2010 proposed payment rate of approximately $4,903. In addition, the commenter provided an analysis of CY 2008 claims data for cases reported under the unlisted CPT code that would previously have been reported for these procedures (CPT code 49659 (Unlisted laparoscopy procedure, hernioplasty, herniorrhaphy, herniotomy)), combined with the ICD–9 diagnoses codes specific to hernia of the abdominal cavity. Because these codes were new for CY 2009, they were assigned comment indicator “NI” in the CY 2009 OPPS/ASC final rule with comment period to indicate that they were subject to comment. For the CY 2009 OPPS/ASC final rule with comment period, the same commenter submitted a similar comment and data analysis of CY 2007 OPPS claims. The commenter found 3,456 claims that met the same case criteria in the analysis, with a median cost of approximately $4,261. The commenter believed that this cost from historical hospital claims data represented the hospital cost of procedures that would be reported with one of the laparoscopic hernia repair CPT codes in CY 2010.
Response: We have no hospital claims data for CPT codes 49652 through 49657 because these CPT codes were new for CY 2009. However, we agree with the commenter that procedures described by these CPT codes were likely commonly furnished in the HOPD in CY 2008 and reported under CPT code 49659. Taking into consideration the commenter's analyses of CY 2007 and CY 2008 claims and performing a detailed clinical review, we agree with the commenter that the resource costs for the six laparoscopic hernia repair CPT codes, specifically CPT codes 49652 through 49657, more closely align with other services assigned to APC 0132. In addition, from a clinical perspective, we also believe that APC 0132 is an appropriate APC assignment for these codes because APC 0132 most accurately recognizes the complexity of the laparoscopic hernia repair codes.
After consideration of the public comments we received, we are modifying our CY 2010 proposals and reassigning CPT codes 49652, 49653, 49654, 49655, 49656, and 49657 from APC 0130 to APC 0132, which has a final CY 2010 APC median cost of approximately $4,873.
3. Genitourinary Services
a. Percutaneous Renal Cryoablation (APC 0423)
For CY 2010, we proposed to continue to assign CPT code 50593 (Ablation, renal tumor(s), unilateral, percutaneous, cryotherapy) to APC 0423 (Level II Percutaneous Abdominal and Biliary Procedures), with a proposed payment rate of approximately $3,329. This CPT code was new in CY 2008. However, the same service was previously described by CPT code 0135T (Ablation renal tumor(s), unilateral, percutaneous, cryotherapy). We note that, for CY 2007, based upon the APC Panel's recommendation made at its March 2006 meeting, we reassigned CPT code 50593 (then CPT code 0135T) from APC 0163 (Level IV Cystourethroscopy and other Genitourinary Procedures) to APC 0423, effective January 1, 2007.
Comment: One commenter expressed concern that the proposed payment rate of approximately $3,329 for CPT code 50593 is inadequate because the payment does not accurately account for the costs incurred by hospitals in performing this procedure. The commenter argued that the low proposed payment rate for CPT code 50593 is attributable to claims data that do not accurately capture the full costs of CPT code 50593 because almost half of the single claims do not contain the HCPCS code and associated charge for the required device, specifically HCPCS code C2618 (Probe, cryoablation). The commenter requested that CMS designate CPT code 50593 as a device-dependent procedure, which would require hospitals to submit claims with the appropriate device HCPCS code, assign the procedure to its own APC, and set the payment rate for that APC based on claims for CPT code 50593 reported with HCPCS code C2618. The commenter's analysis concluded that the median cost on which payment for CPT code 50593 would be based if these recommendations were adopted would be approximately $5,469, resulting in more accurate payment for the procedure and continued Medicare beneficiary access to percutaneous renal cryoablation in the HOPD.
Response: We believe that CPT code 50593 is appropriately assigned to APC 0423 based on clinical and resource considerations when compared to other procedures also proposed for assignment to APC 0423 for CY 2010. As we stated in the CY 2007 OPPS final rule with comment period (71 FR 68049 through 68050), the CY 2008 OPPS/ASC final rule with comment period (72 FR 66709), and the CY 2009 OPPS/ASC final rule with comment period (73 FR 68611), we initially revised the APC assignment for the percutaneous renal cryoablation procedure from APC 0163 to APC 0423 in CY 2007 based on the APC Panel's recommendation to reassign the procedure to APC 0423. The median costs of the four HCPCS codes assigned to APC 0423 for CY 2010 range from approximately $3,159 to $4,670, well within the 2 times rule for the OPPS payment groups. Even if we were to calculate the median cost for CPT code 50593 using only claims that also contain HCPCS code C2618, estimated by the commenter to be approximately $5,469 using proposed rule data, the grouping of these procedures in the same APC would not violate the 2 times rule. Further, we note that all four of these procedures are relatively low volume, with fewer than 1,100 total claims each for CY 2008 and fewer than 700 single claims each for ratesetting. We believe that grouping these clinically similar, low volume procedures for the percutaneous ablation of renal, liver, or pulmonary tumors in the same payment group helps to promote payment stability for these low volume services.
We also do not agree that CPT code 50593 should be designated as a device-dependent procedure and assigned to its own separate APC. We have only 226 single claims (out of 513 total claims) for CPT code 50593 from CY 2008 and, as such, the procedure has the second lowest frequency of the four procedures assigned to APC 0423. We believe this relatively low volume procedure should be assigned to a payment group with similar services, as we have proposed, in order to promote payment stability and encourage hospital efficiency. In addition, we do not identify individual Start Printed Page 60445 HCPCS codes as device-dependent HCPCS codes under the OPPS. Rather, we first consider the clinical and resource characteristics of a procedure and determine the most appropriate APC assignment. When we determine that we should assign a procedure to an APC that is device-dependent, based on whether that APC has been historically identified under the OPPS as having very high device costs, we then consider the implementation of device edits, as appropriate. We note that the identification of device-dependent APCs was particularly important in the early years of the OPPS when separate pass-through payment for many implantable devices expired. At that time, a variety of methodologies to package the costs of those devices into procedural APCs was utilized over several years to ensure appropriate incorporation of the device costs into the procedure payments. At this point in time, hospitals have significantly more experience reporting HCPCS codes for packaged and separately payable items and services under the OPPS and the payment groups are more mature. We believe our standard ratesetting methodology typically results in appropriate payment rates for new procedures that utilize devices, as well as those that do not use high cost devices. In recent years, we have not encountered circumstances whereby we have had to establish new device-dependent APCs because we were not able to accommodate the clinical and resource characteristics of a procedure by assigning it to an existing APC (whether device-dependent or non-device-dependent), and the procedure described by CPT code 50593 is no exception.
While all of the procedures assigned to APC 0423 require the use of implantable devices, for many of the procedures, there are no Level II HCPCS codes that describe all of the technologies that may be used in the procedures. Therefore, it would not be possible for us to develop procedure-to-device edits for all of the CPT codes assigned to APC 0423. Under the OPPS, there are many other procedures that require the use of implantable devices that, because they are assigned to OPPS APCs that are not device-dependent, do not have procedure-to-device edits applied, even if those claims processing edits would be feasible. We believe that our payments for procedures that utilize high cost devices are appropriate for those services, even when those services are grouped with other procedures that either do not require the use of implantable devices or that utilize devices that are not described by specific Level II HCPCS codes.
When reporting CPT code 50593, we expect hospitals to also report the device HCPCS code C2618, which is associated with this procedure. We also remind hospitals that they must report all of the HCPCS codes that appropriately describe the items used to provide services, regardless of whether the HCPCS codes are packaged or paid separately. If hospitals use more than one probe in performing CPT code 50593, we expect hospitals to report this information on the claim and adjust their charges accordingly. Hospitals should report the number of cryoablation probes used to perform CPT code 50593 as the units of HCPCS code C2618 which describes these devices, with their charges for the probes. Since CY 2005, we have required hospitals to report device HCPCS codes for all devices used in procedures if there are appropriate HCPCS codes available. In this way, we can be confident that hospitals have included charges on their claims for costly devices used in procedures when they submit claims for those procedures.
After consideration of the public comment we received, we are finalizing our CY 2010 proposal, without modification, to continue to assign CPT code 50593 to APC 0423, which has a final CY 2010 APC median cost of approximately $3,430.
b. Hemodialysis (APC 0170)
Currently, APC 0170 (Dialysis) contains two HCPCS codes: CPT code 90935 (Hemodialysis procedure with single physician evaluation) and HCPCS code G0257 (Unscheduled or emergency dialysis treatment for an ESRD patient in an HOPD that is not certified as an ESRD facility). Hospital outpatient and emergency departments sometimes must furnish hemodialysis to patients who do not have ESRD and, in these cases, they would report CPT code 90935 for the service. Under the Medicare ESRD benefit, to be covered by Medicare, routine dialysis required by a Medicare ESRD beneficiary must be furnished in a certified ESRD facility. Most HOPDs and emergency departments are not certified by Medicare to furnish routine dialysis to Medicare ESRD patients and, therefore, are not paid under the ESRD benefit. However, there are a limited number of specific cases in which Medicare pays under the OPPS for an ESRD patient to receive unscheduled dialysis in an outpatient department of a hospital that does not have an ESRD-certified facility. These provisions were established in the CY 2003 OPPS final rule with comment period (67 FR 66803 through 66805). Specifically, Medicare pays hospitals under the OPPS for dialysis for ESRD patients under the following limited circumstances as specified in the Medicare Claims Processing Manual, Pub. 100–04, Chapter 4, Section 200.2:
- Dialysis is performed following or in connection with a dialysis-related procedure such as a vascular access procedure or a blood transfusion;
- Dialysis is performed following treatment for an unrelated medical emergency; or
- Emergency dialysis is performed for ESRD patients who would otherwise have to be admitted as inpatients in order for the hospital to receive payment.
When these criteria are met, the hospital reports HCPCS code G0257, which we proposed to assign to APC 0170 for CY 2010, with a proposed payment of approximately $442.
Comment: One commenter, who recognized that Medicare pays under the OPPS for hemodialysis for ESRD patients under very specific, limited circumstances, asked whether hospitals are permitted to bill and be paid under the OPPS for routine hemodialysis for ESRD patients who are unable to arrange for routine hemodialysis at a Medicare-certified ESRD facility.
Response: As the commenter noted, Medicare pays under OPPS for dialysis for a beneficiary with ESRD only under the exceptional circumstances specified in the Medicare Claims Processing Manual, Pub. 100–04, Chapter 4, Section 200.2 that are listed above. Routine treatments in hospitals that do not have an ESRD facility are not payable under the OPPS. A hospital that would like to provide routine hemodialysis to ESRD patients should contact the State survey and certification agency to pursue ESRD certification of an outpatient dialysis unit.
After consideration of the public comment we received, we continue to believe that our policy governing the payment of dialysis services in HOPDs and emergency departments is appropriate. We are not making any change to this policy for CY 2010. The final CY 2010 median cost of APC 0170 for hemodialysis is approximately $456.
c. Radiofrequency Remodeling of Bladder Neck (APC 0165)
For CY 2010, we proposed to continue to assign Category III CPT code 0193T (Transurethral, radiofrequency micro-remodeling of the female bladder neck and proximal urethra for stress urinary incontinence) to APC 0165 (Level IV Urinary and Anal Procedures) with a Start Printed Page 60446 proposed payment rate of approximately $1,353. This CPT code was new for CY 2009 and was assigned to APC 0165 on an interim final basis in the CY 2009 OPPS/ASC final rule with comment period.
At the August 2009 APC Panel meeting, a presenter requested that the APC Panel recommend that CMS reassign CPT code 0193T to either APC 0202 (Level VII Female Reproductive Procedures) or APC 0168 (Level II Urethral Procedures) for CY 2010 based on resource intensity and therapeutic benefit. The presenter claimed that the device cost associated with CPT code 0193T is comparable to the single-use devices that are used with certain procedures assigned to APC 0202, specifically those procedures described by CPT codes 58356 (Endometrial cryoablation with ultrasonic guidance, including endometrial curettage, when performed); 58565 (Hysteroscopy, surgical; with bilateral fallopian tube cannulation to induce occlusion by placement of permanent implants); and 57288 (Sling operation for stress incontinence (e.g., fascia or synthetic)). The presenter indicated that, unlike procedures assigned to APC 0202 that require costly medical devices, the costs of single-use medical devices for procedures assigned to APC 0165 are very minimal. After a discussion, the APC Panel recommended that CMS maintain the APC assignment of CPT code 0193T to APC 0165, as proposed, for CY 2010.
Comment: Some commenters disagreed with CMS' proposal to continue to assign CPT code 0193T to APC 0165. The commenters believed that the proposed payment for the procedure would not pay appropriately for the costs incurred by hospitals to perform the procedure, especially because the procedure utilizes a costly, single-use, disposable medical device. The commenters argued that APC 0202, which had a proposed CY 2010 proposed payment rate of approximately $2,991, contains procedures that are very similar to CPT code 0193T. Specifically, the commenters indicated that CPT code 0193T is similar in clinical characteristics and resource costs to CPT codes 58356, 58565, and 57288. The commenters added that the probe used in the procedure reported with CPT code 0193T costs $1,095 and, overall, the total procedure cost that includes the cost of the probe is approximately $2,473, which is comparable to the proposed CY 2010 payment rate for APC 0202.
Another commenter was concerned that, at the August 2009 APC Panel meeting, the APC Panel members may have been confused about the surgical nature of CPT code 0193T. Specifically, the commenter believed that the APC Panel concluded that all of the procedures assigned to APC 0202 are surgical in nature, whereas the procedure described by CPT code 0193T is not, which resulted in the APC Panel's recommendation to continue to assign this code to APC 0165. The commenter clarified that CPT code 0193T is similar to surgical CPT codes 58565 and 58356, which are both assigned to APC 0202 based on their resource use and clinical characteristics. The commenter further noted that, although CPT code 0193T may be performed in the physician's office, in the Medicare population, this procedure is more likely to be performed in the hospital outpatient setting because of medical conditions and comorbidities experienced by Medicare patients.
Response: As a new Category III CPT code for CY 2009, we do not yet have hospital claims data for the procedure. Category III CPT codes are temporary codes that describe emerging technology, procedures, and services, and they are created by the AMA to allow for data collection for new services or procedures. Under the OPPS, we generally assign a payment rate to a new Category III CPT code based on input from a variety of sources, including but not limited to, review of resource costs and clinical homogeneity of the service to existing procedures, information from specialty societies, input from CMS medical advisors, and other information available to us. Based on our review of the clinical characteristics of CPT code 0193T, as well as the other procedures assigned to APC 0165 and APC 0202 that was recommended by the commenters, and the APC Panel discussion and recommendation regarding the procedure, we continue to believe that APC 0165 is the most appropriate APC assignment for CPT code 0193T for CY 2010. We understand that CPT code 0193T is a minimally invasive procedure for female stress urinary incontinence that requires a relative brief time in the procedure room. We do not agree with the commenters that the procedures assigned to APC 0202 that involve fallopian tube cannulation, endometrial ablation, or implantation of a sling for stress urinary incontinence are sufficiently similar to the procedure described by CPT code 0193T based on procedure duration, device utilization, use of guidance, or other characteristics to warrant reassignment of CPT code 0193T to APC 0202 based on considerations of clinical homogeneity. Rather, we believe that assignment to APC 0165 will appropriately account for the device and procedure costs of CPT code 0193T.
After consideration of the public comments we received and the APC Panel recommendation from the August 2009 meeting, we are finalizing our CY 2010 proposal, without modification, to continue to assign CPT code 0193T to APC 0165, which has a final CY 2010 APC median cost of approximately $1,337.
d. Change of Bladder Tube (APC 0121)
For CY 2010, we proposed to reassign CPT code 51710 (Change of cystostomy tube; complicated) from APC 0427 (Level II Tube or Catheter Changes or Repositioning) to APC 0121 (Level I Tube or Catheter Changes or Repositioning), with a proposed CY 2010 payment rate of approximately $428.
Comment: One commenter supported the proposed APC reassignment of CPT code 51710 from APC 0427 to APC 0121.
Response: We appreciate the commenter's support. Hospital outpatient claims data revealed that we have approximately 267 single claims (out of 431 total claims) for CPT code 51710, with a final CY 2010 median cost of approximately $446. The final CY 2010 median cost for CPT code 51710 closely aligns with the final CY 2010 median cost of approximately $426 for APC 0121. We believe that CPT code 51710 is appropriately reassigned to APC 0121 based on clinical and resource considerations.
After consideration of the public comment we received, we are finalizing our CY 2010 proposal, without modification, to reassign CPT code 51710 from APC 0427 to APC 0121, which has a final CY 2010 APC median cost of approximately $426.
4. Nervous System Services
a. Pain-Related Procedures (APCs 0203, 0204, 0206, 0207, 0221, 0224, and 0388)
We proposed to set the CY 2010 payment rates for APCs 0203 (Level IV Nerve Injections), 0204 (Level I Nerve Injections), 0206 (Level II Nerve Injections), 0207 (Level III Nerve Injections), 0221 (Level II Nerve Procedures), 0224 (Implantation of Catheter/Reservoir/Shunt) and 0388 (Discography) based on the median costs determined under the OPPS standard ratesetting. Among the CPT codes included in these APCs are: 62350 (Implantation, revision, or repositioning of tunneled intrathecal or epidural catheter for long-term medication Start Printed Page 60447 administration via an external pump or implantable reservoir/infusion pump; with laminectomy); 62355 (Removal of previously implanted intrathecal or epidural catheter); 62365 (Removal of subcutaneous reservoir or pump, previously implanted for intrathecal or epidural infusion); 64472 (Injection, anesthetic agent and/or steroid, paravertebral facet joint or facet joint nerve; cervical or thoracic, each additional level (list separately in addition to code for primary procedure)); 64476 (Injection, anesthetic agent and/or steroid, paravertebral facet joint or facet joint nerve; lumbar or sacral, each additional level (list separately in addition to code for primary procedure)); 64480 (Injection, anesthetic agent and/or steroid, transforminal epidural; cervical or thoracic, each additional level (list separately in addition to code for primary procedure)); 64623, (Destruction by neurolytic agent, paravertebral facet joint nerve; lumbar or sacral, each additional level, (list separately in addition to code for primary procedure)); 64627 (Destruction by neurolytic agent, paravertebral facet joint nerve; cervical or thoracic, each additional level, (list separately in addition to code for primary procedure)); 72285 (Discography, cervical or thoracic, radiological supervision and interpretation); and 72295 (Discography, lumbar, radiological supervision and interpretation).
Comment: One commenter objected to the proposed CY 2010 payment rates for CPT codes 64472, 64476, 64480, 64623, and 64627, which the commenter believed have declined 28 percent to 48 percent since CY 2007. The commenter also objected to the proposed CY 2010 increase in payments for CPT codes 72285 and 72295 on the basis that their proposed payment rates are unreasonable because they are not procedures. The commenter added that CPT codes 62290 (Injection procedure for discography, each level, lumbar) and 62291 (Injection procedure for discography, each level, cervical or thoracic) are the related procedures, which are paid at an unreasonably low rate.
Response: OPPS payment rates fluctuate based on a variety of factors, including, but not limited to, changes in the mix of hospitals billing the services, differential changes in hospital charges and costs for the services, and changes in the volumes of services reported. Therefore, the median costs on which the OPPS payment rates are based vary from one year to another. We note that the median costs of all of the APCs to which CPT codes 64472, 64476, 64480, 64623, and 64627 are assigned increased between CY 2009 and CY 2010. For CPT codes 64472 and 64480, the median cost of APC 0206 to which they are assigned increased from approximately $236 in CY 2009 to approximately $249 in CY 2010. In the case of CPT codes 64476 and 64627, the median cost of APC 0204 to which they are assigned increased from approximately $161 in CY 2009 to approximately $171 in CY 2010. Lastly, for CPT code 64623, the median cost of APC 0207 to which the code is assigned increased from approximately $463 in CY 2009 to approximately $481 in CY 2010.
CPT codes 72285 and 72295, both of which are assigned to APC 0388, are “T” packaged codes and, as such, are paid separately only if there is no separately paid surgical procedure with a status indicator of “T” on the same claim. When there is a separate payment made for these codes, the payment is not only payment for the code itself but also includes payment for all services reported on the claim that are always packaged (that is, those with a status indicator of “N”). The median cost of APC 0388 to which CPT codes 72285 and 72295 are assigned for payment when separate payment can be made increased from approximately $1,470 in CY 2009 to approximately $1,727 in CY 2010, reflecting the cost of all conditionally and unconditionally packaged services on the claim. Payment for CPT codes 62290 and 62291 is always packaged into payment for the independent, separately paid procedures with which these codes are reported because we believe that these codes are ancillary and supportive to other major separately paid procedures and that they are furnished only as an ancillary and dependent part of an independent separately paid procedure.
Comment: One commenter disagreed with the proposed CY 2010 payment rates for CPT codes 62355, 62350, 62363 (we note that this code did not exist in CY 2008 and does not exist in CY 2009; the commenter did not provide a description of the procedure that would enable us to identify the code and respond to the comment), and 62365. The commenter believed that access to these services is very limited as a result of payment reductions for these procedures.
Response: The final median costs for the APCs to which CPT codes 62350 and 62365 are assigned for CY 2010 increased from CY 2009 to CY 2010, while the final median cost for APC 0203 to which CPT code 62355 is assigned declined over that same time period. Specifically, CPT code 62350 is assigned to APC 0224, which has a CY 2009 median cost of approximately $2,715 and a final CY 2010 median cost of approximately $2,740. Similarly, CPT code 62365 is assigned to APC 0221, which has a CY 2009 median cost of approximately $2,322 and a final CY 2010 median cost of approximately $2,490. In contrast, CPT code 62355 is assigned to APC 0203, which has a CY 2009 median cost of approximately $928 that declined to approximately $885 in CY 2010. The increased median costs of APCs 0221 and 0224 do not create barriers to care for these procedures. Moreover, we do not believe that the modest reduction in median cost for APC 0203 would cause hospitals to cease to furnish the service.
After consideration of the public comments we received, we are finalizing our CY 2010 proposals, without modification, to pay for CPT codes 62350, 62355, 62365, 64472, 64476, 64480, 64623, 64627, 72285, and 72295 through APCs 0203, 0204, 0206, 0207, 0221, 0224, and 0388. The final CY 2010 median costs of the relevant APCs are displayed in Table 24 below. For comparative purposes, we also are showing in the table the median costs on which the CY 2009 OPPS payments are based.
| APC | APC title | CY 2009 approximate median cost | Proposed CY 2010 approximate median cost | Final CY 2010 approximate median cost |
|---|---|---|---|---|
| 0203 | Level IV Nerve Injections | $929 | $1,066 | $885 |
| 0204 | Level I Nerve Injections | 161 | 181 | 171 |
| 0206 | Level IV Nerve Injections | 236 | 254 | 249 |
| 0207 | Level III Nerve Injections | 463 | 504 | 481 |
| Start Printed Page 60448 | ||||
| 0221 | Level II Nerve Procedures | 2,322 | 2,521 | 2,490 |
| 0224 | Implantation of Catheter/Reservoir/Shunt | 2,715 | 2,769 | 2,740 |
| 0388 | Discography | 1,470 | 1,769 | 1,727 |
b. Magnetoencephalography (APCs 0065 and 0067)
Three CPT codes describe magnetoencephalography services: 95965 (Magnetoencephalography (MEG), recording and analysis; for spontaneous brain magnetic activity (e.g. epileptic cerebral cortex localization)); 95966 (Magnetoencephalography (MEG), recording and analysis; for spontaneous brain magnetic activity (e.g. epileptic cerebral cortex localization) for evoked magnetic fields, single modality (e.g. sensory, motor, language or visual cortex localization)); and 95967 (Magnetoencephalography (MEG), recording and analysis; for spontaneous brain magnetic activity (e.g. epileptic cerebral cortex localization), for evoked magnetic fields, each additional modality (e.g. sensory, motor language, or visual cortex localization (List separately in addition to code for primary procedure)). These CPT codes were originally assigned to New Technology APCs but, beginning in CY 2006 and for every year thereafter, these codes have been assigned to clinical APCs on the basis of the clinical and resource characteristics of the services. For CY 2010, we proposed to continue to assign CPT code 95965 to APC 0067 (Level III Stereotactic Radiosurgery, MRgFUS and MEG) with a proposed payment rate of approximately $3,507, and we proposed to continue to assign CPT codes 95966 and 96967 to APC 0065 (Level II Stereotactic Radiosurgery, MRgFUS and MEG), with a proposed payment rate of approximately $894.
Comment: Several commenters requested that CMS restore the payment rates for CPT codes 95965, 95966, and 95967 to the levels at which they were paid under New Technology APCs in CY 2005 of $5,250, $1,450, and $950, respectively. They believed the payment rates for CYs 2006, 2007, 2008, and 2009 and the proposed rate for CY 2010 were based on median cost calculations that understated the full costs of the services. The commenters asked CMS to create a cost center on the Medicare cost report that would be used solely to house hospitals' costs of MEG and indicated that the NUBC had approved a request for a dedicated revenue code for the reporting of charges for MEG. The commenters argued that if CMS would create a cost center for the costs of MEG from which a specific CCR could be developed for application to MEG charges, the resulting median cost would be a more accurate reflection of the cost of MEG and would, therefore, result in more appropriate payment. One commenter submitted eight claims for MEG, stating that its Medicare contractor had approved use of a subscript for a specific cost center on the cost report to house the costs of MEG. The commenter asked if these claims were used in ratesetting, provided the CCR the commenter calculated using the costs and charges for MEG that would be reported on the cost report line that contained only MEG costs, and asked if CMS calculated the costs of these claims using the specific CCR for MEG services.
Response: We assign new services to New Technology APCs only until we believe that we have sufficient historical hospital claims data reflecting hospital costs to reassign them to appropriate clinical APCs. We initially assigned MEG services to New Technology APCs based on the information available to us at the time about the expected hospital costs. For CY 2006, we believed that we had sufficient claims data to enable us to make informed decisions regarding the proper clinical APCs for assignment of MEG services. We note that the volumes of claims for MEG services have remained stable since we moved them to clinical APCs in CY 2006. We have no reason to believe that the costs that we have derived from our standard cost estimation process for the CY 2010 OPPS fail to appropriately reflect the relative costs of MEG services in relation to the costs of other services paid under the OPPS, nor do we have reason to believe that payment at the rates under which these services were paid under the New Technology APCs in CY 2005 are justified.
With regard to whether individual claims that were submitted by one commenter were used to set the median costs on which the CY 2010 MEG payment rates are based, we note that the claims we use to set the payment rates under the OPPS are available for purchase and a provider that wishes to see if particular claims were used can attain the claims file and perform any analysis they choose. We are not able to create provider-specific revenue code-to-cost center crosswalks that would use unique cost report subscripts that hospitals choose to create for particular services. In the case of a hospital reporting MEG costs on a subscripted line 54.01, the costs would be included as costs in cost center 5400 (the cost center to which 54.01 is a subscripted line), the standard cost center for electroencephalography. In accordance with our standard revenue code-to-cost center crosswalk, we would apply the CCR for this cost center to the charges reported under revenue code 0740 (EEG (Electroencephalogram); General Classification)) if there is no CCR available for nonstandard cost center 3280 (EKG and EEG).
We recognize that the NUBC created a new revenue code for MEG on August 11, 2009, to be effective for services reported on or after April 1, 2010, if a hospital chooses to use it. We anticipate that we will propose to use claims for services furnished in CY 2010 to calculate OPPS payment rates for CY 2012. Therefore, for the CY 2012 OPPS, we expect that we will propose to determine the primary, secondary and tertiary (if any) CCRs to be applied to the new revenue code as part of our standard ratesetting process for the CY 2012 OPPS. With regard to requests for a dedicated cost center for MEG services, the revised draft hospital cost report Form CMS–2552–10 went on public display through the Federal Register (74 FR 31738), with a comment period that ended on August 31, 2009. We will consider whether creation of such a cost center is appropriate in our review of all public comments on the proposed revisions to the cost report.
After consideration of the public comments we received, we are finalizing our CY 2010 proposals, without modification, to continue to Start Printed Page 60449 assign CPT code 95965 to APC 0067, with a final CY 2010 median cost of approximately $3,539, and to continue to assign CPT codes 95966 and 96967 to APC 0065, with a final CY 2010 median cost of approximately $954.
5. Ocular Services
a. Insertion of Anterior Segment Aqueous Drainage Device (APC 0234)
The CPT Editorial Panel created Category III CPT code 0191T (Insertion of anterior segment aqueous drainage device, without extraocular reservoir; internal approach), effective on July 1, 2008. We assigned CPT code 0191T to APC 0234 (Level III Anterior Segment Eye Procedures), effective July 1, 2008, and maintained this APC assignment for CY 2009. For CY 2010, we proposed to continue the assignment of CPT code 0191T to APC 0234, with a proposed payment rate of approximately $1,639.
Comment: One commenter asserted that the assignment of CPT code 0191T to APC 0234 for CY 2010 would not provide sufficient payment to hospitals and ASCs to cover the cost of the procedure and, therefore, is inappropriate. The commenter indicated that the manufacturer of the device system inserted in the procedure reported by CPT code 0191T currently has an Investigational Device Exemption (IDE) from the FDA and has filed a premarket approval (PMA) application with the FDA with the expectation that the device will be available for use in the United States as early as the first quarter of CY 2010. The commenter noted that the CY 2010 proposed median cost of CPT code 0191T of approximately $2,380, based on only three single claims, was much higher than the CY 2010 proposed median cost of APC 0234 of approximately $1,639 and the CY 2010 proposed ASC payment of approximately $962. The commenter explained that the relatively low number of Medicare hospital outpatient claims for CPT code 0191T resulted from the limited use of the procedure in IDE studies and its predominant performance in ASCs in association with cataract surgery. The commenter also noted that none of the other procedures assigned to APC 0234 involve the placement of an implantable device, while CPT code 0191T requires the insertion of a device that costs about $2,500.
Response: CPT code 0191T is a new CPT code with very few Medicare claims from CY 2008, possibly because this procedure has been limited to IDE studies, as noted by the commenter. Furthermore, because this CPT code was effective on July 1, 2008, CY 2008 claims reflect only 6 months of hospital data, rather than a full year. We note that there are a number of other surgical eye procedures to treat glaucoma that are also assigned to APC 0234 for CY 2010. Moreover, the final CY 2010 median cost of CPT code 0191T based on a small number of CY 2008 claims is approximately $1,962, close to the final CY 2010 median cost of APC 0234 of approximately $1,630. Therefore, based on considerations of clinical and resource homogeneity, we continue to believe that APC 0234 is the most appropriate APC assignment for CPT code 0191T for CY 2010.
After consideration of the public comment we received, we are finalizing our CY 2010 proposal, without modification, to continue to assign CPT code 0191T to APC 0234, with a final CY 2010 APC median cost of approximately $1,630.
b. Backbench Preparation of Corneal Allograft
For CY 2010, we proposed to continue to assign CPT code 65757 (Backbench preparation of corneal endothelial allograft prior to transplantation) status indicator “N” (Items and Services Packaged into APC Rates). In the CY 2009 OPPS/ASC final rule with comment period (73 FR 69076), we assigned CPT code 65757 status indicator “N” and flagged the code with comment indicator “NI” to indicate that, as a new CPT code for CY 2009, its interim final CY 2009 OPPS treatment was subject to comment on that final rule with comment period.
Comment: One commenter requested that CMS pay separately for CPT code 65757 under the OPPS through an APC. According to the commenter, this service represents the preparation process for corneal transplants. The commenter argued that because this service is time-consuming and requires specialized skills and equipment, CPT code 65757 should not be packaged under the OPPS but, instead, should be paid separately.
Response: We packaged CPT code 65757 because we consider it to be an intraoperative service that is ancillary and supportive to another service that is paid separately under the OPPS, specifically the corneal transplant. Our general packaging policies for certain categories of services are discussed in section II.A.4. of this final rule with comment period. Although OPPS payment for CPT code 65757 is packaged, we will consider its costs in setting the payment rates for the associated surgical procedures under the OPPS, according to the standard OPPS cost estimation methodology that is discussed in section II.A.2. of this final rule with comment period.
After consideration of the public comment we received, we are finalizing our CY 2010 proposal, without modification, to continue to assign CPT code 65757 status indicator “N.”
6. Orthopedic and Musculoskeletal Services
a. Arthroscopic Procedures (APCs 0041 and 0042)
For CY 2010, we proposed to continue the assignment of various arthroscopy procedures to APCs 0041 (Level I Arthroscopy) and APC 0042 (Level II Arthroscopy), with proposed payment rates of approximately $2,014 and $3,279, respectively.
Comment: One commenter expressed concern about the variety of procedures assigned to APC 0041, whose HCPCS code-specific median costs ranged from $50 to $22,000, and to APC 0042, whose HCPCS code-specific median costs ranged from $143 to $20,000. In particular, the commenter indicated that the current designation of only two APCs for the more than 60 distinct arthroscopic procedures assigned to these APCs does not appropriately reflect the unique clinical and resource characteristics associated with arthroscopic procedures that are provided to Medicare beneficiaries. The commenter urged CMS to create several new APCs to ensure clinical homogeneity and similar resource utilization for the arthroscopy procedures assigned to them and provided recommended APC configurations.
To pay appropriately for arthroscopic procedures under the OPPS, the commenter recommended that CMS restructure the arthroscopy procedures into 11 new APCs based on the following three clinical categories: (1) Diagnostic arthroscopies; (2) lower extremity versus upper extremity arthroscopies; and (3) arthroscopies with implants. The commenter further recommended specific payment rates associated with each of the 11 recommended APCs, ranging from $1,400 to $5,400. According to the commenter, the recommended clinical distinctions parallel the distinctions CMS has created for other classes of procedures, including other orthopedic procedures, and would more accurately reflect the clinical characteristics and resource utilization of the services provided.
Alternatively, the commenter provided, in the event a reconfiguration of APCs 0041 and 0042 is not possible at this time, two more limited Start Printed Page 60450 suggestions: Finalize the proposal to reassign CPT codes 29888 (Arthroscopically aided anterior cruciate ligament repair/augmentation or reconstruction) and 29889 (Arthroscopically aided posterior cruciate ligament repair/augmentation or reconstruction) from APC 0042 to APC 0052 (Level IV Musculoskeletal Procedures Except Hand and Foot) and reassign CPT code 29892 (Arthroscopically aided repair of large osteochondritis dissecans lesion, talar dome fracture, or tibial plafond fracture, with or without internal fixation (includes arthroscopy)) from APC 0042 to APC 0052.
Response: We believe the existing clinical APCs 0041 and 0042 sufficiently account for the different clinical and resource characteristics of the procedures assigned to them. To reduce the size of the APC payment groups and establish new APC payment groups to pay more precisely would be inconsistent with our overall strategy to encourage hospitals to use resources more efficiently by increasing the size of the payment bundles. Moreover, many of the services that are assigned to APCs 0041 and 0042 are low volume services, with even fewer single claims available for ratesetting. Including low volume services in APCs with clinically similar higher volume services and similar median costs generates more stability in the payment rates that are set for these low volume services.
For APC 0041, based on significant services with a total claim frequency of greater than 1,000 or a frequency of greater than 99 and percentage of single claims equal to or greater than 2 percent, CY 2008 hospital outpatient claims data showed that the median cost of the lowest cost service is approximately $1,463 and the median cost of the highest cost service is approximately $2,086. Likewise, for APC 0042, claims data showed that the median cost of the lowest cost significant procedure is approximately $2,730 and the median cost of the highest cost significant procedure is approximately $4,592. Based on the CY 2008 claims data, there is no 2 times violation in either APC 0041 or APC 0042. Therefore, we see no reason for a reconfiguration into many more APCs in light of our interest in promoting hospital efficiency, as discussed earlier.
With respect to the reassignment of CPT code 29892 from APC 0042 to APC 0052 as recommended by the commenter, we agree that this reassignment would be appropriate for CY 2010. While we have very few claims for this procedure upon which to accurately estimate its cost, we reviewed the clinical characteristics associated with CPT code 29892 and agree that, based on the complexity of this procedure, it would be more appropriately assigned to APC 0052 based on its clinical characteristics and expected resource utilization. Furthermore, we appreciate the commenter's support for our proposed reassignment of CPT codes 29888 and 29889 from APC 0042 to APC 0052 for CY 2010.
After consideration of the public comment we received, we are finalizing our CY 2010 proposals to reassign CPT codes 29888 and 29889 from APC 0042 to APC 0052, with a final CY 2010 APC median cost of approximately $5,921. In addition, we are also finalizing the reassignment of CPT code 29892 from APC 0042 to APC 0052 for CY 2010. We are making no other changes to the proposed configurations of APC 0041 and 0042 for CY 2010. The final CY 2010 APC median cost for APC 0041 is approximately $1,998 and approximately $3,261 for APC 0042.
b. Knee Arthroscopy (APCs 0041 and 0042)
For CY 2010, we proposed to continue to assign CPT codes 29882 (Arthroscopy, knee, surgical; with meniscus repair (medial or lateral)) and 29883 (Arthroscopy, knee, surgical; with meniscus repair (medial and lateral)) to APC 0041 (Level I Arthroscopy), with a proposed payment rate of approximately $2,014. In addition, we proposed to continue to assign CPT code 29867 (Arthroscopy, knee, surgical; osteochondral allograft (eg, mosaicplasty)) to APC 0042 (Level II Arthroscopy), with a proposed payment rate of approximately $3,279.
Comment: One commenter recommended that CMS reassign CPT code 29882 and 29883 from APC 0041 to APC 0042 because of their similarity to procedures assigned to APC 0042. The commenter also requested that CMS reassign CPT code 29867 from APC 0042 to APC 0052 (Level IV Musculoskeletal Procedures Except Hand and Foot), with a proposed payment rate of approximately $5,889. The commenter believed that CPT code 29867 is clinically comparable to the other procedures assigned to APC 0052.
Response: We reviewed the clinical and resource characteristics of CPT codes 29882 and 29883 and continue to believe these CPT codes are appropriately assigned to APC 0041 for CY 2010. Analysis of CY 2008 claims data showed that the median cost for CPT code 29882, based on 165 single claims (out of 334 total claims), is approximately $2,224 and for CPT code 29883, based on 116 claims (out of 182 total claims), is approximately $2,075. These median costs are consistent with the final CY 2010 median cost of APC 0041, which is approximately $1,998. Furthermore, these procedures are clinically similar to the majority of other knee arthroscopy procedures that are also assigned to APC 0041.
In addition, we do not agree with the commenter's assertion that CPT code 29867 is similar to the other procedures in APC 0052. Our claims data show that CPT code 29867 has a median cost of approximately $3,652, which is significantly lower than the median cost of approximately $5,921 for APC 0052, but close to the median cost of approximately $3,261 for APC 0042, where we proposed to assign the code for CY 2010. Furthermore, the knee arthroscopy procedure described by CPT code 29867 is not clinically similar to other procedures assigned to APC 0052, which are generally not performed arthoscopically.
After consideration of the public comment we received, we are finalizing our CY 2010 proposals, without modification, to continue to assign CPT codes 29882 and 29883 to APC 0041, which has a final CY 2010 APC median cost of approximately $1,998, and to continue to assign CPT code 29867 to APC 0042, which has a final CY 2010 APC median cost of approximately $3,261.
c. Shoulder Arthroscopy (APC 0042)
For CY 2010, we proposed to continue to assign CPT codes 29806 (Arthroscopy, shoulder, surgical; capsulorrhaphy) and 29807 (Arthroscopy, shoulder, surgical; repair of slap lesion) to APC 0042 (Level II Arthroscopy), with a proposed payment rate of approximately $3,279.
Comment: One commenter recommended that CMS reassign CPT codes 29806 and 29807 to APC 0052 (Level IV Musculoskeletal Procedures Except Hand and Foot), which had a proposed payment rate of approximately $5,889. The commenter believed that these procedures are clinically similar to the other procedures in APC 0052.
Response: We continue to believe that CPT codes 29806 and 29807 are appropriately assigned to APC 0042 based on clinical and resource considerations. We note that most other shoulder arthroscopy procedures that are similar to CPT codes 29806 and 29807 are assigned to APC 0042, while most procedures assigned to APC 0052 are bone procedures that are not performed arthroscopically. Analysis of our claims data revealed that the median cost of CPT code 29806, based Start Printed Page 60451 on 161 single claims (out of 759 total claims), is approximately $4,003, which is significantly lower than the median cost of approximately $5,921 for APC 0052. Likewise, our claims data showed that the median cost of CPT code 29807, based on 199 single claims (out of 3,802 total claims), is approximately $4,202, which is also significantly lower than the median cost for APC 0052. The CPT code-specific median costs of these two procedure codes fall within the range of median costs (approximately $2,730 to $4,592) of significant procedures that are also assigned to APC 0042 for CY 2010. Therefore, we believe that CPT codes 29806 and 29807 are most similar clinically and with respect to resource costs to other procedures assigned to APC 0042.
After consideration of the public comment we received, we are finalizing our CY 2010 proposal, without modification, to continue to assign CPT codes 29806 and 29807 to APC 0042, which has a final CY 2010 APC median cost of approximately $3,261.
d. Fasciotomy Procedures (APC 0049)
For CY 2010, we proposed to continue to assign the following seven CPT codes for fasciotomy procedures to APC 0049 (Level I Musculoskeletal Procedures Except Hand and Foot): CPT code 25020 (Decompression fasciotomy, forearm and/or wrist, flexor or extensor compartment; without debridement of nonviable muscle and/or nerve); CPT code 27496 (Decompression fasciotomy, thigh and/or knee, one compartment (flexor or extensor or adductor)); CPT code 27498 (Decompression fasciotomy, thigh and/or knee, multiple compartments); CPT code 27499 (Decompression fasciotomy, thigh and/or knee, multiple compartments; with debridement of nonviable muscle and/or nerve); CPT code 27892 (Decompression fasciotomy, leg; anterior and/or lateral compartments only, with debridement of nonviable muscle and/or nerve); CPT code 27893 ('Decompression fasciotomy, leg; posterior compartment(s) only, with debridement of nonviable muscle and/or nerve); and CPT code 27894 (Decompression fasciotomy, leg; anterior and/or lateral, and posterior compartment(s), with debridement of nonviable muscle and/or nerve). The CY 2010 proposed payment rate for APC 0049 was approximately $1,490.
Comment: One commenter recommended that CMS reassign CPT codes 25020, 27496, 27498, 27599, 27892, 27893, and 27894 from APC 0049 to APC 0050 (Level II Musculoskeletal Procedures Except Hand and Foot) based on their clinical and resource similarity to the other fasciotomy procedures proposed for assignment to APC 0050. Although the commenter recommended assignment of CPT code 27599 (Unlisted procedure, femur or knee) among its list of codes for assignment to APC 0050, we believe that the commenter may have intended to reference CPT code 27499 instead. CPT code 27499 describes a decompression fasciotomy on the thigh and/or knee and was proposed for assignment to APC 0049. CPT code 27599 was proposed for assignment to APC 0129 (Level I Closed Treatment Fracture Finger/Toe/Trunk) and does not describe a fasciotomy procedure.
Response: We reviewed the clinical characteristics associated with each of the seven fasciotomy procedures, and based on our analysis, we agree with the commenter's recommendation. We note that, while we have no or very limited hospital claims data for these services that reflect hospital costs, a number of other similar fasciotomy procedures are already assigned to APC 0050. Based on further analysis, we believe that CPT codes 25020, 27496, 27498, 27499, 27892, 27893, and 27894 are sufficiently similar to those other fasciotomy procedures to warrant reassignment to APC 0050.
After consideration of the public comment we received, for CY 2010, we are reassigning CPT codes 25020, 27496, 27498, 27499, 27892, 27893, and 27894 from APC 0049 to APC 0050, which has a final CY 2010 APC median cost of approximately $2,122.
e. Fibula Repair (APC 0062)
For CY 2010, we proposed to continue to assign CPT code 27726 (Repair of fibula nonunion and/or malunion with internal fixation) to APC 0062 (Level I Treatment Fracture/Dislocation), with a proposed payment rate of approximately $1,735.
Comment: One commenter recommended that CMS reassign CPT code 27726 from APC 0062 to APC 0063 (Level II Treatment Fracture/Dislocation) because the procedure is comparable in clinical and resource characteristics to CPT code 27760 (Closed treatment of medial malleolus fracture; without manipulation), which was proposed for assignment to APC 0063, with a proposed payment rate of approximately $3,023. In particular, the commenter argued that repair of a fibular nonunion is similar clinically and with respect to resource costs to repair of a tibial nonunion and, therefore, the two procedures should be assigned to the same clinical APC. Although the commenter compared CPT code 27726 to CPT code 27760, we believe that the commenter may have intended to reference CPT code 27720 (Repair of nonunion or malunion, tibia; without graft, (eg, compression technique)), which describes a repair of a tibial nonunion and was proposed for assignment to APC 0063, instead of CPT code 27760. CPT code 27760 describes a closed treatment of an ankle fracture and was proposed for assignment to APC 0129 (Level I closed Treatment Fracture Finger/Toe/Trunk).
Response: We reviewed the clinical characteristics and resource use associated with CPT code 27726, and based on our analysis, we agree with the commenter's recommendation. For CY 2010, our claims data showed a median cost of approximately $3,486 for CPT code 27726, based on 59 single claims (of 121 total claims), which is significantly higher than the median cost of approximately $1,726 for APC 0062. Further, our claims data showed that the median cost of CPT code 27726 is similar to that of APC 0063, which has an APC median cost of approximately $3,037. In addition, CPT code 27726 clinically resembles CPT code 27720, which is also assigned to APC 0063.
After consideration of the public comment we received, for CY 2010, we are modifying our CY 2010 proposal and reassigning CPT code 27726 to APC 0063 for CY 2010, which has a final CY 2010 APC median cost of approximately $3,037.
f. Forearm Orthopedic Procedures (APCs 0050, 0051, and 0052)
For CY 2010, we proposed to assign the 14 forearm fracture procedures listed in Table 25 below to APC 0050 (Level II Musculoskeletal Procedures Except Hand and Foot), APC 0051, (Level III Musculoskeletal Procedures Except Hand and Foot), or APC 0052 (Level IV Musculoskeletal Procedures Except Hand and Foot). The CY 2010 proposed payment rate for APCs 0050 was approximately $2,135; for APC 0051, approximately $3,156; and for APC 0052, approximately $5,889.
Comment: One commenter recommended that CMS reassign six forearm fracture procedures to APC 0051. In particular, the commenter stated that CPT codes 25350, 25355, 25360, 25370, 25390, and 25400 describe forearm surgical procedures involving only one bone and the hospital resource costs for the procedures are similar to those of procedures assigned to APC 0051. In addition, the commenter suggested that CMS reassign both CPT codes 24400 and 24410 to APC 0051 because these procedures are similar in clinical Start Printed Page 60452 characteristics and resource costs to other procedures in APC 0051. Further, the commenter recommended that CPT codes 25365, 25375, 25393, 25405, 25415, and 25420 be reassigned to APC 0052 based on considerations of clinical and resource homogeneity.
Response: We reviewed the clinical characteristics and resource costs associated with each surgical procedure discussed by the commenter. Based on our analysis of hospital claims data and clinical review, we agree with the commenter's recommendation that CPT codes 24400, 24410, 25350, 25355, 25360, 25370, 25390, and 25400 should be assigned to APC 0051. We have very few hospital outpatient claims for these procedures upon which to estimate their hospital costs. We note that these procedures are all performed on only one forearm bone, either the radius or the ulna, and we believe they share significant clinical and resource characteristics with other procedures assigned to APC 0051. Therefore, we are reassigning CPT codes 24400, 24410, 25350, 25360, and 25390 to APC 0051 for CY 2010. As we proposed, we are continuing to assign CPT codes 25355, 25370, and 25400 to APC 0051 for CY 2010.
With regard to the procedures that were recommended for reassignment to APC 0052, we agree with the commenter's argument that CPT codes 25405, 25415, and 25420 have similar resource costs to other procedures already assigned to APC 0052. These procedures were assigned to APC 0052 for CY 2009 and, as we proposed, for CY 2010, we are continuing their assignment to APC 0052.
However, we do not agree with the commenter's recommendation to reassign CPT codes 25365, 25375, and 25393 to APC 0052. We have very few claims for these procedures from CY 2008, but we believe their clinical and resource characteristics are sufficiently similar to other procedures assigned to APC 0051 that they should all be assigned to APC 0051 for CY 2010. While we proposed to assign CPT codes 25375 and 25393 to APC 0051 for CY 2010, we proposed to assign CPT code 25365 to APC 0050. In this final rule with comment period, we are modifying the assignment of CPT code 25365 to APC 0051, where it will reside along with CPT codes 25375 and 25393.
After consideration of the public comment we received, we are finalizing our CY 2010 proposals, without modification, to continue to assign CPT codes 25355, 25370, 25375, and 25393, and 25400 to APC 0051, which has a final CY 2010 APC median cost of approximately $3,111, and CPT codes 25405, 25415, and 25420 to APC 0052, which has a final CY 2010 APC median cost of approximately $5,921. We are modifying our CY 2010 proposals and assigning CPT codes 24400, 24410, 25350, 25360, 25365, and 25390 to APC 0051, which has a final CY 2010 APC median cost of approximately $3,111. Table 25 below lists the final APC assignments for the 14 forearm fracture procedures discussed in this section.
| CY 2010 HCPCS code | CY 2010 Long descriptor | Proposed CY 2010 APC | Final CY 2010 APC |
|---|---|---|---|
| 24400 | Osteotomy, humerus, with or without internal fixation | 0050 | 0051 |
| 24410 | Multiple osteotomies with realignment on intramedullary rod, humeral shaft (sofield type procedure) | 0050 | 0051 |
| 25350 | Osteotomy, radius; distal third | 0052 | 0051 |
| 25355 | Osteotomy, radius; middle or proximal third | 0051 | 0051 |
| 25360 | Osteotomy; ulna | 0050 | 0051 |
| 25365 | Osteotomy; radius and ulna | 0050 | 0051 |
| 25370 | Multiple osteotomies, with realignment on intramedullary rod (sofield type procedure); radius or ulna | 0051 | 0051 |
| 25375 | Multiple osteotomies, with realignment on intramedullary rod (sofield type procedure); radius and ulna | 0051 | 0051 |
| 25390 | Osteoplasty, radius or ulna; shortening | 0050 | 0051 |
| 25393 | Osteoplasty, radius and ulna; lengthening with autograft | 0051 | 0051 |
| 25400 | Repair of nonunion or malunion, radius or ulna; without graft (eg, compression technique | 0051 | 0051 |
| 25405 | Repair of nonunion or malunion, radius or ulna; with autograft (includes obtaining graft) | 0052 | 0052 |
| 25415 | Repair of nonunion or malunion, radius and ulna; without graft (eg, compression technique) | 0052 | 0052 |
| 25420 | Repair of nonunion or malunion, radius and ulna; with autograft (includes obtaining graft) | 0052 | 0052 |
g. Low Energy Extracorporeal Shock Wave Therapy (Low Energy ESWT)
For CY 2010, we proposed to continue to assign CPT code 0019T (Extracorporeal shock wave involving musculoskeletal system, not otherwise specified, low energy) status indicator “A” (Services furnished to a hospital outpatient that are paid under a fee schedule or payment system other than OPPS).
Comment: One commenter urged CMS to assign CPT code 0019T status indicator “T” (Significant Procedure: Multiple Reduction Applies), and to place the CPT code in an APC that pays appropriately. The commenter indicated that high energy ESWT, specifically CPT code 0101T (Extracorporeal shock wave involving musculoskeletal system, not otherwise specified, high energy), is assigned to APC 0050 (Level II Musculoskeletal Procedures Except Hand and Foot), with a proposed CY 2010 payment rate of approximately $2,135. The commenter argued that both the low energy and high energy ESWT treat similar conditions and both use Class III medical devices that are subject to the most stringent FDA approval process that restricts the sale of the device to by or on the order of a physician. Because of this similarity, the commenter urged CMS to be consistent in its payment policy and recommended that both CPT codes 0101T and 0019T be assigned the same status indicator to specify their separate payment under the OPPS.
Response: We do not agree that low energy ESWT is similar to high energy ESWT. High energy ESWT requires the Start Printed Page 60453 use of anesthesia during the procedure and usually involves only one treatment session. Alternatively, low energy ESWT does not require anesthesia and usually is furnished over several sessions. Because of the complexity of high energy ESWT, we believe that it is appropriate to pay for CPT code 0101T as a hospital outpatient service under the OPPS through APC 0050. However, CPT code 0019T is assigned status indicator “A” because it is designated as a “sometimes therapy” service to indicate that it is a therapy service when furnished by a therapist. When performed in the HOPD, we believe CPT code 0019T would be furnished as a therapy service paid under the MPFS and, therefore, the service is appropriately assigned status indicator “A” for hospital outpatient payment purposes. Regulation of the device by the FDA as a Class III medical device for sale by or on the order of a physician and the need for special training to use the technology for its approved use are not inconsistent with our considering CPT code 0019T to be a “sometimes therapy” service, that is, a therapy service when furnished by a therapist.
After consideration of the public comment we received, we are finalizing our CY 2010 proposal, without modification, to continue to assign CPT code 0019T to status indicator “A” for CY 2010.
h. Insertion of Posterior Spinous Process Distraction Device (APC 0052)
For CY 2009 (73 FR 68620), we reassigned CPT codes 0171T (Insertion of posterior spinous process distraction device (including necessary removal of bone or ligament for insertion and imaging guidance), lumbar, single level) and 0172T (Insertion of posterior spinous process distraction device (including necessary removal of bone or ligament for insertion and imaging guidance), lumbar, each additional level) from APC 0050 (Level II Musculoskeletal Procedures Except Hand and Foot) to APC 0052 (Level IV Musculoskeletal Procedures Except Hand and Foot). For CY 2007 and CY 2008, the device implanted in procedures described by CPT codes 0171T and 0172T, HCPCS code C1821 (Interspinous process distraction device (implantable)), was assigned pass-through payment status and, therefore, was paid separately at charges adjusted to cost. The period of pass-through payment for HCPCS code C1821 expired after December 31, 2008. According to our established methodology, the costs of devices no longer eligible for pass-through payments are packaged into the costs of the procedures with which the devices are reported in the claims data used to set the payment rates for those procedures. Therefore, the costs of the implanted device identified by HCPCS code C1821 are packaged into the costs of CPT codes 0171T and 0172T beginning in CY 2009.
At the February 2009 meeting, the APC Panel heard a public presentation that recommended reassignment of CPT codes 0171T and 0172T from APC 0052 to APC 0425 (Level II Arthroplasty or Implantation with Prosthesis). The presenter believed that APC resource homogeneity would be improved if CPT codes 0171T and 0172T were reassigned to APC 0425. The presenter asserted, based on its analysis of CY 2007 claims data, that the median cost of CPT code 0171T was significantly higher than the median cost of APC 0052, while only slightly lower than the median cost of APC 0425. The presenter indicated that, while the median cost of APC 0052 was significantly higher than the median cost of device HCPCS code C1821, device costs are only one element of the overall procedure cost and other associated procedure costs are more than $3,200. Regarding clinical homogeneity, the presenter stated that kyphoplasty is the only spine procedure currently assigned to APC 0052 other than CPT codes 0171T and 0172T. The presenter also claimed that 36 percent of claims for CPT code 0171T are reported without HCPCS code C1821, which identified a device that is always implanted in procedures reported with CPT codes 0171T and 0172T. The presenter requested reassignment of CPT codes 0171T and 0172T to APC 0425 because this APC is a device-dependent APC, and CPT codes 0171T and 0172T would then be subject to procedure-to-device claims processing edits.
The APC Panel recommended that CMS continue the assignment of CPT codes 0171T and 0172T to APC 0052 for CY 2010, institute procedure-to-device claims processing edits for HCPCS code C1821, and then reevaluate the APC assignments of CPT codes 0171T and 0172T in one year.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35305), we stated that under our existing policy, we generally do not identify any individual HCPCS codes as device-dependent codes under the OPPS. We create device edits, when appropriate, for procedures assigned to device-dependent APCs, where those APCs have been historically identified under the OPPS as having very high device costs. We noted in the CY 2009 OPPS/ASC final rule with comment period regarding APC 0052 (73 FR 68621) that we typically do not implement procedure-to-device edits for an APC where there are not device HCPCS codes for all possible devices that could be used to perform a procedure that always requires a device, and the APC is not designated as a device-dependent APC. APC 0052 is not a device-dependent APC because a number of the procedures assigned to the APC do not require the use of implantable devices. Furthermore, in some cases, there may not be HCPCS codes that describe all devices that may be used to perform the procedures in APC 0052.
We examined the CY 2008 claims data available for the CY 2010 proposed rule to determine the frequency of billing CPT code 0171T (which is the main procedure code reported with HCPCS code C1821) with and without device HCPCS code C1821. CPT code 0172T is an add-on code to CPT code 0171T. We recognize that our single claims for CPT code 0172T may not be correctly coded claims and, therefore, our cost estimation for CPT code 0172T may not be accurate. Our analysis showed that the CY 2010 proposed rule median cost for CPT code 0171T was approximately $7,717 based on over 800 single claims. The CY 2010 proposed rule claims data for CPT code 0171T revealed a median cost of approximately $7,916 based on over 500 single claims with HCPCS code C1821, and a median cost of approximately $7,387 based on approximately 300 single claims without HCPCS code C1821. Therefore, we concluded that the median cost of claims for CPT code 0171T reported with HCPCS code C1821 is similar to the median cost of claims for the procedure reported without HCPCS code C1821. We stated in the CY 2010 OPPS/ASC proposed rule (74 FR 35305) that we have no reason to believe that those hospitals not reporting the device HCPCS code had failed to consider the cost of the device in charging for the procedure. Furthermore, claims for CPT code 0171T reported with HCPCS code C1821 accounted for about two-thirds of the single claims available for ratesetting. For the CY 2010 OPPS/ASC proposed rule, we concluded that the overall median cost of CPT code 0171T fell within an appropriate range of HCPCS code-specific median costs for those services proposed for CY 2010 assignment to APC 0052, which had a proposed APC median cost of approximately $5,939 and no 2 times violation. Moreover, in the CY 2010 OPPS/ASC proposed rule (74 FR 35305), we indicated that we do not believe that procedure-to-device claims processing Start Printed Page 60454 edits are necessary to ensure accurate cost estimation for CPT code 0171T.
The CY 2010 OPPS/ASC proposed rule line-item median cost for HCPCS code C1821 was approximately $4,625, while the CY 2010 OPPS/ASC proposed rule median cost of APC 0052 was approximately $1,300 more than this device cost. We stated in the proposed rule (74 FR 35305) that previous estimates of procedure time presented to us at the time of the device pass-through application for the interspinous process distraction device described by HCPCS code C1821 were approximately 30 to 60 minutes of procedure time for the service currently described by CPT code 0171T. This is reasonably comparable to the typical procedure time for kyphoplasty described by CPT code 22523 (Percutaneous vertebral augmentation, including cavity creation (fracture reduction and bone biopsy included when performed) using mechanical device, one vertebral body, unilateral or bilateral cannulation (eg, kyphoplasty); thoracic) and CPT code 22524 (Percutaneous vertebral augmentation, including cavity creation (fracture reduction and bone biopsy included when performed) using mechanical device, one vertebral body, unilateral or bilateral cannulation (eg, kyphoplasty); lumbar), which are also assigned to APC 0052.
Because we reasoned that APC 0052 pays appropriately for the procedure cost of CPT codes 0171T and 0172T, we proposed to maintain the assignment of CPT codes 0171T and 0172T to APC 0052 for CY 2010 and not to implement device edits for these procedures. We proposed to accept one part of the APC Panel's recommendation regarding the continued assignment of CPT codes 0171T and 0172T to APC 0052, but we proposed to not accept the APC Panel's further recommendation to institute procedure-to-device edits for these services for CY 2010. As we do for all OPPS services, we stated that we would reevaluate the APC assignments of CPT codes 0171T and 0172T when additional claims data become available for CY 2011 ratesetting, in accordance with the final part of the APC Panel's recommendation for these procedures (74 FR 35305).
Comment: Some commenters recommended that CMS reassign CPT codes 0171T and 0172T from APC 0052 to APC 0425 for CY 2010, arguing that the resource costs associated with these procedures are more similar to the resource costs of procedures assigned to APC 0425 than to procedures assigned to APC 0052. One commenter noted, for example, that the median cost for CPT code 0171T is approximately 30 percent higher than the median cost for APC 0052, but only two percent lower than the median cost for APC 0425. In response to CMS' observation in the CY 2010 OPPS/ASC proposed rule that the proposed median cost of APC 0052 was approximately $1,300 more than the line-item median cost for HCPCS code C1821 of approximately $4,625, the commenter pointed out that device costs are but one element of the overall procedure costs. The commenter presented data to demonstrate that the service costs associated with CPT code 0171T are greater than this $1,300 difference. The commenter agreed that the 30 to 60 minute procedure time associated with CPT code 0171T that CMS noted in the proposed rule is reasonable, but argued that intraservice time should not be used as a sole basis for judging resource homogeneity because there is not a direct correlation between intraservice time and hospital costs.
The commenters also disagreed with CMS' assertion that the procedures described by CPT codes 0171T and 0172T are more similar clinically to procedures assigned to APC 0052 than to procedures assigned to APC 0425. One commenter argued that kyphoplasty is the only spine procedure assigned to APC 0052, and that, like all of the other procedures assigned to APC 0052, it does not involve the implantation of a device. The commenter acknowledged that, while CMS' statement of clinical similarity for APC 0052 is true to some extent, the procedure described by CPT code 0171T is more similar to procedures assigned to APC 0425 because it is orthopedic in nature and requires the use of a device that is classified as a prosthesis by the FDA.
Moreover, the commenter claimed that there are relevant precedents for reassignment of CPT codes 0171T and 0172T to APC 0425, such as CMS' proposed reassignment of CPT code 27446 (Arthroplasty, knee, condyle and plateau; medial OR lateral compartment) to APC 0425 for CY 2010. The commenter also argued that reassigning CPT 0171T and 0172T to device-dependent APC 0425, to which procedure-to-device edits apply, would help ensure that only correctly coded claims are used in ratesetting.
Response: We continue to believe that APC 0052 is an appropriate APC assignment for CPT codes 0171T and 0172T based on consideration of the procedures' clinical and resource characteristics. We do not agree with the commenters that the resource costs of providing the procedures described by CPT codes 0171T and 0172T are substantially different from the resource costs of providing other procedures assigned to APC 0052 and that they should not be assigned to APC 0052, which has a final CY 2010 APC median cost of approximately $5,921. Based on the CY 2008 claims data reviewed for this final rule with comment period, the final median costs of CPT codes 0171T and 0172T are approximately $7,522 (based on 939 single claims) and approximately $14,617 (based on 6 single claims), respectively. As we have noted previously (73 FR 68620), we recognize that our single claims for CPT code 0172T may not be correctly coded and, therefore, our cost estimation for CPT code 0172T may not be accurate. CPT code 0171T has the highest median cost of the significant procedures (defined as those procedures with a frequency of greater than 1,000 single claims or a frequency of greater than 99 and more than 2 percent of the single claims in the APC) assigned to APC 0052, while the lowest cost significant procedure has a median cost of approximately $5,072. Therefore, the configuration of APC 0052 does not violate the 2 times rule. We continue to believe that, based on resource considerations, assignment to APC 0052 would provide appropriate payment for CPT codes 0171T and 0172T. We agree with the commenters that we should consider factors such as line-item median costs for devices and intraservice times as two data elements among several when we evaluate the clinical and resource homogeneity of APCs. In this case, we continue to believe that, as described in the proposed rule, both the line-item median cost for HCPCS code C1821 and the intraservice time for the procedure described by CPT code 0171T support our assessment that this procedure is similar in terms of resource utilization to other procedures assigned to APC 0052, consistent with the fact there is no 2 times violation within this APC.
We continue to believe the posterior spinous process distraction device procedures described by CPT codes 0171T and 0172T are clinically similar to other procedures, such as the kyphoplasty procedures, that are assigned to APC 0052. We disagree with the commenter that the kyphoplasty procedures described by CPT codes 22523 and 22524 do not involve the implantation of a device. Our definition of an implantable device includes surgically inserted or implanted devices that may not remain with the patient following the procedure, and thus may include expensive devices used in kyphoplasty such as expanders and Start Printed Page 60455 other single-use disposal devices used to create a cavity in the vertebral body. We note the code descriptor of kyphoplasty CPT code 22523 states, “using mechanical device.” Based on a kyphoplasty New Technology APC application we received in CY 2004, the prices for these implantable devices are approximately $3,000. Moreover, the kyphoplasty procedures are clinical substitutes for vertebroplasty procedures, such as the procedure described by CPT code 22520 (Percutaneous vertebroplasty, one vertebral body, unilateral or bilateral injection; thoracic) and are assigned to APC 0050 (Level II Musculoskeletal Procedures Except Hand and Foot). CPT code 22520 has a CY 2010 final rule median cost of approximately $2,181, which is nearly $3,800 less than the final rule median cost of approximately $5,976 calculated for the kyphoplasty procedure described by CPT code 22523. This differential appears to be largely attributable to implantable device costs in kyphoplasty procedures. Therefore, we continue to believe that kyphoplasty and posterior spinous process distraction device procedures are clinically similar in that they are spinal procedures involving implantable devices. We note that there are no procedures involving the spine assigned to APC 0425.
We do not agree with the commenter that our reassignment of the knee arthroplasty procedure described by CPT code 27446 to APC 0425 serves as a precedent for the reassignment of CPT codes 0171T and 0172T to APC 0425. As discussed in section II.A.2.d.(1) of this final rule with comment period, we reassigned CPT code 27446 from APC 0681 (Knee Arthroplasty) to APC 0425 for CY 2010 in order to consolidate APC 0425 with APC 0681, in which CPT code 27446 was the only code. As noted in section II.A.2.d.(1) of this final rule with comment period, over the past several years, the median cost for CPT code 27446 has fluctuated due to a low volume of services being performed by a small number of providers in the HOPD, and to a single provider performing the majority of services. We believe that by reassigning CPT code 27446 to APC 0425 and deleting APC 0681, we can maintain greater stability from year to year in the payment rate for CPT code 27446. Therefore, we do not believe this is a similar situation to that of CPT codes 0171T and 0172T, as the commenter argued. Furthermore, we do not believe that implantation of an interspinous process distraction device, a minimally invasive procedure, is clinically comparable to a knee replacement procedure that is performed on the majority of Medicare beneficiaries on a hospital inpatient basis. We also do not agree that we should reassign CPT codes 0171T and 0172T to APC 0425 in order to implement device edits for these procedures. As we described in the proposed rule (74 FR 35305), based upon analysis of CY 2010 proposed rule claims data for CPT code 0171T, we have no reason to believe that the minority of hospitals that do not bill HCPCS code C1821 along with CPT code 0171T are not already considering the costs of the interspinous process distraction device in charging for the procedure.
After consideration of the public comments we received, we are finalizing our CY 2010 proposals, without modification, to continue to assign CPT codes 0171T and 0172T to APC 0052, which has a final CY 2010 APC median cost of approximately $5,921.
7. Radiation Therapy Services
a. Proton Beam Therapy (APCs 0664 and 0667)
For CY 2010, we proposed to continue to assign CPT codes 77520 (Proton treatment delivery; simple, without compensation) and 77522 (Proton treatment delivery; simple, with compensation) to APC 0664 (Level I Proton Beam Radiation Therapy), which had a proposed payment rate of approximately $713. We also proposed to continue to assign CPT codes 77523 (Proton treatment delivery; intermediate) and 77525 (Proton treatment delivery; complex) to APC 0667 (Level II Proton Beam Radiation Therapy), which had a proposed payment rate of approximately $933.
Comment: Several commenters supported the proposed payment increases for the proton beam treatment CPT codes. The commenters cited a payment increase of 1.43 percent for CPT codes 77520 and 77522, and a payment increase of 11.02 percent for CPT codes 77523 and 77525.
Response: We appreciate the commenters' support for our proposals. The CY 2010 OPPS payment rates for CPT codes 77520, 77522, 77523, and 77525 are based on the APC median costs calculated from CY 2008 hospital claims data and the most current cost reports, according to the standard OPPS ratesetting methodology. We are confident that the observed costs in the claims data are representative of the costs of the proton beam therapy services provided in CY 2008 because almost all of the claims are single claims that can be used for ratesetting.
After consideration of the public comments we received, we are finalizing our CY 2010 proposals, without modification, to assign CPT codes 77520 and 77522 to APC 0664, with a final CY 2010 APC median cost of approximately $934, and CPT codes 77523 and 77525 to APC 0667, with a final CY 2010 APC median cost of approximately $1,221.
b. Stereotactic Radiosurgery (SRS) Treatment Delivery Services (APCs 0065, 0066, 0067, and 0127)
For CY 2010, we proposed to continue to assign CPT code 77371 (Radiation treatment delivery, stereotactic radiosurgery (SRS), complete course of treatment of cranial lesion(s) consisting of 1 session; multi-source Cobalt 60 based) to APC 0127 (Level IV Stereotactic Radiosurgery, MRgFUS, and MEG), with a proposed payment rate of approximately $7,714.
We also proposed to continue to recognize for separate payment in CY 2010 four existing HCPCS G-codes that describe linear accelerator-based SRS treatment delivery services. Specifically, we proposed the following: to assign HCPCS code G0173 (Linear accelerator based stereotactic radiosurgery, complete course of therapy in one session) to APC 0067 (Level III Stereotactic Radiosurgery, MRgFUS, and MEG), with a proposed payment rate of approximately $3,507; to assign HCPCS code G0251 (Linear accelerator-based stereotactic radiosurgery, delivery including collimator changes and custom plugging, fractionated treatment, all lesions, per session, maximum five sessions per course of treatment) to APC 0065 (Level I Stereotactic Radiosurgery, MRgFUS, and MEG), with a proposed payment rate of approximately $894; to assign HCPCS code G0339 (Image-guided robotic linear accelerator-based stereotactic radiosurgery, complete course of therapy in one session or first session of fractionated treatment) to APC 0067, with a proposed payment rate of approximately $3,507; and to assign HCPCS code G0340 (Image-guided robotic linear accelerator-based stereotactic radiosurgery, delivery including collimator changes and custom plugging, fractionated treatment, all lesions, per session, second through fifth sessions, maximum five sessions per course of treatment) to APC 0066 (Level II Stereotactic Radiosurgery, MRgFUS, and MEG), with a proposed payment rate of approximately $2,505.
Further, we proposed to continue to assign CPT codes 77372 (Radiation treatment delivery, stereotactic Start Printed Page 60456 radiosurgery (SRS) (complete course of treatment of cerebral lesion(s) consisting of 1 session); linear accelerator based) and 77373 (Stereotactic body radiation therapy, treatment delivery, per fraction to 1 or more lesions, including image guidance, entire course not to exceed 5 fractions) status indicator “B” (Codes that are not recognized by OPPS when submitted on an outpatient hospital Part B bill type (12x and 13x)) under the OPPS, to indicate that these CPT codes are not payable under the OPPS.
Comment: Several commenters expressed concern about their belief that payment for HCPCS code G0173 and CPT code 77371 is based on the utilization of specific SRS equipment. The commenters stated that no clinical data exist to support the need for differential payment for linear accelerator-based and Cobalt-60 SRS procedures. The commenters further explained that current medical literature cites no difference in clinical effectiveness for the systems associated with linear accelerator-based and Cobalt-60 SRS procedures. One commenter provided an extensive bibliography of relevant peer-reviewed articles supporting this finding. The commenters recommended that CMS assign HCPCS code G0173 and CPT code 77371 to the same APC so that payment for both services would be the same. Specifically, the commenters suggested capping the payment rate for CPT code 77371 at the payment rate for HCPCS code G0173. One commenter added that, based on an internal analysis of CY 2007 claims data using the CY 2009 OPPS payment rates for CPT code 77371 and HCPCS code G0173, paying both procedures at the payment rate for HCPCS code G0173 would lead to Medicare savings of at least $272 million over 10 years and about $104 million over 5 years. The commenters encouraged CMS to consider this payment methodology and, thereby, pay for services appropriately regardless of the specific equipment used to deliver SRS treatment, especially as Medicare moves towards a value-based purchasing system.
Response: Analysis of our claims data shows that the median costs for linear accelerator-based and Cobalt-60 SRS procedures vary significantly. Since the creation of CPT code 77371, which was made effective January 1, 2007, our claims data has shown a median cost of more than approximately $7,000 for this procedure. Based on data available for CY 2010 ratesetting, our claims data showed a median cost of approximately $7,277 for CPT code 77371 that is derived from 483 single claims (of 4,142 total claims), which is significantly higher than the median cost of approximately $2,877 for HCPCS code G0173 that is based on 459 single claims (of 1,471 total claims). Likewise, for claims submitted for CY 2007, the data year used for CY 2009 ratesetting, our claims data showed a median cost of approximately $7,470 based on 518 single claims (of 4,208 total claims) for CPT code 77371, which is much higher than the median cost of approximately $3,523 for HCPCS code G0173, based on 528 single claims (of 1,616 total claims).
The OPPS is a prospective payment system, where APC payment rates are based on the relative costs of services as reported to us by hospitals according to the most recent claims and cost report data as described in section II.A. of this final rule with comment period. The 2 times rule specifies that the median cost of the highest cost item or service within a payment group may be no more than 2 times greater than the median cost of the lowest cost item or service within the same group. Based on application of the 2 times rule, we cannot assign HCPCS code G0173 and CPT code 77371 to the same APC. In addition, because hospitals continue to report very different costs for these services, we believe it is appropriate to maintain the assignment of these two codes to different payment groups for CY 2010. As a matter of payment policy, the OPPS does not set payment rates for services based on considerations of clinical effectiveness. Furthermore, in accordance with the statute, we budget neutralize payments under the OPPS each year in the annual update so that projected changes in spending for certain services are redistributed to payment for other services.
After consideration of the public comments we received, we are finalizing our CY 2010 proposals, without modification, to continue to assign CPT code 77371 to APC 0127, which has a final CY 2010 APC median cost of approximately $7,277, and to continue to assign HCPCS code G0173 to APC 0067, which has a final CY 2010 APC median cost of approximately $3,539.
Comment: One commenter requested that CMS finalize the proposed APC and status indicator assignments for HCPCS codes G0173, G0251, G0339, G0340, 77372, and 77373 for CY 2010. The commenter also recommended that CMS revise code descriptors of HCPCS code G0173, G0251, G0339, and G0340 for SRS, to distinguish between non-gantry and gantry-based SRS systems. Based on internal analysis, the commenter stated that, within the past year, there has been an increase in the OPPS volume of incorrectly coded claims. The commenter suggested specific code descriptor changes for the four revised HCPCS G-codes, as well as specific language changes to the SRS billing instructions in Chapter 4 of the Medicare Claims Processing Manual.
Response: These HCPCS G-codes for SRS have been in effect for several years and, based on questions brought to our attention by hospitals, we have no reason to believe that hospitals are confused about the reporting of these codes. Further, we see resource differences reflected in the median costs of the four HCPCS G-codes that are reasonably consistent with our expectations for different median costs for the services based on the current code descriptors. We believe it would be confusing to hospitals if we were to revise the code descriptors for HCPCS codes G0173, G0251, G0339, and G0340. Moreover, such a change could lead to instability in our median costs and inaccurate payments for some services. Therefore, we believe that modifying the G-code descriptors is not necessary for us to continue to provide appropriate payment for the services they describe. We also do not believe changes to our current billing instructions for SRS services in the Medicare Claims Processing Manual are necessary.
After consideration of the public comment we received, we are finalizing our CY 2010 proposals, without modification, to maintain the existing code descriptors for HCPCS codes G0173, G0251, G0339, and G0340 for linear accelerator-based SRS. In addition, we are finalizing our proposals, without modification, to continue to assign CPT codes 77372 and 77373 to status indicator “B” under the OPPS, and to continue to assign the four linear accelerator-based SRS HCPCS codes to the same APCs for CY 2010 as CY 2009, specifically APCs 0065, 0066, and 0067, with final CY 2010 APC median costs of approximately $954, $2,465, and $3,539, respectively. Table 26 displays the final APC median costs for the SRS treatment delivery HCPCS codes and CPT code 77371. Start Printed Page 60457
| CY 2010 HCPCS code | CY 2010 Short descriptor | Final CY 2010 SI | Final CY 2010 APC | Final CY 2010 approximate APC median cost |
|---|---|---|---|---|
| G0173 | Linear acc stereo radsur com | S | 0067 | $3,539 |
| G0251 | Linear acc based stero radio | S | 0065 | 954 |
| G0339 | Robot lin-radsurg com, first | S | 0067 | 3,539 |
| G0340 | Robt lin-radsurg fractx 2–5 | S | 0066 | 2,465 |
| 77371 | SRS, multisource | S | 0127 | 7,277 |
c. Clinical Brachytherapy (APCs 0312 and 0651)
For CY 2010, we did not propose any change to the HCPCS codes for assignment to APC 0312 (Radioelement Applications) or APC 0651 (Complex Interstitial Radiation Source Application). The proposed CY 2010 payment rates for these APCs were approximately $298 and $808, respectively.
Comment: Several commenters objected to the proposed reduction in the payment rate for brachytherapy services assigned to APC 0312 from approximately $421 in CY 2009 to approximately $298 in CY 2010, and the proposed reduction in the payment rate for APC 0651 from approximately $847 in CY 2009 to approximately $808 in CY 2010. The commenters believed these reductions in payment rates are the result of reduced numbers of single claims for the services assigned to the APCs, caused by the trimming of lines for which no payment was made on the claim. They objected to the use of only 2 percent of total claims or a 30 percent reduction in single claims for CPT code 77778 (Interstitial radiation source application; complex) that is assigned to APC 0651, and to the use of only 9 percent of total claims for CPT code 77776 (Interstitial radiation source application; simple) and 19 percent of total claims for CPT code 77777 (Interstitial radiation source application; intermediate) that are both assigned to APC 0312. The commenters speculated that the problem could be associated with changes to the bypass list, trimming of unpaid lines, or other general problems with CMS' cost estimation methodology. They believed that, regardless of the source of the problem, CMS must establish appropriate and stable payment rates for these services to allow Medicare beneficiaries consistent access to brachytherapy procedures.
Response: The median cost for APC 0312 for CY 2010, calculated using final rule data, is approximately $300. Our review of final rule claims data indicates that the reduction in median cost for APC 0312 from CY 2009 to CY 2010 appears to be caused by changes in the median costs for the HCPCS codes assigned to the APC that drive the median cost for the APC.
| HCPCS code in APC 0312 | Short descriptor | CY 2009 approximate median cost | CY 2009 frequency of single claims | CY 2009 percentages of single claims | CY 2009 total claims | CY 2010 approximate median cost | CY 2010 single claims | CY 2010 percentages of single claims | CY 2010 total claims |
|---|---|---|---|---|---|---|---|---|---|
| 77776 | Apply interstit radiat simpl | $119 | 23 | 6 | 149 | $522 | 9 | 4 | 104 |
| 77762 | Apply intrcav radiat interm | 180 | 70 | 18 | 161 | 345 | 25 | 11 | 69 |
| 77763 | Apply intrcav radiat compl | 507 | 131 | 34 | 352 | 345 | 112 | 48 | 250 |
| 77777 | Apply interstit radiat inter | 608 | 7 | 2 | 51 | 300 | 11 | 5 | 54 |
| 77761 | Apply intrcav radiat simple | 681 | 158 | 41 | 247 | 85 | 78 | 33 | 124 |
| Totals | 389 | 41 | 960 | 235 | 39 | 601 | |||
| * Data exclude claims for CPT code 77799, which were not used in setting the APC median cost. | |||||||||
Specifically, in CY 2009, CPT codes 77761 and 77763 dominated APC 0312 and the APC median cost was approximately $420. For CY 2010, CPT codes 77761 and 77763 continue to dominate APC 0312 but their HCPCS-specific median costs declined. Hence, the median cost for APC 0312 decreased to approximately $300. We do not believe that the exclusion of the lines for which no payment was made was the controlling factor in the decline of the APC median cost. We excluded 97 lines (including one unlisted line that is not relevant) from the claims containing CPT codes assigned to APC 0312 before we split the claims into single claims. Therefore, it is not possible to know how many of the line-items we trimmed were on claims that might have become single claims that could be used for ratesetting purposes. The total frequency of HCPCS codes reported on claims used for CY 2010 ratesetting declined to 601 from 960 (before the line-item trim). Therefore, a reduction in the number of single claims that are available for calculation of the median cost for the APC is to be expected because the universe of claims assigned to APC 0312 declined by more than one third. However, we note that the single claims used in the APC median calculation, as a percent of the total frequency, was 41 percent in CY 2009 and declined only minimally to 39 percent in CY 2010, notwithstanding the decrease in total frequency from CY 2009 to CY 2010 and the trim of 96 Start Printed Page 60458 relevant lines of the 601 total claims for the codes used to set the APC median cost for APC 0312. We agree that the decline in the median costs for CPT codes 77761 and 77763 is notable. However, we know that, for CY 2007 (the year of the claims used for the CY 2009 OPPS), there were no CPT codes for the insertion of the needles and catheters used to apply brachytherapy sources interstitially to body areas other than the prostate. We believe it is possible that the costs of the needles and catheters may have been incorporated into the CY 2009 payment for some of the CPT codes assigned to APC 0312.
For CY 2008, the AMA's CPT Editorial Panel created CPT code 20555 (Placement of needles or catheters into muscle and/or soft tissue for subsequent interstitial radioelement application (at the time of or subsequent to the procedure)), and payment has been made for that CPT code through APC 0050 (Level II Musculoskeletal Procedures Except Hand and Foot) in CY 2008 and CY 2009. In the updated claims data used for this CY 2010 final rule with comment period, for services furnished in CY 2008, CPT code 20555 has a total frequency of 67 and a single claim frequency of 25. CPT code 20555 is assigned to APC 0050, which has a final CY 2010 median cost of approximately $2,122. Because the needles and catheters must be placed before services reported by certain CPT codes assigned to APC 0312 can be performed, the hospital would receive not only the payment for APC 0312, but would also be paid for the placement of the needles and catheters or other devices, whether reported under CPT code 20555 or another code for placement of needles and catheters or other brachytherapy source delivery devices. Therefore, although the payment rate for APC 0312 has declined between CY 2009 and CY 2010, hospitals will commonly receive a separate payment for the placement of the needles and catheters or other devices that, when added to the payment for the application of the sources, will provide a robust payment for the service in its entirety.
The final CY 2010 median cost of APC 0651 is approximately $885, compared to the median cost of approximately $847 for CY 2009. We note that most claims that report CPT code 77778 are for low dose rate prostate brachytherapy that is paid through APC 8001 (LDR Prostate Brachytherapy Composite) rather than through APC 0651. Therefore, the total claim frequency for APC 0651 of 9,649 includes both the 7,742 claims that meet the criteria for payment through APC 8001 and the 1,907 claims that meet the criteria for payment through APC 0651. For this final rule with comment period, we were able to use approximately 11 percent of the claims (206 of 1,907 total claims) that meet the criteria for payment through APC 0651 in the calculation of the median cost for APC 0651. Not only does the CY 2010 median cost for APC 0651 increase over the CY 2009 median cost, but when the separate payment for the placement of brachytherapy insertion devices is made, the full payment for the comprehensive service is substantial. For example, if CPT code 20555 was reported for placement of needles and catheters, the hospital would be paid for both one unit of APC 0651 (based on a CY 2010 median cost of approximately $885) and one unit of APC 0050 (based on a CY 2010 median cost of approximately $2,122).
After consideration of the public comments we received, we are finalizing our CY 2010 proposals, without modification, to calculate the median costs for APCs 0312 and 0651 according to the standard OPPS ratesetting methodology, applying the final bypass list and line-item trim as discussed in sections II.A.1. and II.A.2. of this final rule with comment period. The final CY 2010 median costs of APCs 0312 and 0651 are approximately $300 and $885, respectively. We believe that when hospitals fully report the services required for brachytherapy treatment, the combined OPPS payment for insertion of the source application devices and application of the sources themselves provides appropriate payment for the comprehensive service.
8. Other Services
a. Low Frequency, Non-Contact, Non-Thermal Ultrasound (APC 0013)
The CPT Editorial Panel created CPT code 0183T (Low frequency, non-contact, non-thermal ultrasound, including topical application(s), when performed, wound assessment, and instruction(s) for ongoing care, per day), effective January 1, 2008. Under the OPPS, we assigned CPT code 0183T to APC 0015 (Level III Debridement & Destruction) for CY 2008 and CY 2009. For CY 2009, APC 0015 has a payment rate of approximately $100. Based upon our review of the first year of hospital claims data for CPT 0183T, for CY 2010 we proposed to reassign CPT code 0183T to APC 0013 (Level II Debridement & Destruction), with a proposed payment rate of approximately $59.
Comment: Several commenters recommended that CMS continue to assign CPT code 0183T to APC 0015. The commenters asserted that the proposed payment for APC 0013 would not cover hospitals' costs for performing the procedure. One commenter stated that the single-use kit for the service costs $55. Another commenter reported that the majority of hospitals with the highest utilization of CPT code 0183T either failed to report or underreported the packaged supply costs associated with CPT code 0183T. The commenter analyzed CMS' claims data according to hospitals' reporting of “packaged” supplies with CPT code 0183T and found that 52 percent of all single claims were from 5 hospitals, and that 4 of these 5 hospitals, representing 39 percent of single claims for CPT code 0183T used in ratesetting, reported $0 or an insignificant (less than $5) packaged supply cost. Moreover, the commenter stated that the analysis indicated that, overall, only one-third of the single claims for CPT code 0183T included any packaged costs, although costly supplies are required for hospitals to furnish the service. In addition, the commenter reported that it surveyed hospitals that provided the service and learned that those hospitals reported a median procedure cost of approximately $153.
One commenter offered several reasons why hospitals might not report packaged supply costs with CPT code 0183T, including the fact that CPT code 0183T was a new CPT code in CY 2008, the year of claims data for the CY 2010 OPPS rates; hospitals' historical failure to consider supply costs in setting their procedure charges; the fact that relatively low cost supplies are often overlooked when hospitals charge for services; and the lack of a specific Level II HCPCS code to report a charge for the applicator kit. The commenter estimated that 32,000 procedures were furnished to Medicare beneficiaries in the HOPD in CY 2008, yet there were far fewer CY 2008 OPPS claims for the service. The commenter cited several examples of contractors providing instructions to report other CPT codes, such as CPT code 97602 (Removal of devitalized tissue from wound(s), non-selective debridement, without anesthesia (eg wet-to-moist dressings, enzymatic, abrasion), including topical application(s), wound assessment, and instruction(s) for ongoing care, per session), when providing the low frequency, non-contact, non-thermal ultrasound procedure. Another commenter argued that APC 0015 is the most clinically appropriate APC for CPT Start Printed Page 60459 code 0183T, and stated that if the service were reassigned to APC 0013 as proposed, it would be the only wound healing procedure and the only procedure requiring a single use disposable supply in APC 0013.
Response: We proposed to reassign CPT code 0183T to APC 0013 for CY 2010 based on clinical and resource considerations. The final CY 2010 median cost of CPT code 0183T is approximately $77, based on 9,335 single claims. The final CY 2010 median cost of APC 0013 is approximately $59, and the final CY 2010 final cost of APC 0015 is approximately $103. The final CY 2010 HCPCS code-specific median costs of other significant services assigned to APC 0013 range from approximately $46 to $82; therefore, the $77 final median cost of CPT code 0183T for CY 2010 is well within that range. While CY 2008 is the first year we have cost information from hospitals for the service, the large number of single claims provides a robust estimate of the service's cost based on claims from those hospitals that furnished the service in CY 2008. While the commenters were concerned that many claims did not include separate charges for the associated supplies, we have found that it is common for hospitals to consider the cost of necessary supplies when setting the procedure charge, rather than reporting a separate line-item charge for the associated supplies. Many supplies where payment is always packaged into procedure payments do not have specific Level II HCPCS codes under which to report the associated charges. Hospitals incorporate the charge for such supplies in the procedure charge or provide a charge on a separate line under an appropriate revenue code without a HCPCS code, and we package the costs from these uncoded line-items into payment for the associated procedure. Therefore, we have no reason to believe that our estimated cost for CPT code 0183T from CY 2008 claims data does not include the cost of the necessary supplies. The final CY 2010 median cost of CPT code 0183T is closer to the final CY 2010 median cost of APC 0013 than APC 0015. In fact, if we were to continue to assign CPT code 0183T to APC 0015 for CY 2010, APC 0015 would violate the 2 times rule. That is, if we maintained CPT code 0183T in APC 0015 for CY 2010 as requested by the commenters, it would be the significant procedure with the lowest median cost assigned to APC 0015. In turn, the median cost of approximately $158 for the highest cost significant procedure, CPT code 11000 (Debridement of extensive eczematous or infected skin; up to 1 of body surface), would be more than 2 times the median cost of CPT code 0183T, resulting in a 2 times violation in APC 0015. We note that the APC Panel heard several public presentations that addressed the proposed CY 2010 APC assignment of CPT code 0183T at the August 2009 meeting but made no recommendation regarding the CY 2010 assignment of the code. In particular, the APC Panel did not make a recommendation to us to maintain an APC configuration that would violate the 2 times rule and require that we except APC 0015 from the 2 times rule for CY 2010.
We also believe that APC 0013 is an appropriate APC assignment for CPT code 0183T based on clinical considerations. Other wound care services with similar median costs are assigned to APC 0013 for CY 2010, specifically CPT codes 97602 and 97605 (Negative pressure wound therapy (eg, vacuum assisted drainage collection), including topical application(s), wound assessment, and instruction(s) for ongoing care, per session; total wound(s) surface area less than 50 square centimeters).
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to reassign CPT 0183T from APC 0015 to APC 0013, with a final CY 2010 APC median cost of approximately $59.
b. Skin Repair (APCs 0134 and 0135)
For CY 2010, we proposed to continue to assign the CPT skin repair codes for the application of Apligraf, Oasis, and Dermagraft skin substitutes to the same procedural APCs for CY 2010 as their CY 2009 assignments. Specifically, we proposed to continue to assign the Apligraf application CPT codes 15340 (Tissue cultured allogeneic skin substitute; first 25 sq cm or less) and 15341 (Tissue cultured allogeneic skin substitute; each additional 25 sq cm, or part thereof) to APC 0134 (Level II Skin Repair), with a proposed payment rate of approximately $214. Likewise, we proposed to continue to assign the Dermagraft application CPT codes 15365 (Tissue cultured allogeneic dermal substitute, face, scalp, eyelids, mouth, neck, ears, orbits, genitalia, hands, feet, and/or multiple digits; first 100 sq cm or less, or 1% of body area of infants and children) and 15366 (Tissue cultured allogeneic dermal substitute, face, scalp, eyelids, mouth, neck, ears, orbits, genitalia, hands, feet, and/or multiple digits; each additional 100 sq cm, or each additional 1% of body area of infants and children, or part thereof) to APC 0134. We proposed to continue to assign the Oasis application CPT codes 15430 (Acellular xenograft implant; first 100 sq cm or less, or 1% of body area of infants and children) and 15431 (Acellular xenograft implant; each additional 100 sq cm, or each additional 1% of body area of infants and children, or part thereof) to APC 0135 (Level III Skin Repair), with a proposed payment rate of approximately $297.
At the August 2009 meeting of the APC Panel, one public presenter requested that the APC Panel recommend that CMS reassign CPT codes 15340 and 15341 from APC 0134 to APC 0135. The presenter stated that the CY 2010 proposal to continue to assign both codes to APC 0134 would create a financial incentive favoring Dermagraft application. Specifically, the presenter explained that CPT instructions allow the separate reporting of the CPT codes for site preparation when Dermagraft is applied, while the CPT instructions for Apligraf application codes specify that site preparation cannot be separately reported. The presenter believed that this reporting difference and the resulting expected differences in the associated application procedure costs could be addressed by assigning the Apligraf application CPT codes to a higher paying APC than the Dermagraft application codes, instead of the same APC as CMS proposed for CY 2010. After discussion, the APC Panel requested that CMS provide data at the next APC Panel meeting on the frequency of primary and add-on CPT codes billed for Apligraf, Oasis, and Dermagraft application in order to assess the apparent variability in billing for the application of these products. In addition, the APC Panel requested median cost data for site preparation and debridement that may be separately reported in preparation for application of Dermagraft.
Comment: Several commenters supported the CY 2010 proposal to continue the CY 2009 APC assignments for the Apligraf, Dermagraft, and Oasis application CPT codes. One commenter argued that reassignment of the Apligraf application codes from APC 0134 to APC 0135 would create a financial incentive for hospitals to choose Apligraf instead of other products. Another commenter stated that the current APC assignments for all three sets of skin repair codes are appropriate based on an assessment of clinical homogeneity and resource costs.
Another commenter requested that CMS reassign the Apligraf application CPT codes 15340 and 15341 from APC Start Printed Page 60460 0134 to APC 0135 because of their similarity, from clinical and resource perspectives, to the Oasis application CPT codes 15430 and 15431 that are currently assigned to APC 0135. The commenter noted that none of these procedures allow separate reporting and payment of site preparation when performed. The commenter expressed concern that the variable APC assignments for similar procedures would create an unlevel playing field that would lead to financial incentives for hospitals to use one product rather than the other, as opposed to the most clinically appropriate product. Further, the commenter indicated that site preparation and debridement procedures are not paid separately when associated with Apligraf application, yet these site preparation services are paid separately when reported with Dermagraft application procedures that are assigned to the same APC as Apligraf application procedures. The commenter also requested that CMS not reassign the Oasis application CPT codes 15430 and 15431 from APC 0135 to APC 0134 because such a reassignment would inappropriately group skin repair procedures that incorporate site preparation with those that allow separate reporting and payment of that preparation.
Response: The current Apligraf, Oasis, and Dermagraft application CPT codes were made effective January 1, 2006. In the CY 2006 OPPS final rule with comment period (70 FR 68762), we assigned the Apligraf application CPT codes 15340 and 15341 and the Dermagraft application CPT codes 15365 and 15366 to the Level I Skin Repair APC (then designated as APC 0024 with a payment rate of approximately $92). We assigned the Oasis application CPT codes 15430 and 15431 to the Level II Skin Repair APC (then designated as APC 0025 with a payment rate of approximately $315) based on consideration of clinical and resource homogeneity.
For CY 2007 (71 FR 68054 through 68056), we assigned the three sets of skin repair CPT codes to the Level II Skin Repair APC (then designated as APC 0025) in response to comments received from the public regarding their clinical and expected resource similarity. However, for CY 2008, because of a 2 times violation in two of the four skin repair APCs that resulted from hospital claims data that were first available for these codes, we reconfigured the APC assignments for the Apligraf, Dermagraft, and Oasis application procedures. This reconfiguration resulted in our again differentiating the APC assignments for the Oasis application CPT codes from the APC assignments for the Apligraf and Dermagraft application procedures, similar to the initial CY 2006 APC configuration. We also renumbered the Skin Repair APCs. We note that, for CY 2008, we made no change to the APC assignments for the Apligraf and Dermagraft application CPT codes, maintaining them in APC 0134, but we reassigned the Oasis application codes to APC 0135.
We retained these configurations for CY 2009 and, for CY 2010, we proposed to continue to assign these procedures to their CY 2009 APCs. We also proposed to pay separately for the Apligraf, Dermagraft, and Oasis products themselves in CY 2010. Analysis of our claims data for the application procedures revealed that the hospital resource costs associated with the Apligraf and Oasis application procedures are different. The median cost of the Apligraf application CPT code 15340 is approximately $234, based on 13,551 single claims (of 17,534 total claims), and approximately $186 for CPT code 15341, based on 1,789 single claims (of 4,424 total claims). For the Oasis application CPT code 15430, the median cost is approximately $276 based on 12,807 single claims (of 14,723 total claims), and approximately $261 for CPT code 15431 based on 150 single claims (of 293 total claims). These CPT code-specific median costs are consistent with the APC 0134 and APC 0135 median costs of approximately $210 and $296, respectively, where the different two sets of procedure codes are assigned.
The OPPS is a payment system that is based on the relativity of costs of procedures as reported to us by hospitals. Hospital costs, based on significant numbers of single claims, have been and continue to be consistently higher for the Oasis application procedures than for Apligraf or Dermagraft application procedures, despite the differences in CPT reporting instructions for Apligraf and Oasis application procedures in comparison with Dermagraft application procedures. We also note that the coverage areas for the Apligraf application codes are based on 25 square centimeter increments, whereas the Oasis and Dermagraft application codes are based on 100 square centimeter increments. While we are not sure of the contribution application of different products to different size wounds may have on hospital costs, we have no reason to believe that our high volume and consistent hospital claims data for these services do not accurately represent the costs of the procedures that have been reported in accordance with their specific code descriptors since CY 2006.
Further, we do not agree that different APC assignments for similar skin repair procedures would create an unlevel playing field that would lead to financial incentives for hospitals to use one product rather than the other, as opposed to the most clinically appropriate product. Payments under the OPPS are based on the relative costs of services as reported to us by hospitals in claims and cost report data. In part, we assign services to APCs based on considerations of resource homogeneity, and hospital resources are reflected in the costs reported to us by hospitals. The skin repair CPT codes differ significantly from one another in terms of the other services that are bundled into them (such as site preparation) and in the coverage areas they describe. The specific Apligraf, Dermagraft, and Oasis application procedures have different median costs based on CY 2008 hospital claims that have led us to continue to assign them to different APCs for CY 2010, and we do not believe that appropriate payment for hospitals' costs for procedures provides incentives for hospitals to use one product instead of another. Instead, accurate payment based on the relative costs of services is an important principle of the OPPS, specifically intended to minimize any financial incentives for use of one product rather than the other in the case of similar procedures. We agree with the commenter that the choice of a patient's treatment should be based on clinical considerations, not financial incentives due to OPPS payment rates. We believe our final CY 2010 APC assignments for the Apligraf, Dermagraft, and Oasis application CPT codes are fully consistent with our interest in hospitals providing the most clinically appropriate treatments in an efficient manner.
After consideration of the public comments we received, we are finalizing our CY 2010 proposals, without modification, to continue to assign the Apligraf and Dermagraft application CPT codes to APC 0134, which has a final CY 2010 APC median cost of approximately $210, and to continue to assign the Oasis application CPY codes to APC 0135, which has a final CY 2010 APC median cost of approximately $296. We note that when hospitals are performing these procedures, they also would report the Level II HCPCS codes that describe the biological products that are used with the Apligraf, Dermagraft, and Oasis application CPT codes, which are paid separately in CY 2010. Further, we are accepting the August 2009 Start Printed Page 60461 recommendation of the APC Panel and will provide information at the winter 2010 APC Panel meeting on the frequency of primary and add-on CPT codes billed for Apligraf, Oasis, and Dermagraft application procedures, in addition to providing median cost data for site preparation and debridement that may be separately reported in preparation for application of Dermagraft.
c. Group Psychotherapy (APC 0325)
For CY 2010, we proposed to continue to assign CPT codes 90849 (Multiple-family group psychotherapy), 90853 (Group psychotherapy (other than of a multiple-family group)), and 90857 (Interactive group psychotherapy) to APC 0325 (Group Psychotherapy), with a proposed payment rate of approximately $61, calculated according to the standard OPPS ratesetting methodology. In CY 2009, these three CPT codes also were the only codes assigned to APC 0325, with a payment rate of approximately $65.
Comment: Several commenters expressed concern that the CY 2010 proposed payment rate for APC 0325 of approximately $61 is 21 percent less than the CY 2006 payment rate for this APC, and 24 percent less than the CY 2004 payment rate for this APC. The commenters stated that the proposed payment rate would be insufficient to cover hospitals' costs for providing group mental health services and, as a result, would threaten beneficiary access to these services. Some commenters recommended that CMS increase the final CY 2010 payment rate for APC 0325 by approximately 17 percent, which the commenters calculated is the average increase from CY 2006 to CY 2010 for the other psychotherapy APCs, specifically APC 0322 (Brief Individual Psychotherapy), APC 0323 (Extended Individual Psychotherapy), and APC 0324 (Family Psychotherapy).
Response: As we have stated in the past regarding APC 0325 (72 FR 66739 and 73 FR 68627), we cannot speculate as to why the median cost of group psychotherapy services has decreased significantly since CY 2004. We again note that we have robust claims data for the CPT codes that map to APC 0325. Specifically, we were able to use more than 99 percent of the approximately 1.6 million claims submitted by hospitals to report group psychotherapy services. We set the payment rates for APC 0325 using our standard OPPS methodology based on relative costs from hospital outpatient claims. We have no reason to believe that our claims data, as reported by hospitals, do not accurately reflect the hospital costs of group psychotherapy services. It would appear that the relative cost of providing these mental health services, in comparison with other HOPD services has decreased in recent years.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to calculate the payment rate for APC 0325 by applying our standard OPPS ratesetting methodology that relies on all single claims for all procedures assigned to the APC. The final CY 2010 APC median cost of APC 0325 is approximately $59.
d. Portable X-Ray Services
Consistent with applicable requirements, hospitals may bill and be paid under the OPPS for diagnostic x-ray tests performed in locations other than HOPDs, such as a skilled nursing facility (SNF), if the patient is receiving the x-ray as a covered outpatient department service and not in the course of a Medicare-covered SNF stay. The charge for the x-ray (but not the transportation and set-up charges) is billed on a hospital outpatient claim. Medicare does not pay under the OPPS for transportation or set-up when the x-ray equipment is transported to another location where the x-ray is taken.
Comment: One commenter objected to the assignment of status indicator “B” (Codes that are not recognized by OPPS when submitted on an outpatient hospital Part B bill type (12X or 13X)) to HCPCS codes R0070 (Transportation of portable x-ray equipment and personnel to home or nursing home, per trip to facility or location, one patient seen); R0075 (Transportation of portable x-ray equipment and personnel to home or nursing home, per trip to facility or location, more than one patient seen); and Q0092 (Set up portable x-ray equipment) under the OPPS when a hospital transports and sets up a portable x-ray machine in a SNF or other nonhospital site of service to furnish an x-ray to a patient who is not in the course of a SNF stay that is covered by Medicare. The commenter indicated that to be paid for the transportation and set-up of the portable x-ray, the hospital must enroll as a supplier and bill the Medicare carrier or MAC on a HCFA 1500 claim for the transportation and set-up services, although the hospital may bill the fiscal intermediary or MAC on a UB–04 claim for the x-ray service itself. The commenter requested that CMS revise its billing instructions so that the transportation and set-up charges for portable x-ray services could be reported on the same claim as the hospital's charge for the x-ray.
Response: In the case in which a patient receiving the portable x-ray service is not in a Medicare-covered SNF stay but a hospital furnishes the portable x-ray service in the SNF as a covered outpatient department service consistent with all applicable requirements, the HCPCS code and charge for the x-ray service (but not transportation and set-up charges) are billed to the fiscal intermediary or MAC. Payment is made under the OPPS for the x-ray service under such circumstances. The transportation and set-up of the portable x-ray are also covered services which are currently reported on the HCFA 1500 claim and are carrier-priced. We assign status indicator “B” to HCPCS codes R0070, R0075, and Q0092 because these services (transportation and set-up of the portable x-ray) are not paid under the OPPS and are rejected by the I/OCE if they are billed in an outpatient hospital bill type. We will explore whether it is feasible to revise the billing instructions to enable hospitals to bill for these transportation and set-up services on the same claim on which they report the charge for the x-ray service to which the transportation and set-up charges are ancillary. If we determine that it would be feasible and desirable to propose this change, we would propose to change the status indicators of these codes accordingly.
After consideration of the public comment we received, we are finalizing our CY 2010 proposals, without modification, to continue to assign the status indicator of “B” to HCPCS codes R0070, R0075, and Q0092. We will explore the feasibility of alternatives for billing and payment of these services that could reduce the hospital administrative burden associated with billing for the services.
e. Home Sleep Study Tests (APC 0213)
For CY 2010, we proposed to continue to assign Level II HCPCS codes G0398 (Home sleep study test (HST) with type II portable monitor, unattended; minimum of 7 channels: EEG, EOG, EMG, ECG/heart rate, airflow, respiratory effort and oxygen saturation), G0399 (Home sleep test (HST) with type III portable monitor, unattended; minimum of 4 channels: 2 respiratory movement/airflow, 1 ECG/heart rate and 1 oxygen saturation), and G0400 (Home sleep test (HST) with type IV portable monitor, unattended; minimum of 3 channels) to APC 0213 (Level I Extended EEG, Sleep, and Cardiovascular Studies), with a Start Printed Page 60462 proposed payment rate of approximately $160.
Comment: One commenter urged CMS to pay appropriately for Level II HCPCS codes G0398, G0399, and G0400 to adequately cover the cost of devices used in performing these procedures. Specifically, the commenter stated that the acquisition costs for the devices used with these procedures are significant and vary between $4,400 and $16,500. The commenter argued that it was unreasonable for CMS to assign all three HCPCS G-codes to the same APC because the devices used for the procedures vary significantly in their costs and, therefore, payment at the same rate for all three services would violate the 2 times rule. The commenter urged CMS to review the proposed payment rates for HCPCS G-codes G0398, G0399, and G0400.
Response: As we explained in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68602), we created these three HCPCS G-codes to describe the various types of home sleep tests that Medicare determined could be used to allow for coverage of continuous positive airway pressure (CPAP) therapy based upon a diagnosis of obstructive sleep apnea (OSA) according to a home sleep study. We further explained that we decided to assign these HCPCS G-codes to an APC under the OPPS because we believe these diagnostic services may be provided by HOPDs to Medicare beneficiaries.
HCPCS codes G0398, G0399, and G0400 were made effective in March 2008. Analysis of our claims data from CY 2008 reveals that these services are not commonly performed in the hospital outpatient setting for Medicare beneficiaries. Our claims data show no single claims and only three total claims for HCPCS code G0398. The median cost of HCPCS code G0399 is approximately $236 based on 12 single claims (of 13 total claims), and the median cost of HCPCS code G0400 is approximately $80 based on 11 single claims (of 12 total claims). We believe it would be difficult to draw any conclusions about the resource differences among these three services based upon such limited claims data from a single year.
With regard to the commenter's concern about a violation of the 2 times rule, there is no 2 times violation in APC 0213 because none of the sleep study HCPCS G-codes are significant procedures in the APC. Generally, we review, on an annual basis, the items and services within an APC group to determine, with respect to comparability of the use of resources, if the median cost of the highest cost item or service within an APC group is more than 2 times greater than the median cost of the lowest cost item or service within that same group, thereby assessing for 2 times rule violations. We make exceptions to the 2 times rule in unusual cases, such as low-volume items and services, and we only consider significant procedures for purposes of the 2 times assessment. We define significant procedures as those with a single claim frequency of greater than 1,000 or those with a frequency of greater than 99 and that constitute at least 2 percent of single claims in the APC. For APC 0213, our CY 2008 hospital outpatient claims used for CY 2010 ratesetting show that the median cost of the lowest cost significant service is approximately $150 compared to approximately $241 for the highest cost service. Based on our claims data, there is no 2 times violation in APC 0213.
After consideration of the public comment we received, we are finalizing our CY 2010 proposal, without modification, to continue to assign HCPCS codes G0398, G0399, and G0400 to APC 0213, which has a final CY 2010 APC median cost of approximately $161.
IV. OPPS Payment for Devices
A. Pass-Through Payments for Devices
1. Expiration of Transitional Pass-Through Payments for Certain Devices
Section 1833(t)(6)(B)(iii) of the Act requires that, under the OPPS, a category of devices be eligible for transitional pass-through payments for at least 2, but not more than 3, years. This pass-through payment eligibility period begins with the first date on which transitional pass-through payments may be made for any medical device that is described by the category. We may establish a new device category for pass-through payment in any quarter. Under our established policy, we base the pass-through status expiration dates for the category codes on the date on which a category is in effect. The date on which a category is in effect is the first date on which pass-through payment may be made for any medical device that is described by such category. We propose and finalize the dates for expiration of pass-through status for device categories as part of the OPPS annual update.
We also have an established policy to package the costs of the devices no longer eligible for pass-through payments into the costs of the procedures with which the devices are reported in the claims data used to set the payment rates (67 FR 66763). Brachytherapy sources, which are now separately paid in accordance with section 1833(t)(2)(H) of the Act, are an exception to this established policy.
There currently are no device categories eligible for pass-through payment, and there are no categories for which we proposed expiration of pass-through status. If we create new device categories for pass-through payment status during the remainder of CY 2009 or during CY 2010, we will propose future expiration dates in accordance with the statutory requirement that they be eligible for pass-through payments for at least 2, but not more than 3, years from the date on which pass-through payment for any medical device described by the category may first be made.
2. Provisions for Reducing Transitional Pass-Through Payments To Offset Costs Packaged Into APC Groups
a. Background
We have an established policy to estimate the portion of each APC payment rate that could reasonably be attributed to the cost of the associated devices that are eligible for pass-through payments (66 FR 59904). We deduct from the pass-through payments for identified device categories eligible for pass-through payments an amount that reflects the portion of the APC payment amount that we determine is associated with the cost of the device, defined as the device APC offset amount, as required by section 1833(t)(6)(D)(ii) of the Act. We have consistently employed an established methodology to estimate the portion of each APC payment rate that could reasonably be attributed to the cost of an associated device eligible for pass-through payment, using claims data from the period used for the most recent recalibration of the APC rates (72 FR 66751 through 66752). We establish and update the applicable device APC offset amounts for eligible pass-through device categories through the transmittals that implement the quarterly OPPS updates.
We currently have published a list of all procedural APCs with the CY 2009 portions (both percentages and dollar amounts) of the APC payment amounts that we determine are associated with the cost of devices, on the CMS Web site at: http://www.cms.hhs.gov/HospitalOutpatientPPS/01_overview.asp. The dollar amounts are used as the device APC offset amounts. In addition, in accordance with our established practice, the device APC offset amounts in a related APC are used in order to evaluate whether the cost of a device in an application for a new Start Printed Page 60463 device category for pass-through payment is not insignificant in relation to the APC payment amount for the service related to the category of devices, as specified in our regulations at § 419.66(d).
b. Final Policy
In the CY 2010 OPPS/ASC proposed rule (74 FR 35306), for CY 2010, we proposed to continue our established policies for calculating and setting the device APC offset amounts for each device category eligible for pass-through payment. We also proposed to continue to review each new device category on a case-by-case basis to determine whether device costs associated with the new category are already packaged into the existing APC structure. If device costs packaged into the existing APC structure are associated with the new category, we proposed to deduct the device APC offset amount from the pass-through payment for the device category. As stated earlier, these device APC offset amounts also would be used in order to evaluate whether the cost of a device in an application for a new device category for pass-through payment is not insignificant in relation to the APC payment amount for the service related to the category of devices (§ 419.66(d)).
In section V.A.4. of the CY 2010 OPPS/ASC proposed rule (74 FR 35311 through 35314), we proposed to specify that, beginning in CY 2010, the pass-through evaluation process and pass-through payment methodology for implantable biologicals, that are surgically inserted or implanted (through a surgical incision or a natural orifice) and that are newly approved for pass-through status beginning on or after January 1, 2010, would be the device pass-through process and payment methodology only. As a result of that proposal, we then proposed that, beginning in CY 2010, we would include implantable biologicals in our calculation of the device APC offset amounts. As of CY 2009, the costs of implantable biologicals not eligible for pass-through payment are packaged into the costs of the procedures in which they are implanted because nonpass-through implantable biologicals are not separately paid. We proposed to calculate and set any device APC offset amount for a new device pass-through category that includes a newly eligible implantable biological beginning in CY 2010 using the same methodology we have historically used to calculate and set device APC offset amounts for device categories eligible for pass-through payment (72 FR 66751 through 66752), with one modification. Because implantable biologicals would be considered devices rather than drugs for purposes of pass-through evaluation and payment under this proposal for CY 2010, the device APC offset amounts would include the costs of implantable biologicals for the first time. We also proposed to utilize these revised device APC offset amounts to evaluate whether the cost of an implantable biological in an application for a new device category for pass-through payment is not insignificant in relation to the APC payment amount for the service related to the category of devices. Further, we proposed to no longer use the “policy-packaged” drug APC offset amounts for evaluating the cost significance of implantable biological pass-through applications under review and for setting the APC offset amounts that would apply to pass-through payment for those implantable biologicals, effective for new pass-through status determinations beginning in CY 2010. In addition, we proposed to update, on the CMS Web site at http://www.cms.hhs.gov/HospitalOutpatientPPS, the list of all procedural APCs with the final CY 2010 portions of the APC payment amounts that we determine are associated with the cost of devices so that this information is available for use by the public in developing potential CY 2010 device pass-through payment applications and by CMS in reviewing those applications.
Comment: One commenter noted that a significant consequence of paying for new implantable biologicals under the device pass-through payment methodology would be that the payment for the implantable biological would be reduced by the estimated cost of any predecessor devices included in the APC payment rate. The commenter believed that it is reasonable for CMS to reduce the payment for the pass-through implantable biological when the biological is used in lieu of a predecessor device whose cost is already incorporated into payment for the associated procedure. However, the commenter also stated that if the hospital implanted the predecessor device during the procedure in addition to the pass-through implantable biological, a reduction in the pass-through payment for the implantable biological by the predecessor device cost should not be taken.
Response: Concerning the commenter's request that we not take a reduction (that is, device APC offset) when both a predecessor device and an implantable biological that is on pass-through status are used in a procedure in the case of medical necessity, we note that our standard policy when establishing a new device category for pass-through payment is to determine whether device costs associated with the new category are already packaged into the relevant existing clinical APC. If device costs packaged into the existing clinical APC are associated with the new pass-through device category and these predecessor devices would generally not be used when a device described by the new device category was implanted, we identify the device APC offset that would be deducted from the pass-through payment amount each time the new category is reported with the related clinical APC. We make determinations about the applicability of a device APC offset based on our overall clinical understanding of the device category and its associated procedures, rather than on a claim-by-claim basis for each different scenario. In the rare case where an implantable biological that is described by a device category with pass-through status was used in addition to a predecessor device in the performance of a procedure for which we had determined that a device APC offset was applicable, we would still apply the device APC offset to the pass-through payment for the implantable biological. With respect to a prospective payment system such as the OPPS, in some individual cases, payment exceeds the average cost; in other cases, payment is less than the average cost of an individual case. On balance, however, payment should approximate the relative cost of the average case, recognizing that, as a prospective payment system, the OPPS is a system of averages. We would not expect the scenario of implanting both a new implantable biological and the predecessor device described by the commenter to be common. If such a clinical scenario were common, we would determine that no device APC offset would apply to the new device category because the implantable biological was typically used in addition to the predecessor device in performing the associated procedure.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to reduce device pass-through payments based on device costs already included in the associated procedural APCs, when we determine that device costs associated with the new category are already packaged into the existing APC structure. Start Printed Page 60464
B. Adjustment to OPPS Payment for No Cost/Full Credit and Partial Credit Devices
1. Background
In recent years, there have been several field actions on and recalls of medical devices as a result of implantable device failures. In many of these cases, the manufacturers have offered devices without cost to the hospital or with credit for the device being replaced if the patient required a more expensive device. In order to ensure that payment rates for procedures involving devices reflect only the full costs of those devices, our standard ratesetting methodology for device-dependent APCs uses only claims that contain the correct device code for the procedure, do not contain token charges, and do not contain the “FB” modifier signifying that the device was furnished without cost or with a full credit. As discussed in section II.A.2.d.(1) of the CY 2010 OPPS/ASC proposed rule (74 FR 35267) and this final rule with comment period, we are further refining our standard ratesetting methodology for device-dependent APCs for CY 2010 by also excluding claims with the “FC” modifier signifying that the device was furnished with partial credit.
To ensure equitable payment when the hospital receives a device without cost or with full credit, in CY 2007 we implemented a policy to reduce the payment for specified device-dependent APCs by the estimated portion of the APC payment attributable to device costs (that is, the device offset) when the hospital receives a specified device at no cost or with full credit (71 FR 68071 through 68077). Hospitals are instructed to report no cost/full credit cases using the “FB” modifier on the line with the procedure code in which the no cost/full credit device is used. In cases in which the device is furnished without cost or with full credit, the hospital is instructed to report a token device charge of less than $1.01. In cases in which the device being inserted is an upgrade (either of the same type of device or to a different type of device) with a full credit for the device being replaced, the hospital is instructed to report as the device charge the difference between its usual charge for the device being implanted and its usual charge for the device for which it received full credit. In CY 2008, we expanded this payment adjustment policy to include cases in which hospitals receive partial credit of 50 percent or more of the cost of a specified device. Hospitals are instructed to append the “FC” modifier to the procedure code that reports the service provided to furnish the device when they receive a partial credit of 50 percent or more of the cost of the new device. We reduce the OPPS payment for the implantation procedure by 100 percent of the device offset for no cost/full credit cases when both a specified device code is present on the claim and the procedure code maps to a specified APC. Payment for the implantation procedure is reduced by 50 percent of the device offset for partial credit cases when both a specified device code is present on the claim and the procedure code maps to a specified APC. Beneficiary copayment is based on the reduced payment amount when either the “FB” or the “FC” modifier is billed and the procedure and device codes appear on the lists of procedures and devices to which this policy applies. We refer readers to the CY 2008 OPPS/ASC final rule with comment period for more background information on the “FB” and “FC” payment adjustment policies (72 FR 66743 through 66749).
2. APCs and Devices Subject to the Adjustment Policy
In the CY 2010 OPPS/ASC proposed rule (74 FR 35307), we proposed for CY 2010 to continue the policy of reducing OPPS payment for specified APCs by 100 percent of the device offset amount when a hospital furnishes a specified device without cost or with a full credit and by 50 percent of the device offset amount when the hospital receives partial credit in the amount of 50 percent or more of the cost for the specified device. Because the APC payments for the related services are specifically constructed to ensure that the full cost of the device is included in the payment, we stated in the CY 2010 OPPS/ASC proposed rule (74 FR 35307) that we continue to believe it is appropriate to reduce the APC payment in cases in which the hospital receives a device without cost, with full credit, or with partial credit, in order to provide equitable payment in these cases. (We refer readers to section II.A.2.d.(1) of this final rule with comment period for a description of our standard ratesetting methodology for device-dependent APCs.) Moreover, the payment for these devices comprises a large part of the APC payment on which the beneficiary copayment is based, and we continue to believe it is equitable that the beneficiary cost sharing reflects the reduced costs in these cases.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35307), we also proposed to continue using the three criteria established in the CY 2007 OPPS/ASC final rule with comment period for determining the APCs to which this policy applies (71 FR 68072 through 68077). Specifically, (1) all procedures assigned to the selected APCs must involve implantable devices that would be reported if device insertion procedures were performed; (2) the required devices must be surgically inserted or implanted devices that remain in the patient's body after the conclusion of the procedure (at least temporarily); and (3) the device offset amount must be significant, which, for purposes of this policy, is defined as exceeding 40 percent of the APC cost. We proposed to continue to restrict the devices to which the APC payment adjustment would apply to a specific set of costly devices to ensure that the adjustment would not be triggered by the implantation of an inexpensive device whose cost would not constitute a significant proportion of the total payment rate for an APC. We stated in the CY 2010 OPPS/ASC proposed rule (74 FR 35307) that we continue to believe these criteria are appropriate because free devices and device credits are likely to be associated with particular cases only when the device must be reported on the claim and is of a type that is implanted and remains in the body when the beneficiary leaves the hospital. We believe that the reduction in payment is appropriate only when the cost of the device is a significant part of the total cost of the APC into which the device cost is packaged, and that the 40-percent threshold is a reasonable definition of a significant cost.
As indicated in the CY 2010 OPPS/ASC proposed rule (74 FR 35307), we examined the offset amounts calculated from the CY 2010 proposed rule data and the clinical characteristics of APCs to determine whether the APCs to which the no cost/full credit and partial credit device adjustment policy applies in CY 2009 continue to meet the criteria for CY 2010, and to determine whether other APCs to which the policy does not apply in CY 2009 would meet the criteria for CY 2010. Based on the CY 2008 claims data available for the CY 2010 proposed rule, we did not propose any changes to the APCs and devices to which this policy applies. Table 19 of the CY 2010 OPPS/ASC proposed rule (74 FR 35307 through 35308) listed the proposed APCs to which the payment adjustment policy for no cost/full credit and partial credit devices would apply in CY 2010 and displayed the proposed payment adjustment percentages for both no cost/full credit and partial credit circumstances. Table 20 of the CY 2010 OPPS/ASC proposed rule (74 FR Start Printed Page 60465 35308) listed the proposed devices to which this policy would apply in CY 2010. We stated in the CY 2010 OPPS/ASC proposed rule (74 FR 35307) that we would update the lists of APCs and devices to which the no cost/full credit and partial credit device adjustment policy would apply in CY 2010, consistent with the three selection criteria discussed earlier in this section and based on the final CY 2008 claims data available for this CY 2010 OPPS/ASC final rule with comment period.
We did not receive any public comments on our CY 2010 proposal to continue the policy of reducing OPPS payment for specified APCs by 100 percent of the device offset amount when a hospital furnishes a specified device without cost or with a full credit and by 50 percent of the device offset amount when the hospital receives partial credit in the amount of 50 percent or more of the cost for the specified device. We also did not receive any public comments on our CY 2010 proposal to continue using the three criteria established in the CY 2007 OPPS/ASC final rule with comment period for determining the APCs to which this policy applies (71 FR 68072 through 68077). Therefore, we are finalizing our CY 2010 proposals, without modification, to continue the established no cost/full credit and partial credit device adjustment policy. For CY 2010, OPPS payments for implantation procedures to which the “FB” modifier is appended are reduced by 100 percent of the device offset for no cost/full credit cases when both a device code listed in Table 29, below, is present on the claim and the procedure code maps to an APC listed in Table 28 below. OPPS payments for implantation procedures to which the “FC” modifier is appended are reduced by 50 percent of the device offset when both a device code listed in Table 29 is present on the claim and the procedure code maps to an APC listed in Table 28. Beneficiary copayment is based on the reduced amount when either the “FB” or “FC” modifier is billed and the procedure and device codes appear on the lists of procedures and devices to which this policy applies.
We are adding device HCPCS code L8680 (Implantable neurostimulator electrode, each) to the list of devices in Table 29 because we are changing the status indicator for this code from “B” (Codes that are not recognized by OPPS when submitted on an outpatient hospital Part B bill type (12x and13x)) to “N” (Items and Services Packaged into APC Rates) for CY 2010, as reflected in Addendum B to this final rule with comment period. We are recognizing HCPCS code L8680 for payment purposes under the OPPS because it appropriately describes neurostimulator electrodes, and we typically try to recognize all valid HCPCS codes that hospitals may use to report items and services provided to hospital outpatients that are packaged or otherwise payable under the OPPS. This change in status indicator for HCPCS code L8680 for CY 2010 does not require hospitals to change their current billing practices in any way, but it does provide them with the flexibility to use this code if they choose to do so.
| Final CY 2010 APC | CY 2010 APC title | Final CY 2010 device offset percentage for no cost/ full credit case | Final CY 2010 device offset percentage for partial credit case |
|---|---|---|---|
| 0039 | Level I Implantation of Neurostimulator Generator | 85 | 43 |
| 0040 | Percutaneous Implantation of Neurostimulator Electrodes | 58 | 29 |
| 0061 | Laminectomy, Laparoscopy, or Incision for Implantation of Neurostimulator Electrodes | 64 | 32 |
| 0089 | Insertion/Replacement of Permanent Pacemaker and Electrodes | 72 | 36 |
| 0090 | Insertion/Replacement of Pacemaker Pulse Generator | 74 | 37 |
| 0106 | Insertion/Replacement of Pacemaker Leads and/or Electrodes | 44 | 22 |
| 0107 | Insertion of Cardioverter-Defibrillator | 89 | 44 |
| 0108 | Insertion/Replacement/Repair of Cardioverter-Defibrillator Leads | 88 | 44 |
| 0225 | Implantation of Neurostimulator Electrodes, Cranial Nerve | 73 | 37 |
| 0227 | Implantation of Drug Infusion Device | 83 | 41 |
| 0259 | Level VII ENT Procedures | 85 | 42 |
| 0315 | Level II Implantation of Neurostimulator Generator | 88 | 44 |
| 0385 | Level I Prosthetic Urological Procedures | 59 | 30 |
| 0386 | Level II Prosthetic Urological Procedures | 71 | 35 |
| 0418 | Insertion of Left Ventricular Pacing Elect. | 81 | 41 |
| 0425 | Level II Arthroplasty or Implantation with Prosthesis | 58 | 29 |
| 0648 | Level IV Breast Surgery | 48 | 24 |
| 0654 | Insertion/Replacement of a permanent dual chamber pacemaker | 75 | 37 |
| 0655 | Insertion/Replacement/Conversion of a permanent dual chamber pacemaker | 75 | 37 |
| 0680 | Insertion of Patient Activated Event Recorders | 73 | 36 |
| CY 2010 device HCPCS code | CY 2010 short descriptor |
|---|---|
| C1721 | AICD, dual chamber. |
| C1722 | AICD, single chamber. |
| C1728 | Cath, brachytx seed adm. |
| C1764 | Event recorder, cardiac. |
| C1767 | Generator, neurostim, imp. |
| C1771 | Rep dev, urinary, w/sling. |
| C1772 | Infusion pump, programmable. |
| C1776 | Joint device (implantable). |
| C1777 | Lead, AICD, endo single coil. |
| C1778 | Lead, neurostimulator. |
| C1779 | Lead, pmkr, transvenous VDD. |
| C1785 | Pmkr, dual, rate-resp. |
| C1786 | Pmkr, single, rate-resp. |
| C1789 | Prosthesis, breast, imp. |
| C1813 | Prosthesis, penile, inflatab. |
| C1815 | Pros, urinary sph, imp. |
| C1820 | Generator, neuro rechg bat sys. |
| C1881 | Dialysis access system. |
| C1882 | AICD, other than sing/dual. |
| C1891 | Infusion pump, non-prog, perm. |
| C1895 | Lead, AICD, endo dual coil. |
| C1896 | Lead, AICD, non sing/dual. |
| C1897 | Lead, neurostim, test kit. |
| C1898 | Lead, pmkr, other than trans. |
| C1899 | Lead, pmkr/AICD combination. |
| C1900 | Lead coronary venous. |
| C2619 | Pmkr, dual, non rate-resp. |
| C2620 | Pmkr, single, non rate-resp. |
| C2621 | Pmkr, other than sing/dual. |
| C2622 | Prosthesis, penile, non-inf. |
| C2626 | Infusion pump, non-prog, temp. |
| C2631 | Rep dev, urinary, w/o sling. |
| L8600 | Implant breast silicone/eq. |
| L8614 | Cochlear device/system. |
| L8680 | Implt neurostim elctr each. |
| L8685 | Implt nrostm pls gen sng rec. |
| L8686 | Implt nrostm pls gen sng non. |
| L8687 | Implt nrostm pls gen dua rec. |
| L8688 | Implt nrostm pls gen dua non. |
| L8690 | Aud osseo dev, int/ext comp. |
V. OPPS Payment Changes for Drugs, Biologicals, and Radiopharmaceuticals
A. OPPS Transitional Pass-Through Payment for Additional Costs of Drugs, Biologicals, and Radiopharmaceuticals
1. Background
Section 1833(t)(6) of the Act provides for temporary additional payments or “transitional pass-through payments” for certain drugs and biological agents. As enacted by the Medicare, Medicaid, and SCHIP Balanced Budget Refinement Act (BBRA) of 1999 (Pub. L. 106–113), this provision requires the Secretary to make additional payments to hospitals for current orphan drugs, as designated under section 526 of the Federal Food, Drug, and Cosmetic Act (Pub. L. 107–186); current drugs and biological agents and brachytherapy sources used for the treatment of cancer; and current radiopharmaceutical drugs and biological products. For those drugs and biological agents referred to as “current,” the transitional pass-through payment began on the first date the hospital OPPS was implemented.
Transitional pass-through payments also are provided for certain “new” drugs and biological agents that were not being paid for as an HOPD service as of December 31, 1996, and whose cost is “not insignificant” in relation to the OPPS payments for the procedures or services associated with the new drug or biological. For pass-through payment purposes, radiopharmaceuticals are included as “drugs.” Under the statute, transitional pass-through payments for a drug or biological described in section 1833(t)(6)(C)(i)(II) of the Act can be made for at least 2 years but not more than 3 years after the product's first payment as a hospital outpatient service under Part B. The pass-through payment eligibility period is discussed in detail in section V.A.5. of this final rule with comment period. CY 2010 pass-through drugs and biologicals and their designated APCs are assigned status indicator “G” in Addenda A and B to this final rule with comment period.
Section 1833(t)(6)(D)(i) of the Act specifies that the pass-through payment amount, in the case of a drug or biological, is the amount by which the amount determined under section 1842(o) of the Act (or, if the drug or biological is covered under a competitive acquisition contract under section 1847B of the Act, an amount determined by the Secretary to be equal to the average price for the drug or biological for all competitive acquisition areas and the year established under such section as calculated and adjusted by the Secretary) for the drug or biological exceeds the portion of the otherwise applicable Medicare OPD fee schedule that the Secretary determines is associated with the drug or biological. This methodology for determining the pass-through payment amount is set forth in § 419.64 of the regulations, which specifies that the pass-through payment equals the amount determined under section 1842(o) of the Act minus the portion of the APC payment that CMS determines is associated with the drug or biological. Section 1847A of the Act establishes the use of the average sales price (ASP) methodology as the basis for payment for drugs and biologicals described in section 1842(o)(1)(C) of the Act that are furnished on or after January 1, 2005. The ASP methodology, as applied under the OPPS, uses several sources of data as a basis for payment, including the ASP, wholesale acquisition cost (WAC), and average wholesale price (AWP). In this final rule with comment period, the term “ASP methodology” and “ASP-based” are inclusive of all data sources and methodologies described therein. Additional information on the ASP methodology can be found on the CMS Web site at: http://www.cms.hhs.gov/McrPartBDrugAvgSalesPrice.
As noted above, section 1833(t)(6)(D)(i) of the Act also states that if a drug or biological is covered under a competitive acquisition contract under section 1847B of the Act, the payment rate is equal to the average price for the drug or biological for all competitive acquisition areas and the year established as calculated and adjusted by the Secretary. Section 1847B of the Act establishes the payment methodology for Medicare Part B drugs and biologicals under the competitive acquisition program (CAP). The Part B drug CAP was implemented on July 1, 2006, and included approximately 190 of the most common Part B drugs provided in the physician's office setting. As we noted in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68633), the Part B drug CAP program was suspended beginning in CY 2009 (Medicare Learning Network (MLN) Matters Special Edition 0833, available via the Web site: http://www.medicare.gov). Therefore, there is no effective Part B drug CAP rate for pass-through drugs and biologicals as of January 1, 2009. As we indicated in the CY 2010 OPPS/ASC proposed rule (74 FR 35309), if the program is reinstituted during CY 2010 and Part B drug CAP rates become available, we would again use the Part B drug CAP rate for pass-through drugs and biologicals if they are a part of the Part B drug CAP program. Otherwise, we would continue to use the rate that would be paid in the physician's office setting for drugs and biologicals with pass-through status. We note that the CY 2010 MPFS proposed rule (74 FR 33623 through 33633) included proposed changes to the operation of the Part B drug CAP program, including a proposed change in the frequency of CAP drug pricing updates. A discussion of the final CAP policies is available in the CY 2010 MPFS final rule with comment period.
For CYs 2005, 2006, and 2007, we estimated the OPPS pass-through Start Printed Page 60467 payment amount for drugs and biologicals to be zero based on our interpretation that the “otherwise applicable Medicare OPD fee schedule” amount was equivalent to the amount to be paid for pass-through drugs and biologicals under section 1842(o) of the Act (or section 1847B of the Act, if the drug or biological is covered under a competitive acquisition contract). We concluded for those years that the resulting difference between these two rates would be zero. For CYs 2008 and 2009, we estimated the OPPS pass-through payment amount for drugs and biologicals to be $6.6 million and $23.3 million, respectively. Our final OPPS pass-through payment estimate for drugs and biologicals in CY 2010 is $35.5 million, which is discussed in section VI.B. of this final rule with comment period.
The pass-through application and review process for drugs and biologicals is explained on the CMS Web site at: http://www.cms.hhs.gov/HospitalOutpatientPPS/04_passthrough_payment.asp.
2. Drugs and Biologicals With Expiring Pass-Through Status in CY 2009
In the CY 2010 OPPS/ASC proposed rule (74 FR 35309), we proposed that the pass-through status of 6 drugs and biologicals would expire on December 31, 2009, as listed in Table 21 of the proposed rule (74 FR 35309 through 35310). These items were approved for pass-through status on or before January 1, 2008 and, therefore, all of these drugs and biologicals will have received OPPS pass-through payment for at least 2 years and no more than 3 years by December 31, 2009.
Two of the products with proposed expiring pass-through status for CY 2010 are biologicals that are solely surgically implanted according to their Food and Drug Administration approved indications. As discussed in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68634), we package payment for those implantable biologicals that have expiring pass-through status into payment for the associated surgical procedure. In the CY 2010 OPPS/ASC proposed rule, we proposed to package payment for two products described by HCPCS codes C9354 (Acellular pericardial tissue matrix of non-human origin (Veritas), per square centimeter) and C9355 (Collagen nerve cuff (NeuroMatrix), per 0.5 centimeter length).
To date, for other nonpass-through biologicals paid under the OPPS that may sometimes be used as implantable devices, we have instructed hospitals, via Transmittal 1336, Change Request 5718, dated September 14, 2007, to not separately bill for the HCPCS codes for the products when using these items as implantable devices (including as a scaffold or an alternative to human or nonhuman connective tissue or mesh used in a graft) during surgical procedures. In such cases, we consider payment for the biological used as an implantable device in a specific clinical case to be included in payment for the surgical procedure.
As we established in the CY 2003 OPPS final rule with comment period (67 FR 66763), when the pass-through payment period for an implantable device ends, it is standard OPPS policy to package payment for the implantable device into payment for its associated surgical procedure. We consider nonpass-through implantable devices to be integral and supportive items and services for which packaged payment is most appropriate. According to our regulations at § 419.2(b), as a prospective payment system, the OPPS establishes a national payment rate that includes operating and capital-related costs that are directly related and integral to performing a procedure or furnishing a service on an outpatient basis including, but not limited to, implantable prosthetics, implantable durable medical equipment, and medical and surgical supplies. Therefore, when the period of nonbiological device pass-through payment ends, we package the costs of the devices no longer eligible for pass-through payment into the costs of the procedures with which the devices were reported in the claims data used to set the payment rates for the upcoming calendar year. As described in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68634), we believed that this policy to package payment for implantable devices that are integral to the performance of separately paid procedures should also apply to payment for implantable biologicals without pass-through status, when those biologicals function as implantable devices. As stated above, implantable biologicals may be used in place of other implantable nonbiological devices whose costs are already accounted for in the associated procedural APC payments for surgical procedures. If we were to provide separate payment for these implantable biologicals without pass-through status, we would potentially be providing duplicate device payment, both through the packaged nonbiological device cost included in the surgical procedure's payment and separate biological payment. We indicated in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68634) that we saw no basis for treating implantable biological and nonbiological devices without pass-through status differently for OPPS payment purposes because both are integral to and supportive of the separately paid surgical procedures in which either may be used.
With the exception of those groups of drugs and biologicals that are always packaged when they do not have pass-through status, specifically diagnostic radiopharmaceuticals, contrast agents, and implantable biologicals, our standard methodology of providing payment for drugs and biologicals with expiring pass-through status in an upcoming calendar year is to determine the product's estimated per day cost and compare it with the OPPS drug packaging threshold for that calendar year (which is $65 for CY 2010), as discussed further in section V.B.2. of this final rule with comment period. If the drug's or biological's estimated per day cost is less than or equal to the applicable OPPS drug packaging threshold, we would package payment for the drug or biological into the payment for the associated procedure in the upcoming calendar year. If the estimated per day cost of the drug or biological is greater than the OPPS drug packaging threshold, we would provide separate payment at the applicable relative ASP-based payment amount (which is at ASP+4 percent for CY 2010, as discussed further in section V.B.3. of this final rule with comment period). Section V.B.2.d. of this final rule with comment period discusses the packaging of all nonpass-through contrast agents, diagnostic radiopharmaceuticals, and implantable biologicals.
We did not receive any public comments on our proposal to expire certain drugs and biologicals from pass-through status, effective December 31, 2009. Therefore, we are finalizing our proposal, without modification, to expire the pass-through status of the six drugs and biologicals listed in Table 30 below, effective December 31, 2009. Start Printed Page 60468
| CY 2009 HCPCS code | CY 2010 HCPCS code | CY 2010 long descriptor | Final CY 2010 SI | Final CY 2010 APC |
|---|---|---|---|---|
| C9354 | C9354 | Acellular pericardial tissue matrix of non-human origin (Veritas), per square centimeter | N | N/A |
| C9355 | C9355 | Collagen nerve cuff (NeuroMatrix), per 0.5 centimeter length | N | N/A |
| J1300 | J1300 | Injection, eculizumab, 10 mg | K | 9236 |
| J3488 | J3488 | Injection, zoledronic acid (Reclast), 1 mg | K | 0951 |
| J9261 | J9261 | Injection, nelarabine, 50 mg | K | 0825 |
| J9330 | J9330 | Injection, temsirolimus, 1 mg | K | 1168 |
3. Drugs, Biologicals, and Radiopharmaceuticals With New or Continuing Pass-Through Status in CY 2010
In the CY 2010 OPPS/ASC proposed rule (74 FR 35310), we proposed to continue pass-through status in CY 2010 for 31 drugs and biologicals. These items, which were approved for pass-through status between April 1, 2008 and July 1, 2009, were listed in Table 22 of the proposed rule (74 FR 35310 through 35311). None of these products will have received OPPS pass-through payment for at least 2 years and no more than 3 years by December 31, 2009. The APCs and HCPCS codes for these drugs and biologicals were assigned status indicator “G” in Addenda A and B to the proposed rule.
Section 1833(t)(6)(D)(i) of the Act sets the amount of pass-through payment for pass-through drugs and biologicals (the pass-through payment amount) as the difference between the amount authorized under section 1842(o) of the Act (or, if the drug or biological is covered under a CAP under section 1847B of the Act, an amount determined by the Secretary equal to the average price for the drug or biological for all competitive acquisition areas and the year established under such section as calculated and adjusted by the Secretary) and the portion of the otherwise applicable OPD fee schedule that the Secretary determines is associated with the drug or biological. Payment for drugs and biologicals with pass-through status under the OPPS is currently made at the physician's office payment rate of ASP+6 percent. We believe it is consistent with the statute to continue to provide payment for drugs and biologicals with pass-through status at a rate of ASP+6 percent in CY 2010, the amount that drugs and biologicals receive under section 1842(o) of the Act. Thus, for CY 2010, we proposed to pay for pass-through drugs and biologicals at ASP+6 percent, equivalent to the rate these drugs and biologicals would receive in the physician's office setting in CY 2010. The difference between ASP+4 percent that we proposed to pay for nonpass-through separately payable drugs under the CY 2010 OPPS and ASP+6 percent, therefore, would be the CY 2010 pass-through payment amount for these drugs and biologicals. In the case of pass-through contrast agents, diagnostic radiopharmaceuticals, and implantable biologicals, their pass-through payment amount would be equal to ASP+6 percent because, if not on pass-through status, payment for these products would be packaged into the associated procedures.
As discussed in more detail in section V.B.2.d. of this final rule with comment period, over the last 2 years, we implemented a policy whereby payment for all nonpass-through diagnostic radiopharmaceuticals, contrast agents, and implantable biologicals is packaged into payment for the associated procedure, and we proposed to continue the packaging of these items, regardless of their per day cost, in CY 2010. As stated earlier, pass-through payment is the difference between the amount authorized under section 1842(o) of the Act (or, if the drug or biological is covered under a CAP under section 1847B of the Act, an amount determined by the Secretary equal to the average price for the drug or biological for all competitive acquisition areas and the year established under such section as calculated and adjusted by the Secretary) and the portion of the otherwise applicable OPD fee schedule that the Secretary determines is associated with the drug or biological. Because payment for a drug that is either a diagnostic radiopharmaceutical or a contrast agent (identified as a “policy-packaged” drug, first described in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68639)) or for an implantable biological (which we proposed to consider to be a device for all payment purposes beginning in CY 2010 as discussed in sections V.A.4. and V.B.2.d. of the CY 2010 OPPS/ASC proposed rule (74 FR 35311 through 35314 and 74 FR 35323 through 35324) and this final rule with comment period) would otherwise be packaged if the product did not have pass-through status, we believe the otherwise applicable OPPS payment amount would be equal to the “policy-packaged” drug or device APC offset amount for the associated clinical APC in which the drug or biological is utilized. The calculation of the “policy-packaged” drug and device APC offset amounts are described in more detail in sections V.A.6.b. and IV.A.2. of this final rule with comment period, respectively. It follows that the copayment for the nonpass-through payment portion (the otherwise applicable fee schedule amount that we would also offset from payment for the drug or biological if a payment offset applies) of the total OPPS payment for those drugs and biologicals would, therefore, be accounted for in the copayment for the associated clinical APC in which the drug or biological is used. According to section 1833(t)(8)(E) of the Act, the amount of copayment associated with pass-through items is equal to the amount of copayment that would be applicable if the pass-through adjustment was not applied. Therefore, beginning in CY 2010, we proposed to set the associated copayment amount for pass-through diagnostic radiopharmaceuticals, contrast agents, and implantable biologicals that would otherwise be packaged if the item did not have pass-through status to zero. The separate OPPS payment to a hospital for the pass-through diagnostic radiopharmaceutical, contrast agent, or implantable biological, after taking into account any applicable payment offset for the item due to the device or “policy-packaged” APC offset policy, is the item's pass-through payment, which is not subject to a copayment according to the statute. Therefore, we did not publish a copayment amount for these items in Addendum A and B to the proposed rule. Start Printed Page 60469
We also proposed to update pass-through payment rates on a quarterly basis on the CMS Web site during CY 2010 if later quarter ASP submissions (or more recent WAC or AWP information, as applicable) indicate that adjustments to the payment rates for these pass-through drugs or biologicals are necessary. If the Part B drug CAP is reinstated during CY 2010, and a drug or biological that has been granted pass-through status for CY 2010 becomes covered under the Part B drug CAP, we proposed to provide pass-through payment at the Part B drug CAP rate and to make the appropriate adjustments to the payment rates for these drugs and biologicals on a quarterly basis as appropriate.
As is our standard methodology, we annually review new permanent HCPCS codes and delete temporary HCPCS C-codes if an alternate permanent HCPCS code is available for purposes of OPPS billing and payment. For our CY 2010 review, we have determined that HCPCS code J2796 (Injection, romiplostim, 10 micrograms) describes the product reported under HCPCS code C9245 (Injection, romiplostim, 10 mcg); HCPCS code A9581 (Injection, gadoxetate disodium, 1 ml) describes the product reported under HCPCS code C9246 (Injection, gadoxetate disodium, per ml); HCPCS code A9582 (Iodine I-123 iobenguane, diagnostic, per study dose, up to 15 millicuries) describes the product reported under HCPCS code C9247 (Iobenguane, I-123, diagnostic, per study dose, up to 10 millicuries); HCPCS code J0718 (Injection, certolizumab pegol, 1 mg) describes the product reported under HCPCS code C9249 (Injection, certolizumab pegol, 1 mg); HCPCS code J0598 (Injection, C1 esterase inhibitor (human), 10 units) describes the product reported under HCPCS code C9251 (Injection, C1 esterase inhibitor (human), 10 units); HCPCS code J2562 (Injection, plerixafor, 1 mg) describes the product reported under HCPCS code C9252 (Injection, plerixafor, 1 mg); and HCPCS code J9328 (Injection, temozolomide, 1 mg) describes the product reported under HCPCS code C9253 (Injection, temozolomide, 1 mg). These new CY 2010 HCPCS codes are included in Table 31 below.
Comment: Several commenters supported CMS' proposal to provide payment at ASP+6 percent for drugs, biologicals, contrast agents, and radiopharmaceuticals that are granted pass-through status. Further, the commenters approved of the proposal to use the ASP methodology that would provide payment based on WAC if ASP information is not available, and payment at 95 percent of AWP if WAC information is not available. Some commenters requested that CMS provide an additional payment for radiopharmaceuticals that are granted pass-through status because radiopharmaceuticals typically have higher overhead and pharmacy handling costs associated with their preparation than the overhead costs of other drugs and biologicals.
Response: As discussed above, the statutorily mandated pass-through payment equals the amount determined under section 1842(o) of the Act minus the portion of the APC payment that CMS determines is associated with the drug or biological. Therefore, the pass-through payment is determined by subtracting the otherwise applicable payment amount under the OPPS (determined to be ASP+4 percent for CY 2010) from the amount determined under section 1842(o) (ASP+6 percent).
For CY 2010, consistent with our CY 2009 policy for diagnostic radiopharmaceuticals, we proposed to provide payment for both diagnostic and therapeutic radiopharmaceuticals with pass-through status based on the ASP methodology. As stated above, the ASP methodology, as applied under the OPPS, uses several sources of data as a basis for payment, including the ASP, WAC if ASP is unavailable, and AWP if ASP and WAC are unavailable. For purposes of pass-through payment, we consider radiopharmaceuticals to be drugs under the OPPS and, therefore, if a diagnostic or therapeutic radiopharmaceutical receives pass-through status during CY 2010, we proposed to follow the standard ASP methodology to determine its pass-through payment rate under the OPPS. We have routinely provided a single payment for drugs, biologicals, and radiopharmaceuticals under the OPPS to account for the acquisition and pharmacy overhead costs, including compounding costs. We continue to believe that a single payment is appropriate for diagnostic radiopharmaceuticals with pass-through status in CY 2009, and that the payment rate of ASP+6 (or payment based on the ASP methodology) is appropriate to provide payment for both the radiopharmaceutical acquisition cost and any associated nuclear medicine handling and compounding costs. We refer readers to section V.B.5.b. of this final rule with comment period for further discussion of payment for radiopharmaceuticals based on ASP information submitted by manufacturers.
Comment: Several commenters expressed concern that a pass-through payment period of possibly only 2 years discourages new product development, especially for radiopharmaceutical products. One commenter recommended providing pass-through payment for approved radiopharmaceuticals for a full 3-year time period to allow hospitals time to incorporate new products into their chargemasters and billing practices.
Response: The pass-through statute specifically allows for pass-through payment of drugs and biologicals to be made for at least 2 years, but no more than 3 years. We believe this period of payment facilitates dissemination of these new products into clinical practice and collection of hospital claims data reflective of their costs for future OPPS ratesetting. Our longstanding practice has been to provide pass-through payment for a period of 2 to 3 years, with expiration of pass-through status proposed and finalized through the annual rulemaking process. Each year when proposing to expire the pass-through status of certain drugs and biologicals, we examine our claims data for these products and we have generally seen no evidence that hospitals have not fully incorporated these items into their chargemasters based on the utilization and costs observed in our claims data. As discussed further in section V.A.5. of this final rule with comment period, we are making no operational changes to the drug and biological pass-through program for CY 2010 and plan to continue to expire pass-through status on an annual basis through rulemaking. Under this existing operational policy, which was generally supported by the commenters, because we begin pass-through payment on a quarterly basis that depends on when applications are submitted to us for consideration and we expire pass-through status only on an annual basis, there is no way to ensure that all pass-through drugs and biologicals receive pass-through payment for a full 3 years, while also providing pass-through payment for no more than 3 years as the statute requires. Therefore, we will continue to provide drug and biological pass-through payment for at least 2 years, but no more than 3 years, as required by the statute. We continue to receive numerous pass-through applications for drugs and biologicals for consideration each quarter, and we have no evidence that our current pass-through payment policies discourage new product development.
There is currently one diagnostic radiopharmaceutical, HCPCS code C9247 (Iodine I-123 iobenguane, diagnostic, per study dose, up to 15 Start Printed Page 60470 millicuries), that has been granted pass-through status at the time of this final rule with comment period. We proposed to continue pass-through status for this diagnostic radiopharmaceutical as it would not have received at least 2 but not more than 3 years of pass-through payment by December 31, 2009. This is consistent with the OPPS provision that provides for at least 2 but not more than 3 years of pass-through payment for drugs and biologicals that are approved for pass-through payments.
We provide an opportunity through the annual OPPS/ASC rulemaking cycle for public comment on those drugs and biologicals that are proposed for expiration of pass-through payment at the end of the next calendar year. We have often received public comments related to our proposed expiration of pass-through status for particular drugs and biologicals, and we expect to continue to receive public comments regarding the proposed expiration of pass-through status for drugs and biologicals in the future. In this manner, we would address specific concerns about the pass-through payment period for individual drugs and biologicals in the future, including radiopharmaceuticals.
Comment: A few commenters supported the CY 2010 proposal to set the associated copayment amounts for pass-through diagnostic radiopharmaceuticals, contrast agents, and implantable biologicals that would otherwise be packaged if the product did not have pass-through status to zero. The commenters noted increased beneficiary savings by setting the copayment amount to zero.
Response: We appreciate the commenters' support. As discussed in the CY 2010 OPPS/ASC proposed rule (74 FR 35311), we believe that for drugs and biologicals that are “policy-packaged,” the copayment for the nonpass-through payment portion of the total OPPS payment for this subset of drugs and biologicals is accounted for in the copayment for the associated clinical APC in which the drug or biological is used. According to section 1833(t)(8)(E) of the Act, the amount of copayment associated with pass-through items is equal to the amount of copayment that would be applicable if the pass-through adjustment was not applied. Therefore, it is our belief that the amount should be zero for drugs and biologicals that are “policy-packaged,” including diagnostic radiopharmaceuticals.
We did not receive any public comments on our proposal to update pass-through payment rates on a quarterly basis during CY 2010 if later quarter ASP submissions (or more recent WAC or AWP information, as applicable) indicate that adjustments to the payment rates for these pass-through drugs and biologicals are necessary.
After consideration of the public comments we received, we are finalizing our CY 2010 pass-through payment proposals, without modification. Specifically, we will provide pass-through payment in CY 2010 for those drugs, biologicals and radiopharmaceuticals listed in Table 31 below. Pass-through payment for drugs, biologicals, and radiopharmaceuticals granted pass-through status will be made at the payment rate indicated in section 1842(o) of the Act, that is, ASP+6 percent. If ASP data are not available, pass-through payment will be based on the OPPS ASP methodology—that is, payment at WAC+6 percent if ASP data are not available and payment at 95 percent of the pass-through radiopharmaceutical's most recent AWP if WAC information is not available. We will update pass-through payment rates on a quarterly basis during CY 2010 if later ASP submissions (or more recent WAC or AWP information, as applicable) indicate that adjustments to the payment rates for pass-through drugs and biologicals are necessary. We will set the associated copayment amount for pass-through diagnostic radiopharmaceuticals, contrast agents, and implantable biologicals that would otherwise be packaged if the item did not have pass-through status to zero. Finally, if a drug or biological that has been granted pass-through status for CY 2010 becomes covered under the Part B drug CAP if the program is reinstituted, we will provide payment for Part B drugs that are granted pass-through status and are covered under the Part B drug CAP at the Part B drug CAP rate.
The drugs and biologicals that are continuing pass-through status for CY 2010 or that have been granted pass-through status as of January 2010 are displayed in Table 31 below.
| CY 2009 HCPCS code | CY 2010 HCPCS code | CY 2010 long descriptor | Final CY 2010 SI | Final CY 2010 APC |
|---|---|---|---|---|
| C9245 | J2796 | Injection, romiplostim, 10 micrograms | G | 9245 |
| C9246 | A9581 | Injection, gadoxetate disodium, 1 ml | G | 9246 |
| C9247 | A9582 | Iodine I–123 iobenguane, diagnostic, per study dose, up to 15 millicuries | G | 9247 |
| A9583 | Injection, gadofosveset trisodium, 1 ml | G | 1299 | |
| C9248 | C9248 | Injection, clevidipien butyrate, 1 mg | G | 9248 |
| C9249 | J0718 | Injection, certolizumab pegol, 1 mg | G | 9249 |
| C9250 | C9250 | Human plasma fibrin sealant, vapor-heated, solvent-detergent (Artiss), 2ml | G | 9250 |
| C9251 | J0598 | Injection, C1 esterase inhibitor (human), 10 units | G | 9251 |
| C9252 | J2562 | Injection, plerixafor, 1 mg | G | 9252 |
| C9253 | J9328 | Injection, temozolomide, 1 mg | G | 9253 |
| C9255 | Injection, paliperidone palmitate, 1 mg | G | 1300 | |
| C9256 | Injection, dexamethasone intravitreal implant, 0.1 mg | G | 9256 | |
| C9356 | C9356 | Tendon, porous matrix of cross-linked collagen and glycosaminoglycan matrix (TenoGlide Tendon Protector Sheet), per square centimeter | G | 9356 |
| C9358 | C9358 | Dermal substitute, native, non-denatured collagen, fetal bovine origin (SurgiMend Collagen Matrix), per 0.5 square centimeters | G | 9358 |
| C9359 | C9359 | Porous purified collagen matrix bone void filler (Integra Mozaik Osteoconductive Scaffold Putty, Integra OS Osteoconductive Scaffold Putty), per 0.5 cc | G | 9359 |
| Start Printed Page 60471 | ||||
| C9360 | C9360 | Dermal substitute, native, non-denatured collagen, neonatal bovine origin (SurgiMend Collagen Matrix), per 0.5 square centimeters | G | 9360 |
| C9361 | C9361 | Collagen matrix nerve wrap (NeuroMend Collagen Nerve Wrap), per 0.5 centimeter length | G | 9361 |
| C9362 | C9362 | Porous purified collagen matrix bone void filler (Integra Mozaik Osteoconductive Scaffold Strip), per 0.5 cc | G | 9362 |
| C9363 | C9363 | Skin substitute, Integra Meshed Bilayer Wound Matrix, per square centimeter | G | 9363 |
| C9364 | C9364 | Porcine implant, Permacol, per square centimeter | G | 9364 |
| J0641 | J0641 | Injection, levoleucovorin calcium, 0.5 mg | G | 1236 |
| J1267 | J1267 | Injection, doripenem, 10 mg | G | 9241 |
| J1453 | J1453 | Injection, fosaprepitant, 1 mg | G | 9242 |
| J1459 | J1459 | Injection, immune globulin (privigen), intravenous, non-lyophilized (e.g. liquid), 500 mg | G | 1214 |
| J1571 | J1571 | Injection, hepatitis b immune globulin (hepagam b), intramuscular, 0.5 ml | G | 0946 |
| J1573 | J1573 | Injection, hepatitis B immune globulin (Hepagam B), intravenous, 0.5ml | G | 1138 |
| J1680 | Injection, human fibrinogen concentrate, 100 mg | G | 1290 | |
| J1953 | J1953 | Injection, levetiracetam, 10 mg | G | 9238 |
| J2785 | J2785 | Injection, regadenoson, 0.1 mg | G | 9244 |
| J8705 | J8705 | Topotecan, oral, 0.25 mg | G | 1238 |
| J9033 | J9033 | Injection, bendamustine hcl, 1 mg | G | 9243 |
| J9155 | Injection, degarelix, 1 mg | G | 1296 | |
| J9207 | J9207 | Injection, ixabepilone, 1 mg | G | 9240 |
| J9225 | J9225 | Histrelin implant (vantas), 50 mg | G | 1711 |
| J9226 | J9226 | Histrelin implant (supprelin la), 50 mg | G | 1142 |
| Q0138 | Injection, ferumoxytol, for treatment of iron deficiency anemia, 1 mg (non-esrd use) | G | 1297 | |
| Q4114 | Q4114 | Dermal substitute, granulated cross-linked collagen and glycosaminoglycan matrix (Flowable Wound Matrix), 1 cc | G | 1251 |
4. Pass-Through Payment for Implantable Biologicals
a. Background
Section 1833(t)(6)(A)(iv) of the Act authorizes transitional pass-through payments for new medical devices, drugs, and biologicals, for those items where payment was not being made as a hospital outpatient service under Part B as of December 31, 1996, and whose cost is not insignificant in relation to the OPD fee schedule amount payable for the service (or group of services) involved. These pass-through payments are in addition to the usual APC payments for services in which the product is used. Coding and payment for drugs and biologicals with pass-through status are generally provided on a product-specific basis for a period of no less than 2 and no more than 3 years from the date pass-through payment is first made as discussed in section V.A.5. of this final rule with comment period, while coding and payment for devices with pass-through status are provided for categories of devices that may describe numerous products. The Act specifies that the duration of transitional pass-through payments for devices must be no less than 2 and no more than 3 years from the first date on which payment is made for any medical device that is described by the category. Therefore, we utilize separate pass-through application and evaluation processes and criteria for drugs and biologicals and device categories because the statutory provisions are not the same for all items that may receive pass-through payment. These processes and the applicable evaluation criteria are available on the CMS Web site at: http://www.cms.hhs.gov/HospitalOutpatientPPS/04_passthrough_payment.asp#TopOfPage. The regulations that govern pass-through payment for drugs and biologicals are found in § 419.64 and those applicable to pass-through device categories are found in § 419.66.
Section 1833(t)(6)(D)(i) of the Act specifies that the pass-through payment amount, in the case of a drug or biological, is the amount by which the amount determined under section 1842(o) of the Act (or, if the drug or biological is covered under a competitive acquisition contract under section 1847B of the Act, an amount determined by the Secretary equal to the average price for the drug or biological for all competitive acquisition areas and the year established under such section as calculated and adjusted by the Secretary) for the drug or biological exceeds the portion of the otherwise applicable Medicare OPD fee schedule that the Secretary determines is associated with the drug or biological. For the drugs and biologicals that would have otherwise been paid under the Part B drug CAP, because the Part B drug CAP has been suspended beginning January 1, 2009, pass-through payment for these drugs and biologicals is currently made at the physician's office payment rate of ASP+6 percent. In the case of diagnostic radiopharmaceuticals, where all products without pass-through status are packaged into payment for nuclear medicine procedures, the pass-through payment is reduced by an amount that reflects the diagnostic radiopharmaceutical portion of the APC payment amount for the associated nuclear medicine procedure (the “policy-packaged” drug APC offset) that we determine is associated with the cost of predecessor diagnostic radiopharmaceuticals. In the CY 2010 OPPS/ASC proposed rule (74 FR 35318), Start Printed Page 60472 we proposed a similar payment offset policy for contrast agents beginning in CY 2010, as discussed in section V.A.6.c. of the proposed rule, and we are finalizing this policy for CY 2010, as discussed in section V.A.6.c. of this final rule with comment period. Pass-through payment for a category of devices is made at the hospital's charge for the device, adjusted to cost by application of the hospital's CCR. If applicable, the device payment is reduced by an amount that reflects the portion of the APC payment amount for the associated surgical procedure that we determine is associated with the cost of the device, called the device APC offset and discussed further in section IV.A.2. of the proposed rule (74 FR 35306) and this final rule with comment period.
In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68633 through 68636), we finalized a policy to package payment for implantable biologicals without pass-through status that are surgically inserted or implanted (through a surgical incision or a natural orifice) into payment for the associated surgical procedure. Prior to our implementation of this policy for nonpass-through implantable biologicals, we adopted in the CY 2003 OPPS final rule with comment period (67 FR 66763) the current OPPS policy that packages payment for an implantable device into the associated surgical procedures when its pass-through payment period ends because payment for all implantable devices without pass-through status under the OPPS is packaged. We consider nonpass-through implantable devices to be integral and supportive items for which packaged payment is most appropriate. As we stated in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68634), we believe this policy to package payment for implantable devices that are integral to the performance of procedures paid separately through an APC payment should also apply to payment for implantable biologicals without pass-through status, when those biologicals function as implantable devices. Implantable biologicals may be used in place of other implantable nonbiological devices whose costs are already accounted for in the associated procedural APC payments for surgical procedures. We reasoned that if we were to provide separate payment for nonpass-through implantable biologicals, we would potentially be providing duplicate device payment, both through the packaged nonbiological device cost included in the surgical procedure's payment and the separate biological payment.
In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68634), we stated our belief that the three implantable biologicals with expiring pass-through status for CY 2009 differ from other biologicals paid under the OPPS in that they specifically always function as surgically implanted devices. We noted that both implantable nonbiological devices under the OPPS and the three biologicals with expiring pass-through status in CY 2009 are surgically inserted or implanted (including through a surgical incision or a natural orifice). These three biologicals are approved by the FDA as devices, and they are solely surgically implanted according to their FDA-approved indications. Furthermore, in some cases, these implantable biologicals can substitute for implantable nonbiological devices (such as for synthetic nerve conduits or synthetic mesh used in tendon repair).
For other nonpass-through biologicals paid under the OPPS that may sometimes be used as implantable devices, we have instructed hospitals, beginning via Transmittal 1336, Change Request 5718, dated September 14, 2007, to not separately bill the HCPCS codes for the products when using these items as implantable devices (including as a scaffold or an alternative to human or nonhuman connective tissue or mesh used in a graft) during surgical procedures. In such cases, we consider payment for the biological used as an implantable device in a specific clinical case to be included in payment for the surgical procedure. We stated that hospitals may include the charge for the biological in their charge for the procedure, report the charge on an uncoded revenue center line, or report the charge under a device HCPCS code, if one exists, so that the biological costs may be considered in future ratesetting for the associated surgical procedures.
Several commenters who responded to the CY 2009 OPPS/ASC proposed rule supported CMS' proposal to package payment for implantable biologicals without pass-through status into payment for the associated surgical procedure (73 FR 68635). One commenter also recommended that CMS treat biologicals that are always surgically implanted or inserted and have FDA device approval as devices for purposes of pass-through payment, rather than as drugs. The commenter observed that this would allow all implantable devices, biological and otherwise, to be subject to a single pass-through payment policy. The commenter concluded that this policy change would provide consistency in billing and payment for these products functioning as implantable devices during their pass-through payment period, as well as after the expiration of pass-through status.
We finalized in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68635) our proposal to package payment for any nonpass-through biological that is surgically inserted or implanted (through a surgical incision or a natural orifice) into the payment for the associated surgical procedure, just as we package payment for all nonpass-through, implantable, nonbiological devices. As a result of this final policy, the three implantable biologicals with expiring pass-through status in CY 2009 were packaged and assigned status indicator “N” as of January 1, 2009. In addition, any new biologicals without pass-through status that are surgically inserted or implanted (through a surgical incision or a natural orifice) are also packaged beginning in CY 2009. Hospitals continue to report the HCPCS codes that describe biologicals that are always used as implantable devices on their claims, and we package the costs of those biologicals into the associated procedures, according to the standard OPPS ratesetting methodology that is described in section II.A.2. of the CY 2010 OPPS/ASC proposed rule (74 FR 35254 through 35267) and this final rule with comment period. Moreover, for nonpass-through biologicals that may sometimes be used as implantable devices, we continue to instruct hospitals to not bill separately for the HCPCS codes for the products when used as implantable devices. This reporting ensures that the costs of these products that may be, but are not always, used as implanted biologicals are appropriately packaged into payment for the associated implantation procedures when the products are used as implantable devices.
b. Policy for CY 2010
Some implantable biologicals are described by device category codes for expired pass-through categories, including HCPCS code C1781 (Mesh (implantable)), HCPCS code C1762 (Connective tissue, human), and HCPCS code C1763 (Connective tissue, non-human). All implantable devices described by the latter two categories are biologicals, while HCPCS code C1781 describes both implantable biological and nonbiological devices. Historically, these category codes included biological products that we approved for pass-through payment under the device pass-through process, initially when we paid for pass-through Start Printed Page 60473 devices on a brand-specific basis from CY 2000 through March 31, 2001, and later through the device categories described by HCPCS codes C1781, C1762, and C1763, which were developed effective April 1, 2001.
We believe that it is most appropriate for a product to be eligible for a single period of OPPS pass-through payment, rather than a period of device pass-through payment and a period of drug or biological pass-through payment. The limited timeframe for transitional pass-through payment ensures that new devices, drugs, and biologicals may receive special payment consideration under the OPPS for the first few years after their initial use, in order to allow sufficient time for their cost information to be reflected in hospital claims data and, therefore, to be available for OPPS ratesetting. After the pass-through payment period ends, like other existing services, we have cost information regarding these new products provided to us by hospitals from claims and cost report data. We then utilize that information when packaging the costs of the items (all devices, diagnostic radiopharmaceuticals, contrast agents, and implantable biologicals, and other drugs with an estimated per day cost equal to or less than the annual drug packaging threshold) or paying separately for the products (drugs except contrast agents and diagnostic radiopharmaceuticals and also nonimplantable biologicals with estimated per day costs above the annual drug packaging threshold). Further, although implantable biologicals with pass-through status may substitute for nonpass-through implantable devices whose costs are packaged into procedural APC payments, our existing APC offset policies for the costs of predecessor items packaged into APC payment for the associated services do not apply to pass-through payment for biologicals. We note that the APC offset amount that would be most applicable to implantable biologicals, if we determine that an offset applies for a given APC, would be the device APC offset amount, based on their similarity of function to the implantable devices whose costs have been included in establishing the procedural APC payment, not the “policy-packaged” or “threshold-packaged” drug APC offset amounts that one would expect to apply to pass-through drugs and biologicals. Similarly, when we currently evaluate a pass-through implantable biological application for the cost significance of the product, our methodology utilizes the “policy-packaged” APC offset amount to assess the candidate implantable biological, not the device APC offset amount that would be more reflective of the costs of predecessor devices related to the candidate implantable biological, such as those of device category HCPCS codes C1781, C1762, and C1763.
Many implantable biologicals, such as the three biologicals that expired from pass-through status after CY 2008, have FDA approval as devices. A number of other implantable biologicals with FDA approval as devices also have been approved for OPPS pass-through payment over the past several years, based on their product-specific pass-through applications as biologicals, not devices. Moreover, outside of the period of pass-through payment, the costs of implantable biologicals, like the costs of implantable devices, are now packaged into the cost of the procedure in which they are used. Implantable biologicals may be used in place of other implantable nonbiological devices whose costs are already accounted for in the associated procedural APC payments. Payment is made for nonpass-through implantable biologicals, like for devices, through the APC payment for the associated surgical procedure.
In view of these considerations, in the CY 2010 OPPS/ASC proposed rule (74 FR 35313), we proposed that the pass-through evaluation process and pass-through payment methodology for implantable biologicals that are surgically inserted or implanted (through a surgical incision or a natural orifice) and that are newly approved for pass-through status beginning on or after January 1, 2010, be the device pass-through process and payment methodology only. Given the shared payment methodologies for implantable biological and nonbiological devices during their nonpass-through payment periods, as well as their overlapping and sometimes identical clinical uses and their similar regulation by the FDA as devices, we believe that the most consistent pass-through payment policy for these different types of items that are surgically inserted or implanted and that may sometimes substitute for one another is to evaluate all such devices, both biological and nonbiological, only under the device pass-through process. As a result, implantable biologicals would no longer be eligible to submit biological pass-through applications and to receive biological pass-through payment at ASP+6 percent. While we understand that implantable biologicals have characteristics that result in their meeting the definitions of both devices and biologicals, we believe that implantable biologicals are most similar to devices because of their required surgical insertion or implantation and that it would be appropriate to only evaluate them as devices because they share significant clinical similarity with implantable nonbiological devices. We refer readers to the CMS Web site specified previously in this section to view the device pass-through application requirements and review criteria that would apply to the evaluation of all implantable biologicals for pass-through status when their pass-through payment would begin on or after January 1, 2010.
However, those implantable biologicals that are surgically inserted or implanted (through a surgical incision or natural orifice) and that are receiving pass-through payment as biologicals prior to January 1, 2010, would continue to be considered pass-through biologicals for the duration of their period of pass-through payment. These products have already been evaluated for pass-through status based on their applications as biologicals and have been approved for pass-through status based on the established criteria for biological pass-through payment. We believe it would be most appropriate for them to complete their 2- to 3-year period of pass-through payment as biologicals in accordance with the pass-through payment policies that were applicable at the time their pass-through status was initially approved.
We note that, in conducting our pass-through review of implantable biologicals as devices beginning with CY 2010 pass-through payment, we would apply the portions of APC payment amounts associated with devices (that is, the device APC offset amounts) to assess the cost significance of the candidate implantable biologicals, as we do for other devices. The CY 2009 device APC offset amounts are posted on the CMS Web site at: http://www.cms.hhs.gov/HospitalOutpatientPPS/04_passthrough_payment.asp. The result of evaluating all implantable biological items only for device pass-through payment is that payment for implantable biologicals eligible for pass-through payment beginning on or after January 1, 2010, would be based on hospital charges adjusted to cost, rather than the ASP methodology that is applicable to pass-through drugs and biologicals. Treating implantable biologicals as devices for evaluation of pass-through payment eligibility and payment would result in their consistent treatment with respect to coding and payment during their pass-through and nonpass-through periods of Start Printed Page 60474 payment. This proposed policy would allow us to appropriately offset the pass-through payment for an implantable biological using the device APC offset amounts, which would incorporate the costs of predecessor devices (both biological and nonbiological) that are similar to the implantable biological item with pass-through status. Finally, this proposed policy would ensure that each implantable biological is eligible for OPPS pass-through payment for only one 2- to 3-year time period (as a device only, not as a biological), so that once OPPS claims data incorporate cost information for the implantable biological, the product would not be again eligible for OPPS pass-through payment in the future.
Further, because we proposed that the pass-through evaluation process for CY 2010 pass-through status approvals and pass-through payment methodology for implantable biologicals that are surgically inserted or implanted (through a surgical incision or a natural orifice) beginning in CY 2010 be the device pass-through process and payment methodology only, we also proposed to revise our regulations at §§ 419.64 and 419.66 to conform to this new policy. Specifically, we proposed to amend § 419.64 by adding a new paragraph (a)(4)(iii) and language under a new paragraph (c)(3) to exclude implantable biologicals from consideration for drug and biological pass-through payment. Furthermore, under proposed new paragraph (a)(4)(iv) of § 419.64, we proposed to specify the continued inclusion of implantable biologicals for which pass-through payment as a biological is made on or before December 31, 2009, as eligible for biological pass-through payment, consistent with our proposal to allow these products to complete their period of pass-through payment as biologicals.
Moreover, in light of our CY 2010 proposal that implantable biological applications for pass-through status beginning on or after January 1, 2010, would be considered only for device pass-through evaluation and payment, we stated in the proposed rule (74 FR 35314) that we believe it would also be appropriate to clarify the current example in § 419.66(b)(4)(iii) of the regulations regarding the exclusion of materials, for example, biological or synthetic materials, that may be used to replace human skin from device pass-through payment eligibility. While, by definition, implantable biologicals that are surgically implanted or inserted would not be biological materials that replace human skin, we proposed to more precisely state this in the regulations. Therefore, we proposed to revise § 419.66(b)(4)(iii), which currently states that a device is not a material that may be used to replace human skin and provides an example of such a material as “a biological or synthetic material.” We proposed to revise § 419.66(b)(4)(iii) to specify that the biological materials be a “biological skin replacement material” rather than a “biological” and the synthetic materials be a “synthetic skin replacement material” rather than a “synthetic material” because we do not believe this example should refer to biologicals or synthetic materials that are used for purposes other than as a skin replacement material, given that the regulatory provision in § 419.66(b)(4)(iii) applies only to a material that may be used to replace human skin.
Comment: A few commenters requested that CMS continue to pay for all pass-through biologicals under the ASP methodology for drugs and nonimplantable biologicals, and not pay for new implantable biologicals eligible for pass-through payment based on charges adjusted to cost. One commenter believed that the ASP methodology is well understood by hospitals and Medicare contractors and asserted that some new implantable biologicals under development will cost several thousand dollars per procedure. Therefore, the commenter stated, many hospitals will be reluctant to mark up charges for these new implantable biologicals, thereby resulting in charge compression and an underestimate of the costs of biologicals. Furthermore, the commenter claimed that continued payment for pass-through implantable biologicals based on the ASP methodology would ensure consistent payment for new biologicals rather than variable payment based on hospitals' charging practices.
Response: Under our CY 2010 proposal to evaluate and pay for implantable biologicals under the device pass-through methodology, we would use the charges adjusted to cost payment methodology and apply a reduction to payment (that is, the device APC offset) for implantable biologicals eligible for pass-through payment beginning on or after January 1, 2010. Regarding the commenters' request that we continue the ASP payment methodology for pass-through implantable biologicals, we do not agree that payment under this methodology would be appropriate. Payment based on ASP for pass-through implantable biologicals would not provide the similar OPPS payment treatment of biological and nonbiological implantable devices that is our goal for new devices. Given the shared payment methodologies for implantable biological and nonbiological devices during their nonpass-through payment periods, as well as their overlapping and sometimes identical clinical uses and their generally similar regulation by the FDA as devices, we believe that the most consistent pass-through payment policy for these different types of items that are surgically inserted or implanted and that may sometimes substitute for one another is to evaluate and pay for all such devices, both biological and nonbiological, only under the device pass-through process and payment methodology. As we stated in the CY 2010 OPPS/ASC proposed rule (74 FR 35313), we believe that implantable biologicals are most similar to devices because of their required surgical insertion or implantation and that it would be appropriate to only evaluate them as devices because they share significant clinical similarity with implantable nonbiological devices. We note that we will continue pass-through payment under the ASP methodology for any implantable biological for which pass-through payment as a biological begins on or before December 31, 2009.
Comment: A few commenters supported the proposal to treat implantable biologicals and implanted devices the same regarding the pass-through eligibility criteria and payment methodology. Some commenters stated that payment for both implantable biological and nonbiological devices should be made on the same basis for items with both pass-through and nonpass-through status. One commenter asserted that the proposed treatment of implantable biologicals is consistent with CMS' policy to package the costs of implantable devices and would reinforce previous CMS instructions regarding the billing of biologicals when used as implanted devices. Furthermore, another commenter also agreed with CMS' policy that separately payable HCPCS codes not be reported when biologicals that are sometimes implanted are surgically inserted during a procedure. The commenter urged CMS to continue educating providers about when HCPCS codes that describe biologicals that are sometimes implanted should be reported, including publishing a list of procedures with which the HCPCS codes for implantable biologicals would not typically be reported. The commenter encouraged CMS to publish “reverse” device-to-procedure edits for such procedures.
Response: We appreciate the commenters' support for our proposal. We agree that payment for both implantable biological and Start Printed Page 60475 nonbiological devices that may be substitutes for one another should be made on the same basis for items with both pass-through and nonpass-through status, that is, based on charges adjusted to cost while on pass-through status and packaged when not on pass-through status. Concerning the suggestion to publish a list of procedure codes with which the HCPCS codes for biologicals that are implanted would not typically be reported, we believe that creating and maintaining such a list would not be feasible because implantable biologicals may be used in a wide variety of surgical procedures. Moreover, creating and maintaining device-to-procedure edits for implantable biologicals also would not be feasible, given the broad array of surgical procedures in which such biologicals may be implanted.
Comment: One commenter requested that CMS delay the CY 2010 proposal to include implantable biologicals in the calculation of the device APC offset amounts. The commenter also recommended that CMS grandfather all implantable biological applications submitted under the drug and biological pass-through application process prior to the September 1, 2009 application filing deadline. The commenter noted that implantable biological applications submitted prior to September 1, 2009, could have received biological pass-through status if CMS had not proposed and finalized the policy to treat them as devices for pass-through purposes, beginning in CY 2010.
The commenter explained that two implantable biological products that are competitors to the product manufactured by the commenter currently have pass-through status as biologicals, and their pass-through status is proposed to continue for CY 2010. The commenter believed that treating implantable biologicals differently based on the date of their pass-through application would result in a competitive disadvantage for the product manufactured by the commenter.
Response: The commenter recommended delaying the packaging of implantable biologicals in calculating the device offset. As a practical matter, the packaging of nonpass-through implantable biologicals was proposed and finalized for CY 2009 (73 FR 68635) and was implemented beginning in CY 2009. Given our proposal to treat implantable biologicals as devices for pass-through purposes beginning in CY 2010 and our longstanding device APC offset policy for pass-through devices, we believe it is appropriate to consider the costs of implantable biologicals that are packaged in establishing the device APC offset amounts under a policy that considers implantable biologicals to be devices for pass-through evaluation and payment purposes. We rely on the device APC offset amounts to account for the costs of all predecessor devices to a new device category when those predecessor devices are implanted in procedures assigned to an APC to which procedures associated with the new device category would be assigned, and the predecessor devices may now include implantable biologicals.
Concerning the commenter's request to grandfather all implantable biological applications submitted under the drug and biological pass-through application process prior to the September 1, 2009 application filing deadline, we believe it is important to adopt a consistent implantable biological pass-through policy for a full calendar year to provide appropriate payment under a single payment policy for that year and allow consistent use of our CY 2010 claims data for ratesetting in the future. The earliest an application filed for the September 1 deadline (applications are received and processed on a continual basis) could be considered for pass-through status is January 1 of the following year, in this case, CY 2010, as we have established and posted on the CMS Web site for pass-through applications at: http://www.cms.hhs.gov/HospitalOutpatientPPS/04_passthrough_payment.asp#TopOfPage. We do not believe it would be appropriate to implement pass-through evaluation and payment of implantable biologicals as devices later than the quarter beginning January 1, 2010. In order to meet the timeframes required by our claims processing systems, applications for drug and biological pass-through status received by the September 1, 2009 deadline for January 2010 payment have been evaluated based on the policy established in this final rule with comment period to evaluate implantable biologicals for device pass-through payment. We also note that when adopting any significant policy change under the OPPS with a specific effective date, we recognize that similar products or services may be treated differently because of the timing of their FDA approval, pass-through application submission, or other characteristics. Nevertheless, the rulemaking process provides significant opportunity for public notice and comment prior to such policy changes in order to ensure that we give full consideration to all issues and information related to proposals of new policy.
Comment: Several commenters recommended that both implantable and nonimplantable biologicals approved by the FDA under a biologics license application (BLA) be evaluated for pass-through payment status under the drug pass-through evaluation process, and indicated their belief that Congress intended biologicals approved under BLAs to be paid under the specific OPPS statutory provisions that apply to specified covered outpatient drugs (SCODs), including the pass-through provisions. One commenter agreed that CMS should have similar payment methodologies for biological, nonbiological, and composite devices for fairness and consistency and recommended that CMS implement the proposed policy based on FDA approval status, specifically treating as devices for pass-through purposes only those implantable biologicals approved by the FDA as devices. The commenter claimed that CMS determined that several implantable devices that are currently treated as drugs or biologicals must be paid based on their product-specific ASP submissions because the requirement for combining drugs for the purpose of ASP is that the reference materials report them as clinical equivalents. The commenter reasoned that devices do not have equivalents identified in reference materials; therefore, those devices paid as drugs must always receive separate payment. The commenter also requested that CMS clarify when it will treat an implantable device as a biological for ASP payment.
One commenter suggested that CMS not use the device pass-through process for evaluating drugs or biologicals that are implanted using a device as merely a delivery vehicle, simply because the drug is administered through a device. The commenter recommended that CMS base its pass-through payment decision on the identity of the component that exerts the therapeutic effect of the combined product, either the biological component or the delivery vehicle, and provided as an example the practice of FDA's Office of Combination Products to assess combination products in development and assign their FDA regulation based on which component exerts the therapeutic effect claimed by the manufacturer. The commenter believed that there are clinical problems with using implantation to define whether a biological should be treated as a device because, for some drugs, implantation may always be the clinically superior route of administration. Another commenter claimed that some implantable biologicals meet the Act's definition of a biological under section 1861(t)(1) of Start Printed Page 60476 the Act even though they are approved by the FDA as devices.
Response: We proposed to evaluate implantable biologicals that function as and are substitutes for implantable devices, regardless of their category of FDA approval, as devices for OPPS payment purposes. We do not believe it is necessary to make our OPPS payment policies regarding implantable biologicals dependent on categories of FDA approval, the intent of which is to ensure the safety and effectiveness of medical products.
We do not agree with the commenters who asserted that Congress intended biologicals approved under BLAs to be paid under the specific OPPS statutory provisions that apply to SCODs, including the pass-through provisions. Moreover, Congress did not specify that we must pay for implantable biologicals as biologicals rather than devices, if they also meet our criteria for payment as a device. We believe that implantable biologicals meet the definitions of a device and a biological and that, for payment purposes, it is appropriate for us to consider implantable biologicals as implantable devices in all cases, not as biologicals. For example, beginning in CY 2009, we package the costs of implantable biologicals into the costs of the procedures in which they are used, as we do for implantable devices. Therefore, we do not believe that we must pay for implantable biologicals under our OPPS biological payment methodologies, rather than our device payment methodologies. Furthermore, because we consider implantable biologicals to be devices for payment purposes, any interpretation that a biological is unique in the context of the ASP payment methodology for biologicals would not apply. Thus, we disagree with the commenter's conclusion that implantable biologicals treated as devices must receive separate payment because devices do not have equivalents in reference materials, a concept applicable only to the requirements for combining biologicals for payment under the ASP methodology, because we consider these implantable biologicals to be devices under the OPPS, to which packaged payment outside of the pass-through payment period applies.
It is not our intention to consider biologicals under the device pass-through evaluation process and payment methodology when these products are merely administered through the implantation of a delivery system for the biological. Each implantable biological pass-through application for a combination product would be initially evaluated in such a case to determine if the biological or device is the key therapeutic or diagnostic component, after which we would then determine whether to evaluate the item under the device or drug and biological pass-through process. If the key component of the candidate pass-through product is the biological and that biological is only implanted because it is administered through an implanted delivery system for the biological (that is, the biological itself is not functioning as an implantable device), we would evaluate the product under the drug and biological pass-through process. Conversely, if the key component of the candidate pass-through product is the biological and that biological is functioning as an implantable device or the key component of the product is the implantable delivery system for the biological, we would evaluate the product under the device pass-through process.
As we stated in the CY 2010 OPPS/ASC proposed rule (74 FR 35313) and this final rule with comment period, while we understand that implantable biologicals have characteristics that result in their meeting the definitions of both devices and biologicals, we believe that biologicals are most similar to devices because of their required surgical insertion or implantation and that it would be appropriate to only evaluate them as devices because they share significant clinical similarity with implantable nonbiological devices. We do not believe that those implantable biologicals that meet the Act's definition of biological under section 1861(t)(1) necessarily must be evaluated and paid for under the OPPS drug and biological pass-through payment methodology, when they also meet the definition of a device for purposes of pass-through evaluation and payment.
Comment: One commenter requested that CMS clarify certain points regarding the proposal to evaluate and pay for implantable biologicals with pass-through status similarly to pass-through devices. The commenter requested that CMS designate that the types of biologicals that would be affected by the proposal would be connective tissue replacements that function as devices. The commenter also requested that CMS clarify that the proposed changes would apply to pass-through implantable biologicals and not to implantable drugs, and that CMS recognize that it would be inappropriate to treat implantable drugs as devices for pass-through purposes in the future.
Response: Our CY 2010 proposal was not limited to implantable biological connective tissue replacements, but instead it applies to all implantable biologicals. For example, in the proposed rule (74 FR 35313), we cited expired device category HCPCS code C1781 (Mesh (implantable)) as describing implantable biologicals as well as implantable nonbiological devices, yet mesh need not necessarily function as a connective tissue replacement. We did not propose to treat implantable drugs as devices and, therefore, would not treat implantable drugs as devices for pass-through payment program purposes in CY 2010.
Comment: One commenter suggested that implantable biologicals should not be treated as devices, and observed that stakeholders have not had adequate time to consider the long-term implications of the CMS proposal. The commenter recommended that CMS not finalize the proposal at this time and hold a public meeting regarding the proposal.
Response: We believe that all stakeholders have had sufficient time to consider this proposal through the routine notice and comment rulemaking process. We received numerous public comments on our CY 2010 proposal and, while we are always open to meeting with stakeholders who would like to share their views with us, we do not believe a public meeting on this issue is needed.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, that the pass-through evaluation process and payment methodology for implantable biologicals that are surgically inserted or implanted (through a surgical incision or a natural orifice) and that are newly approved for pass-through status beginning on or after January 1, 2010, be the device pass-through process and payment methodology only. However, those implantable biologicals that are surgically inserted or implanted (through a surgical incision or natural orifice) and that are receiving pass-through payment as biologicals prior to January 1, 2010, would continue to be considered pass-through biologicals for the duration of their period of pass-through payment. As proposed, in conducting our pass-through review of implantable biologicals as devices beginning with CY 2010 pass-through payment, we will apply the portions of APC payment amounts associated with devices (that is, the device APC offset amounts) to assess the cost significance of the candidate implantable biologicals, as we do for other devices. Furthermore, we are finalizing our proposal to revise our regulations at §§ 419.64 and 419.66 to conform to this new policy. Start Printed Page 60477
Specifically, we are finalizing our proposal to amend § 419.64 by adding a new paragraph (a)(4)(iii) to exclude implantable biologicals from consideration for drug and biological pass-through payment. However, we note that, as discussed in section V.A.5. of this final rule with comment period, we are not finalizing our proposed addition of a new paragraph (c)(3) to § 419.64 and, therefore, we are not adopting our related proposed change to proposed paragraph (c)(3) that would have excluded implantable biologicals from consideration for drug and biological pass-through payment. Furthermore, we are adopting our proposed new paragraph (a)(4)(iv) of § 419.64, which specifies the continued inclusion of implantable biologicals for which pass-through payment as a biological is made on or before December 31, 2009, as eligible for biological pass-through payment. Finally, we are adopting our proposal stated above that clarifies the current example in § 419.66(b)(4)(iii) of the regulations regarding the exclusion of materials, for example, biological or synthetic materials, that may be used to replace human skin from device pass-through payment eligibility.
5. Definition of Pass-Through Payment Eligibility Period for New Drugs and Biologicals
Section 1833(t)(6) of the Act provides for transitional pass-through payments for medical devices, drugs, and biologicals. Section 1833(t)(6)(A) of the Act generally describes two groups of services—“current” and “new”—that are eligible for pass-through payments, depending, in part, on when they were first paid. One of the criteria for “new” drugs and biologicals to receive pass-through payments under section 1833(t)(6)(A)(iv)(I) of the Act is that payment for the item as an outpatient hospital service under Part B was not being made as of December 31, 1996. For those “new” drugs and biologicals, section 1833(t)(6)(C)(i)(II) of the Act specifies that there is a 2- to 3-year limitation on the pass-through period that begins on the first date on which payment is made under Part B for the drug or biological as an outpatient hospital service.
Section 419.64 of the regulations codifies the transitional pass-through payment provisions for drugs and biologicals. Section 419.64(a) describes the drugs and biologicals that are eligible for pass-through payments, essentially capturing the distinction between “new” and “current” services. Section 419.64(c)(2) provides that the pass-through payment eligibility period for drugs and biologicals that fall into the “new” category begins on the date that CMS makes its first pass-through payment for the drug or biological.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35314), we noted that it had come to our attention that our pass-through payment eligibility period for “new” drugs and biologicals in § 419.64(c)(2) of the regulations might not most accurately reflect the statutory requirements of section 1833(t)(6)(C)(i)(II) of the Act. While our regulations indicate that the pass-through payment eligibility period for “new” drugs and biologicals begins on the first date on which pass-through payment is made for the item, section 1833(t)(6)(C)(i)(II) of the Act specifies that the pass-through period of 2 to 3 years for “new” drugs and biologicals begins on the first date on which payment is made under Part B for the drug or biological as an outpatient hospital service. In order to better reflect the statutory requirement for the pass-through period for a “new” drug or biological, in the CY 2010 OPPS/ASC proposed rule (74 FR 35314), we proposed to revise paragraph (c)(2) of § 419.64 and add a new paragraph (c)(3) to § 419.64.
In order to conform the regulations to the statutory provisions, we proposed to change the start date of the pass-through payment eligibility period for a drug or biological from the first date on which pass-through payment is made to the date on which payment is first made for a drug or biological as an outpatient hospital service under Part B. Under this proposal, we needed to identify a first date of payment for a drug or biological as an outpatient hospital service under Part B. (Under our current policy, we had not established a start date for the eligibility period distinct from the beginning of pass-through payment because our current policy is to begin the pass-through payment eligibility period at the same time as we begin pass-through payment for the drug or biological.)
Due to the 2-year delay in the availability of claims data, under our CY 2010 proposal, we would not be able to identify an exact date of first payment for a drug or biological as an outpatient hospital service under Part B in order to determine the start date of the pass-through payment eligibility period until years after an application for pass-through payment for a “new” drug or biological has been submitted. At that later point in time, the pass-through payment eligibility period may be close to expiring, and the result of relying upon our claims data to evaluate an item for its eligibility for pass-through status could result in a very short period of pass-through payment for the new drug or biological. Consequently, in the proposed rule, we stated our belief that it would be desirable to identify an appropriate and timely proxy for the date of first payment for the drug or biological as an outpatient hospital service under Part B. We proposed the date of first sale for a drug or biological in the United States following FDA approval as an appropriate proxy, as explained below, for the date on which the pass-through payment eligibility period would begin. We also noted that, in light of our CY 2010 proposal to treat implantable biologicals as medical devices for purposes of pass-through eligibility and payment under section 1833(t)(6) of the Act, described in section V.A.4. of the proposed rule (74 FR 35311 through 35314), these proposed revisions to the pass-through payment eligibility period for a drug or biological approved for pass-through payment beginning on or after January 1, 2010, would not apply to implantable biologicals, but rather only to nonimplantable biologicals.
We explained that the date of first sale of the drug or nonimplantable biological in the United States following FDA approval was an appropriate proxy for the first date of payment for the drug or nonimplantable biological as an outpatient hospital service under Part B for several reasons, including our expectation that Medicare beneficiaries would be among the first to use these drugs and nonimplantable biologicals. In addition, we currently rely on the date of first sale of a drug or biological in the United States following FDA approval under the ASP methodology and in the existing OPPS pass-through payment eligibility determination. We stated that we did not believe that there is a more accurate and readily available proxy for the first date of payment for a drug or biological under Part B as an outpatient hospital service than the date of first sale of the drug or nonimplantable biological in the United States following FDA approval and that it was an accepted and available indicator of initial payment for the Medicare program.
For these reasons, we proposed that the date of first sale of a drug or nonimplantable biological in the United States following FDA approval would be the start date of the pass-through payment eligibility period for drugs or nonimplantable biologicals approved for pass-through payment beginning on or after January 1, 2010. We specified that our current policy—that the pass-through payment eligibility period of 2 to 3 years begins on the first date that Start Printed Page 60478 pass-through payment is made for the drug or biological—would apply only to drugs and biologicals approved for and receiving pass-through payment on or before December 31, 2009.
We currently implement new approvals of pass-through status for drugs and biologicals on a quarterly basis, and under our proposal for CY 2010, we stated that we would continue to implement these new approvals on a quarterly basis. We describe our quarterly process for reviewing and approving applications for drugs and biologicals to receive pass-through payment on the CMS Web site at: http://www.cms.hhs.gov/HospitalOutpatientPPS/04_passthrough_payment.asp. Interested parties may submit a complete application at any time. We typically review and make pass-through status approval decisions about complete applications for initiation of pass-through payment within 4 months of their submission and implement new pass-through status approvals on a quarterly basis through the next available OPPS quarterly update. The CMS Web site provides a timeline showing the relationship between the date of submission of a complete application and the earliest date of pass-through payment that would result from approval of pass-through status for the drug or biological.
Under our current policy, the pass-through payment eligibility period and period of pass-through payment are the same. However, the pass-through payment eligibility period and the period of pass-through payment would not have been identical under our proposed policy. For our proposed policy, we identified both the pass-through payment eligibility period, as well as the period during which pass-through payment would be made, including the respective start and expiration dates of the pass-through payment eligibility period and the period of pass-through payment. We stated that the period of pass-through payment would coincide with the time period during which the drug or biological is designated as having pass-through status. (We note that being within the pass-through payment eligibility period alone does not qualify a “new” drug or biological for pass-through payment; the drug or biological must also meet the other requirements for pass-through payment, including a CMS determination that the cost of a drug or biological is not insignificant.) Under our proposal, the pass-through payment eligibility period would run for at least 2 years but no more than 3 years. We proposed to modify § 419.64 accordingly by adding new paragraph (c)(3) to state: “For a drug or nonimplantable biological described in paragraph (a)(4) of this section and approved for pass-through payment beginning on or after January 1, 2010—[the pass-through payment eligibility period begins on] the date of the first sale of the drug or nonimplantable biological in the United States after FDA approval.” Next, we proposed that pass-through payment itself would start on the first day of the calendar quarter following the calendar quarter during which the completed application was approved. We proposed to reflect this in regulation text, in proposed new § 419.64(c)(3), as follows. “Pass-through payment for the drug or nonimplantable biological begins on the first day of the hospital outpatient prospective payment system update following the update period during which the drug or nonimplantable biological was approved for pass-through status.” We noted that this start date for the period of pass-through payment would be specified in a letter to the applicant conveying pass-through status approval for the new drug or biological and would be the first day of the calendar quarter following the calendar quarter during which a complete pass-through application is approved by CMS for pass-through status.
Because the proposed revised definition of the pass-through payment eligibility period could have resulted in the eligibility period beginning well before application is made for pass-through payment for the drug or nonimplantable biological and could have resulted in a shorter period of pass-through payment for some drugs and biologicals than would be the case under our current policy, we also proposed to expire pass-through status for “new” drugs and biologicals on a quarterly basis. This proposal to expire the pass-through status of drugs and nonimplantable biologicals on a quarterly basis was a departure from our current policy for expiring the pass-through status of drugs and biologicals. Presently, we expire the pass-through status of drugs and biologicals at the end of the calendar year preceding the year of the applicable annual OPPS update. Because our current pass-through payment eligibility period policy effectively aligns the start of pass-through payment with the beginning of the 2- to 3-year pass-through payment eligibility period, expiration of pass-through status on a calendar year basis affords those drugs and biologicals at least 2 but not more than 3 years of pass-through payment.
In addition to proposing to expire the pass-through status of “new” drugs and nonimplantable biologicals described by proposed new § 419.64(c)(3) on a quarterly basis, we also proposed to continue our established policy of determining whether a drug or biological would receive separate payment or packaged payment, after the expiration of the period of pass-through payment, on a calendar year basis through the annual OPPS rulemaking process as described in section V.B.2. of the proposed rule (74 FR 35319 through 35321) and this final rule with comment period. Therefore, after the expiration of pass-through status of a “new” drug or biological in a given year's calendar quarter, we proposed to continue to make separate payment through the end of that calendar year for those drugs and nonimplantable biologicals that would be subject to the drug packaging threshold when they did not have pass-through status at the applicable OPPS payment rate for separately payable drugs and biologicals without pass-through status for that year, proposed to be ASP + 4 percent for CY 2010. (This proposal would exclude contrast agents and diagnostic radiopharmaceuticals for CY 2010, which would always be packaged when not on pass-through status.)
Comment: Several commenters disagreed with CMS' proposal to change the pass-through payment eligibility period policy for new drugs and nonimplantable biologicals in CY 2010. Most of the commenters expressed concerns about separating the pass-through payment eligibility period from the period of pass-through payment, noting that delays that may occur between the date of the first sale of a drug in the United States and the date on which payment is first made under Part B would inevitably and inappropriately reduce the period of pass-through payment for new drugs. The commenters cited several examples, including a manufacturer's delay in submitting a pass-through application after receiving FDA approval, the length of CMS' pass-through review and approval process, delays in claim submissions and challenges associated with hospital billing for new services, and lags due to the resale process of a drug from a manufacturer to a wholesaler before the drug is available to the beneficiary. In addition, many commenters argued that non-Medicare beneficiaries, as opposed to Medicare beneficiaries, may be the first to receive a drug or biological, making the date of a drug's first sale in the United States Start Printed Page 60479 after FDA approval irrelevant to the Medicare population.
One commenter acknowledged CMS' need to align the pass-through payment eligibility period policy with the statutory provisions. However, the commenter disagreed with CMS' proposal to use the date of the first sale in the United States following FDA approval as a proxy for the date on which payment is made under Part B. The commenter suggested that, considering all of the potential delays between the date of the first sale in the United States after FDA approval and the first date of payment under Part B as an outpatient hospital service, the date of the first sale in the United States after FDA approval is not a sufficiently precise proxy. The commenter suggested that CMS continue to use the current pass-through payment policy as a proxy for the first date on which payment is made under Part B, specifically the date that CMS first makes pass-through payment for a drug or biological, because it is the most accurate proxy. The commenter reasoned that establishing the date that CMS first makes pass-through payment for a drug or biological as a proxy for the first date on which payment is made under Part B as an outpatient hospital service is appropriate because the date of first pass-through payment would never predate the first payment under Part B as an outpatient hospital service, nor would it likely be made later than the date of first OPPS payment by an appreciable period of time. The commenter noted that, in general, manufacturers have an incentive to submit pass-through applications as quickly as possible and will do whatever they can to minimize any lag time between the date of first outpatient hospital payment and the availability of pass-through payments because pass-through status facilitates the product's introduction into the hospital outpatient setting.
Response: The commenter who urges us to adopt a different proxy than the one we proposed for the date of first payment under part B as an outpatient hospital service makes some very persuasive and compelling points. We have considered the merits and advantages of adopting the commenter's suggested proxy rather than the one we proposed, and we find that we agree with the commenter that the most appropriate policy is one that establishes the date that CMS makes its first pass-through payment for a drug or biological as the proxy for the first date on which payment is made under Part B as an outpatient hospital service. We believe that the date on which pass-through payment is first made for a drug or nonimplantable biological is a more accurate proxy for the date on which payment is first made under Part B as an outpatient hospital service for several reasons. First, we agree with the commenter's points concerning the significant delays that may occur between the date of first sale of a drug or nonimplantable biological in the United States after FDA approval and the first date on which outpatient hospital payment is made under Part B. Such delays may result from numerous transactions in the drug distribution chain, initial use for non-Medicare patients with later diffusion to treatment of Medicare patients, delays in claims submission for new products without specific HCPCS codes, and established timeframes for Medicare processing payment of claims. All of these lags between the date of first sale and the date of first payment under Part B as an outpatient hospital service are cumulative and potentially significant. Therefore, adoption of the proposed proxy could, in some cases, lead to the start of the pass-through payment eligibility period substantially earlier than the start of the period of pass-through payment, thereby resulting in a reduction in the period of pass-through payment.
Second, we believe that utilizing the commenter's recommended proxy would eliminate the potential for delays between the proxy and the actual first date of payment under Part B as an outpatient hospital service, since the date of first pass-through payment would never predate the first payment under Part B as an outpatient hospital service. Although the first date of payment under Part B as an outpatient hospital service potentially could predate the date of first pass-through payment, it is also true that manufacturers have a significant incentive to submit pass-through applications as quickly as possible to minimize any lag between the date of first payment under the OPPS and the availability of pass-through payment. Pass-through payment can facilitate the availability of a product-specific HCPCS code for reporting its use and additional pass-through payment for the drug may allow beneficiaries access to the new drug in the HOPD. Therefore, in the rare circumstance that the date of first pass-through payment under the OPPS lags behind the first payment for the product under Part B as an outpatient hospital service, the delay is likely to be minimal. As a result, adopting this alternative date as a proxy would be unlikely to extend the pass-through payment eligibility period beyond 2 to 3 years from the date of first payment under Part B as an outpatient hospital service as specified in the statute.
In addition, utilizing the date of first pass-through payment under the OPPS as a proxy for the date payment is first made for a product under Part B as an outpatient hospital service would afford drugs and nonimplantable biologicals at least a full 2 years of pass-through payment, whereas the proposed proxy might not have allowed for a full 2 years of pass-through payment in every case. Finally, using the date of first pass-through payment under the OPPS as the proxy for the date of first payment under Part B as an outpatient hospital service would not present an administrative burden to CMS or the public nor would it disrupt or change CMS' current operational practices. This administratively simple proxy would result in a continuation of the same smoothly functioning operational practices that CMS currently utilizes in determining pass-through payment for drugs and biologicals. Therefore, we are finalizing the date on which CMS makes its first pass-through payment as the proxy for the first date on which payment is made under Part B as an outpatient hospital service.
We note that, in the CY 2010 OPPS/ASC proposed rule (74 FR 35315 through 35317), we outlined CMS' pass-through payment policies for approving and expiring pass-through payment status for drugs and nonimplantable biologicals under the OPPS. In adopting the date on which CMS makes its first pass-through payment as a proxy for the first date on which payment is made under Part B as an outpatient hospital service and, therefore, as the start date for pass-through payment eligibility, we are not changing our current practices concerning application, approval, payment, and expiration of pass-through status for drugs and nonimplantable biologicals. In this regard, we will continue to accept applications as is currently described on the CMS Web site at: http://www.cms.hhs.gov/HospitalOutpatientPPS/04_passthrough_payment.asp. We will continue to begin pass-through payment on a quarterly basis through the next available OPPS quarterly update after the approval of a product's pass-through status. In addition, we will continue to expire pass-through status for drugs and nonimplantable biologicals on an annual basis through notice and comment rulemaking. Furthermore, our policy regarding the determination of packaging status after the pass-through status ends for a drug or biological, as discussed in section V.B.2. of this final Start Printed Page 60480 rule with comment period, remains the same. For those drugs with expiring pass-through status that are always packaged when they do not have pass-through status (“policy-packaged”), specifically diagnostic radiopharmaceuticals and contrast agents for CY 2010, we will package payment for these drugs once their pass-through status has expired. We discuss this policy in detail in section V.B.2.d. of this final rule with comment period.
Comment: One commenter argued that CMS' proposed proxy of the date of the first sale of a drug or biological in the United States following FDA approval was contradictory to section 1833(t)(6)(C)(i)(II) of the Act because that section references section 1833(t)(6)(A)(iv) of the Act, which defines a “new” drug or biological eligible for pass-through payment as being new after December 31, 1996, and as meeting the cost significance criteria. The commenter argued that a drug cannot be considered a pass-through drug until cost significance has been determined and that CMS would not determine cost significance until it qualifies a drug for pass-through status. Based on this assessment, the commenter argued that CMS should begin the pass-through payment period on the date CMS begins to treat the product as a pass-through drug, the first date of the pass-through payment period.
Response: We continue to believe that section 1833(t)(6)(C)(i)(II) of the Act requires the start date of the pass-through payment eligibility period for a drug or nonimplantable biological to begin on the date on which payment is first made for a drug or biological as an outpatient hospital service under Part B. As noted in the previous response, however, we are convinced by a commenter to adopt as the proxy for this date, the date on which CMS makes its first pass-through payment for the drug or nonimplantable biological.
Comment: Several commenters recommended that CMS continue to end pass-through status for drugs and nonimplantable biologicals on an annual basis, instead of ending pass-through status on a quarterly basis as CMS proposed. In the context of the specific proposal for the pass-through payment eligibility period, another commenter agreed with CMS' proposal to end pass-through status on a quarterly basis. Several other commenters argued that, because the proposal creates a delay between the beginning of the pass-through payment eligibility period and the period of pass-through payment, drugs and nonimplantable biologicals that are approved for pass-through status should be given pass-through payment for the extent of the full 3-year pass-through eligibility period.
Response: Because we are adopting the date of first pass-through payment as the start of the pass-through payment eligibility period in this final rule with comment period, we will not change, as we proposed, the current operation of our drug and biological pass-through program. As is our current practice, we will continue to expire pass-through status for drugs and biologicals on an annual basis through notice and comment rulemaking. For example, if CMS receives a complete application for pass-through status for a drug on August 1, 2009, and approves the application for pass-through status for the January 1, 2010 OPPS quarterly update, the pass-through payment eligibility period would start on January 1, 2010. The pass-through payment period would extend for 2 but not more than 3 years, as is mandated by the statute, and we would propose to expire pass-through status for the drug on December 31, 2011 in the CY 2012 OPPS/ASC rulemaking process for January 1, 2012.
After consideration of the public comments we received, we are modifying our CY 2010 proposal and adopting the date of first pass-through payment for the drug or nonimplantable biological as the proxy for the first date on which payment for the product is made under Part B as an outpatient hospital service. Therefore, the 2- to 3-year pass-through payment eligibility period will start on the date of first pass-through payment and, consistent with our current policy, the pass-through payment eligibility period and the period of pass-through payment coincide. Finally, we will continue to expire the pass-through status of drugs and nonimplantable biologicals annually through the notice and comment rulemaking process.
Because our final policy reflects our current practice for implementing the pass-through eligibility and payment periods defined in section 1833(t)(6)(C)(i)(II) of the Act, we are not making any changes to § 419.64(c)(2), and we are not adding proposed new § 419.64(c)(3) to our regulations.
6. Provisions for Reducing Transitional Pass-Through Payments for Diagnostic Radiopharmaceuticals and Contrast Agents To Offset Costs Packaged Into APC Groups
a. Background
Prior to CY 2008, diagnostic radiopharmaceuticals and contrast agents were paid separately under the OPPS if their mean per day costs were greater than the applicable year's drug packaging threshold. In CY 2008 (72 FR 66768), we began a policy of packaging payment for all nonpass-through diagnostic radiopharmaceuticals and contrast agents as ancillary and supportive items and services into their associated nuclear medicine procedures. Therefore, beginning in CY 2008, nonpass-through diagnostic radiopharmaceuticals and contrast agents were not subject to the annual OPPS drug packaging threshold to determine their packaged or separately payable payment status, and instead all nonpass-through diagnostic radiopharmaceuticals and contrast agents were packaged as a matter of policy. In the CY 2010 OPPS/ASC proposed rule (74 FR 35323), we proposed to continue to package payment for all nonpass-through diagnostic radiopharmaceuticals and contrast agents for CY 2010 as discussed in section V.B.2.d. of the proposed rule (74 FR 35323 through 35324).
b. Payment Offset Policy for Diagnostic Radiopharmaceuticals
As previously noted, radiopharmaceuticals are considered to be drugs for OPPS pass-through payment purposes. As described above, section 1833(t)(6)(D)(i) of the Act specifies that the transitional pass-through payment amount for pass-through drugs and biologicals is the difference between the amount paid under section 1842(o) (or the Part B drug CAP rate) and the otherwise applicable OPD fee schedule amount. There is currently one radiopharmaceutical with pass-through status under the OPPS, HCPCS code C9247 (Iobenguane, I–123, diagnostic, per study dose, up to 10 millicuries). HCPCS code C9247 was granted pass-through status beginning April 1, 2009 and will continue on pass-through status in CY 2010 under permanent HCPCS code A9582 (Iodine I–123 iobenguane, diagnostic, per study dose, up to 15 millicuries). We currently apply the established radiopharmaceutical payment offset policy to pass-through payment for this product. As described earlier in section V.A.3. of this final rule with comment period, new pass-through diagnostic radiopharmaceuticals will be paid at ASP+6 percent, while those without ASP information will be paid at WAC+6 percent or, if WAC is not available, payment will be based on 95 percent of the product's most recently published AWP.
As a payment offset is necessary in order to provide an appropriate Start Printed Page 60481 transitional pass-through payment, we deduct from the payment for pass-through radiopharmaceuticals an amount that reflects the portion of the APC payment associated with predecessor radiopharmaceuticals in order to ensure no duplicate radiopharmaceutical payment is made. In CY 2009, we established a policy to estimate the portion of each APC payment rate that could reasonably be attributed to the cost of predecessor diagnostic radiopharmaceuticals when considering a new diagnostic radiopharmaceutical for pass-through payment (73 FR 68638 through 68641). Specifically, we utilize the “policy-packaged” drug offset fraction for APCs containing nuclear medicine procedures, calculated as 1 minus (the cost from single procedure claims in the APC after removing the cost for “policy-packaged” drugs divided by the cost from single procedure claims in the APC). We have previously defined “policy-packaged” drugs and biologicals as nonpass-through diagnostic radiopharmaceuticals, contrast agents, and implantable biologicals (73 FR 68639). In the CY 2010 OPPS/ASC proposed rule (74 FR 35323), we proposed for CY 2010 to redefine “policy-packaged” drugs as only nonpass-through diagnostic radiopharmaceuticals and contrast agents, as a result of the CY 2010 proposals discussed in sections V.A.4. and V.B.2.d. of the proposed rule (74 FR 35311 through 35314 and 74 FR 35323 through 35324) that would treat nonpass-through implantable biologicals that are surgically inserted or implanted (through a surgical incision or a natural orifice) and implantable biologicals that are surgically inserted or implanted (through a surgical incision or a natural orifice) with newly approved pass-through status beginning in CY 2010 or later as devices, rather than drugs. To determine the actual APC offset amount for pass-through diagnostic radiopharmaceuticals that takes into consideration the otherwise applicable OPPS payment amount, we multiply the “policy-packaged” drug offset fraction by the APC payment amount for the nuclear medicine procedure with which the pass-through diagnostic radiopharmaceutical is used and, accordingly, reduce the separate OPPS payment for the pass-through diagnostic radiopharmaceutical by this amount.
We will continue to post annually on the CMS Web site at http://www.cms.hhs.gov/HospitalOutpatientPPS, a file that contains the APC offset amounts that would be used for that year for purposes of both evaluating cost significance for candidate pass-through device categories and drugs and biologicals, including diagnostic radiopharmaceuticals, and establishing any appropriate APC offset amounts. Specifically, the file will continue to provide, for every OPPS clinical APC, the amounts and percentages of APC payment associated with packaged implantable devices, “policy-packaged” drugs, and “threshold-packaged” drugs and biologicals.
Table 23 of the proposed rule (74 FR 35318) displayed the proposed APCs to which nuclear medicine procedures would be assigned in CY 2010 and for which we expected that an APC offset could be applicable in the case of new diagnostic radiopharmaceuticals with pass-through status.
Comment: A few commenters supported the continuation of the pass-through diagnostic radiopharmaceutical offset policy for CY 2010.
Response: We continue to believe that a diagnostic radiopharmaceutical offset policy is necessary in order to ensure that duplicate payment is not made for diagnostic radiopharmaceuticals with pass-through status. We believe it is appropriate to remove the radiopharmaceutical payment amount that is already packaged into the payment for the associated nuclear medicine procedure when we provide pass-through payment for a diagnostic radiopharmaceutical with pass-through status.
Therefore, after consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to apply the diagnostic radiopharmaceutical offset policy to payment for pass-through diagnostic radiopharmaceuticals, as described above. Table 32 below displays the APCs to which nuclear medicine procedures are assigned in CY 2010 and for which we expect that an APC offset could be applicable in the case of diagnostic radiopharmaceuticals with pass-through status.
| CY 2010 APC | CY 2010 APC title |
|---|---|
| 0307 | Myocardial Positron Emission Tomography (PET) imaging. |
| 0308 | Non-Myocardial Positron Emission Tomography (PET) imaging. |
| 0377 | Level II Cardiac Imaging. |
| 0378 | Level II Pulmonary Imaging. |
| 0389 | Level I Non-imaging Nuclear Medicine. |
| 0390 | Level I Endocrine Imaging. |
| 0391 | Level II Endocrine Imaging. |
| 0392 | Level II Non-imaging Nuclear Medicine. |
| 0393 | Hematologic Processing & Studies. |
| 0394 | Hepatobiliary Imaging. |
| 0395 | GI Tract Imaging. |
| 0396 | Bone Imaging. |
| 0397 | Vascular Imaging. |
| 0398 | Level I Cardiac Imaging. |
| 0400 | Hematopoietic Imaging. |
| 0401 | Level I Pulmonary Imaging. |
| 0402 | Level II Nervous System Imaging. |
| 0403 | Level I Nervous System Imaging. |
| 0404 | Renal and Genitourinary Studies. |
| 0406 | Level I Tumor/Infection Imaging. |
| 0408 | Level III Tumor/Infection Imaging. |
| 0414 | Level II Tumor/Infection Imaging. |
c. Payment Offset Policy for Contrast Agents
As described above, section 1833(t)(6)(D)(i) of the Act specifies that the transitional pass-through payment amount for pass-through drugs and biologicals is the difference between the amount paid under section 1842(o) (or the Part B drug CAP rate) and the otherwise applicable OPD fee schedule amount. There is currently one contrast agent with pass-through status under the OPPS, HCPCS code C9246 (Injection, gadoxetate disodium, per ml). HCPCS code C9246 was granted pass-through status beginning January 1, 2009, and will continue with pass-through status in CY 2010 under HCPCS code A9581 (Injection, gadoxetate disodium, 1 ml). As described earlier in section V.A.3. of this final rule with comment period, new pass-through contrast agents will be paid at ASP+6 percent, while those without ASP information would be paid at WAC+6 percent or, if WAC is not available, payment would be based on 95 percent of the product's most recently published AWP.
As discussed in the CY 2010 OPPS/ASC proposed rule (74 FR 35318), we believe that a payment offset, similar to the offset currently in place for pass-through devices and diagnostic radiopharmaceuticals, is necessary in order to provide an appropriate transitional pass-through payment for contrast agents because all of these items are packaged when they do not have pass-through status. In accordance with our standard offset methodology, in the CY 2010 OPPS/ASC proposed rule (74 FR 35318), we proposed to deduct from the payment for pass-through contrast agents an amount that reflects the portion of the APC payment associated with predecessor contrast agents in order to ensure no duplicate contrast agent payment is made.
In CY 2009, we established a policy to estimate the portion of each APC payment rate that could reasonably be attributed to the cost of predecessor diagnostic radiopharmaceuticals when considering a new diagnostic radiopharmaceutical for pass-through payment (73 FR 68638 through 68641). For CY 2010, we proposed to apply this same policy to contrast agents. Specifically, we proposed to utilize the “policy-packaged” drug offset fraction for clinical APCs calculated as 1 minus (the cost from single procedure claims in the APC after removing the cost for “policy-packaged” drugs divided by the cost from single procedure claims in the APC). As discussed above, while we have previously defined the “policy-packaged” drugs and biologicals as nonpass-through diagnostic radiopharmaceuticals, contrast agents, and implantable biologicals (73 FR 68639), we proposed for CY 2010 to redefine “policy-packaged” drugs as only nonpass-through diagnostic radiopharmaceuticals and contrast agents, as a result of the CY 2010 proposal discussed in sections V.A.4. and V.B.2.d. of the proposed rule (74 FR 35311 through 35314 and 74 FR 35323 through 35324) that would treat all implantable biologicals as devices, rather than drugs. To determine the actual APC offset amount for pass-through contrast agents that takes into consideration the otherwise applicable OPPS payment amount, we proposed to multiply the “policy-packaged” drug offset fraction by the APC payment amount for the procedure with which the pass-through contrast agent is used and, accordingly, reduce the separate OPPS payment for the pass-through contrast agent by this amount.
We proposed to continue to post annually on the CMS Web site at http://www.cms.hhs.gov/HospitalOutpatientPPS, a file that contains the APC offset amounts that would be used for that year for purposes of both evaluating cost significance for candidate pass-through device categories and drugs and biologicals, including contrast agents, and establishing any appropriate APC offset amounts. Specifically, the file will continue to provide, for every OPPS clinical APC, the amounts and percentages of APC payment associated with packaged implantable devices, “policy-packaged” drugs, and “threshold-packaged” drugs and biologicals.
Comment: One commenter objected to the proposed offset policy for contrast agents, stating that an offset for new contrast agents granted pass-through status, combined with the packaging policy for all nonpass-through contrast agents, would discourage hospitals from providing contrast agents for financial reasons. The commenter argued that an offset policy is not necessary to avoid duplicate payment for pass-through contrast agents as the majority of older contrast agents have costs that are well below the $65 OPPS drug packaging threshold and more expensive contrast agents would be eligible for pass-through status. Finally, the commenter believed that CMS does not have the appropriate contrast agent data available in order to calculate an offset amount for these products. Another commenter objected to CMS' proposed offset methodology for contrast agents and urged CMS to specify the APCs that would be subject to an offset. Further, the commenter requested that CMS implement a contrast offset methodology that would be more similar to the offset methodology currently in place for pass-through devices and diagnostic radiopharmaceuticals.
Response: We have consistently implemented an offset policy for products receiving pass-through payment that would otherwise receive significant packaged payment if not for their pass-through status. An offset methodology ensures that we do not pay twice, first through a packaged payment included in the associated procedure payment and second through an individual separate payment, for the item with pass-through status. The potential for duplicate payment is higher for items such as contrast agents, diagnostic radiopharmaceuticals, and devices where the pass-through item typically substitutes for items that are otherwise always packaged. Furthermore, the potential magnitude of duplicate payment also is higher for these items because they are always packaged when they do not have pass-through status.
As discussed above, this offset policy appropriately provides for pass-through payment for the new product that represents the difference between the physician's office payment amount and the otherwise applicable OPD fee schedule amount, in the case of packaged contrast agents the “policy-packaged” drug APC offset amount, as specified by the statute. We note that the proposed contrast agent offset policy is virtually identical to the offset methodology currently in place for pass-through devices and diagnostic radiopharmaceuticals, consistent with the recommendation by one commenter that we adopt a similar policy for contrast agents. We believe that this methodology would pay appropriately for the cost of pass-through contrast agents and that hospitals should have no payment concerns when determining which contrast agent would be most clinically appropriate and efficient for a particular patient's study. Therefore, we do not believe that the application of a contrast agent offset methodology would discourage hospitals from using pass-through contrast agents insofar as providers determine they are necessary in the care of the patient.
As discussed above, we proposed to deduct from the payment for pass-through contrast agents an amount that reflects the portion of the APC payment associated with predecessor contrast agents in order to ensure no duplicate contrast agent payment is made. As Start Printed Page 60483 discussed above, we identified the “policy-packaged” drug APC offset amount as applicable to our offset policy because we have identified contrast agents as “policy- packaged” drugs in our claims data. To the extent that hospitals reported the HCPCS code for contrast agents when those drugs were administered during procedures, the contrast agent costs are included in our calculation of the “policy-packaged” drug APC offset amounts, and we believe that we have sufficient information regarding the costs of predecessor contrast agents to apply the resulting offset amounts to payment for pass-through contrast agents. To the extent hospitals did not report the use of contrast agents under specific HCPCS codes in CY 2008, we could not fully total the cost of contrast for a given imaging APC and we would underestimate an accurate “policy-packaged” drug APC offset amount. This unknown but potential bias would generally result in higher overall pass-through payment for a new contrast agent so any limitations of our current data on contrast agents for purposes of the offset would not inappropriately reduce pass-through payment for a new contrast agent.
We disagree with the commenter that an offset is unnecessary to avoid duplicate payment for contrast material. All nonpass-through contrast agents, regardless of their per day costs, are packaged into payments for the associated procedures. Therefore, OPPS payment for imaging and other procedures that currently utilize contrast agents already includes packaged payment for the necessary contrast agent. The observation that most contrast agents have per day costs below the $65 threshold does not obviate the need for an offset policy for contrast agents with pass-through status. First, while the CY 2010 drug packaging threshold is low, $65 as the per day cost, this cost may constitute a sizable percentage of a procedural APC's median cost. Paying the full procedural APC amount plus the pass-through contrast agent payment of ASP+6 for an imaging scan with high volume could result in significant overpayment of the new contrast agent. Furthermore, a few contrast agents have per day costs above the $65 drug packaging threshold, so that the amount of contrast agent cost represented in the “policy-packaged” drug amount of an APC median cost could be fairly substantial. Finally, unlike “threshold-packaged” drugs that are packaged based on the relationship of their per day cost to the $65 drug packaging threshold, where the packaged drug cost in a procedural APC may or may not represent predecessor drug costs and where multiple drugs may be administered in a single session paid under one procedural APC, contrast agents typically substitute for one another and hospitals rarely administer multiple contrast agents in the same session. Pass-through contrast agents are paid separately and are billed with procedures that already have costs of predecessor contrast agents packaged into the procedural APC payment, so duplicate contrast agent payment would result in the absence of an offset methodology.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35318), we proposed to utilize the “policy-packaged” drug offset fraction for procedural APCs calculated as 1 minus (the cost from single procedure claims in the APC after removing the cost for “policy-packaged” drugs divided by the cost from single procedure claims in the APC). To determine the actual APC offset amount for pass-through contrast agents that takes into consideration the otherwise applicable OPPS payment amount, we proposed to multiply the “policy-packaged” drug offset fraction by the APC payment amount for the procedure with which the pass-through contrast agent is used and, accordingly, reduce the separate OPPS payment for the pass-through contrast agent by this amount.
In response to the commenters' concerns regarding our proposed methodology and request that we specify the APCs subject to the contrast agent offset policy, we reviewed the methodology and specifically examined the amount of contrast agent offsets associated with procedural APCs to determine which APCs, other than nuclear medicine APCs that contained the costs of diagnostic radiopharmaceuticals, included a significant “policy-packaged” drug amount in the APC payment. First, we excluded all APCs to which nuclear medicine procedures were assigned for CY 2010 from the APCs that would be subject to a contrast agent offset policy, reasoning that the “policy-packaged” drug costs associated with these APCs were for diagnostic radiopharmaceuticals. From a clinical perspective, there is very little overlap in the procedures that use contrast agents or diagnostic radiopharmaceuticals. Next, we reviewed the per day costs for all contrast agents with CY 2008 claims data and compared their aggregate, average per day cost to the “policy-packaged” drug amounts listed in the CY 2010 proposed rule APC offset file that was posted on the CMS Web site in association with the CY 2010 OPPS/ASC proposed rule. When examining those APCs with “policy-packaged” drug amounts equal to or less than the 25th percentile of per day contrast agent cost (approximately $22), we found that the majority of APCs with a “policy- packaged” drug offset amount other than zero but less than $20 (our $22 estimate rounded to the nearest $5 increment) were generally APCs that were not likely to include procedures requiring significant use of contrast agents. We selected the 25th percentile of per day contrast agent cost to identify the majority of APCs with significant contrast agent cost because we believe that the 25th percentile is an appropriate threshold for representing significant contrast agent cost as it captures the lower bound of significant variation around the per day contrast agent cost. The interquartile range, the 25th to 75th percentile, is a typical descriptive statistic used to describe the variation in the center of a distribution. Further, the dollar value of the 25th percentile, $22 was sufficiently high that we believed it would be worth establishing and implementing offset logic in our claims processing Pricer module. This allowed us to establish a meaningful threshold cost for application of a contrast agent offset policy that would identify APCs in which there is significant packaged contrast agent cost. Unlike the case of diagnostic radiopharmaceuticals, which are always administered during a limited number of nuclear medicine procedures so we are able to identify all APCs to which nuclear medicine procedures are assigned as those for which the diagnostic radiopharmaceutical offset policy would apply, contrast agents are utilized much more widely among procedures assigned to many OPPS APCs.
The APCs that we identified as below the threshold of $20 included APC 0384 (GI Procedures with Stents) and APC 0427 (Level II Tube or Catheter Changes or Repositioning). As we would not expect contrast agents to generally be used in the procedures assigned to these APCs, we believe that implementing a threshold that would exclude these APCs from a contrast agent offset policy would be appropriate for administrative simplification of claims processing, while continuing to ensure no duplicate payment is made for pass-through contrast agents. Therefore, we have identified the APCs that would be subject to the contrast offset policy in CY 2010, within the scope of the criteria discussed above.
After consideration of the public comments we received, we are Start Printed Page 60484 finalizing a pass through contrast agent offset policy for CY 2010, with modification to specify the procedural APCs to which offsets for pass-through contrast agents would apply. Procedural APCs for which we expect a contrast agent offset could be applicable in the case of a pass-through contrast agent have been identified as any procedural APC with a “policy-packaged” drug amount greater than $20 that is not a nuclear medicine APC identified in Table 32 above, and these APCs are displayed in Table 33. For CY 2010, when a contrast agent with pass-through status is billed with any procedural APC listed in Table 33, a specific offset based on the procedural APC will be applied to payment for the contrast agent to ensure that duplicate payment is not made for the contrast agent.
| CY 2010 APC | CY 2010 APC title |
|---|---|
| 0080 | Diagnostic Cardiac Catheterization. |
| 0082 | Coronary or Non-Coronary Atherectomy. |
| 0083 | Coronary or Non-Coronary Angioplasty and Percutaneous Valvuloplasty. |
| 0093 | Vascular Reconstruction/Fistula Repair without Device. |
| 0104 | Transcatheter Placement of Intracoronary Stents. |
| 0128 | Echocardiogram with Contrast. |
| 0152 | Level I Percutaneous Abdominal and Biliary Procedures. |
| 0229 | Transcatheter Placement of Intravascular Shunts. |
| 0278 | Diagnostic Urography. |
| 0279 | Level II Angiography and Venography. |
| 0280 | Level III Angiography and Venography. |
| 0283 | Computed Tomography with Contrast. |
| 0284 | Magnetic Resonance Imaging and Magnetic Resonance Angiography with Contrast. |
| 0333 | Computed Tomography without Contrast followed by Contrast. |
| 0337 | Magnetic Resonance Imaging and Magnetic Resonance Angiography without Contrast followed by Contrast. |
| 0375 | Ancillary Outpatient Services When Patient Expires. |
| 0383 | Cardiac Computed Tomographic Imaging. |
| 0388 | Discography. |
| 0418 | Insertion of Left Ventricular Pacing Elect. |
| 0442 | Dosimetric Drug Administration. |
| 0653 | Vascular Reconstruction/Fistula Repair with Device. |
| 0656 | Transcatheter Placement of Intracoronary Drug-Eluting Stents. |
| 0662 | CT Angiography. |
| 0668 | Level I Angiography and Venography. |
| 8006 | CT and CTA with Contrast Composite. |
| 8008 | MRI and MRA with Contrast Composite. |
B. OPPS Payment for Drugs, Biologicals, and Radiopharmaceuticals Without Pass-Through Status
1. Background
Under the CY 2009 OPPS, we currently pay for drugs, biologicals, and radiopharmaceuticals that do not have pass-through status in one of two ways: packaged payment into the payment for the associated service; or separate payment (individual APCs). We explained in the April 7, 2000 OPPS final rule with comment period (65 FR 18450) that we generally package the cost of drugs and radiopharmaceuticals into the APC payment rate for the procedure or treatment with which the products are usually furnished. Hospitals do not receive separate payment for packaged items and supplies, and hospitals may not bill beneficiaries separately for any packaged items and supplies whose costs are recognized and paid within the national OPPS payment rate for the associated procedure or service. (Transmittal A–01–133, issued on November 20, 2001, explains in greater detail the rules regarding separate payment for packaged services.)
Packaging costs into a single aggregate payment for a service, procedure, or episode-of-care is a fundamental principle that distinguishes a prospective payment system from a fee schedule. In general, packaging the costs of items and services into the payment for the primary procedure or service with which they are associated encourages hospital efficiencies and also enables hospitals to manage their resources with maximum flexibility.
Section 1833(t)(16)(B) of the Act, as added by section 621(a)(2) of Public Law 108–173, set the threshold for establishing separate APCs for drugs and biologicals at $50 per administration for CYs 2005 and 2006. Therefore, for CYs 2005 and 2006, we paid separately for drugs, biologicals, and radiopharmaceuticals whose per day cost exceeded $50 and packaged the costs of drugs, biologicals, and radiopharmaceuticals whose per day cost was equal to or less than $50 into the procedures with which they were billed. For CY 2007, the packaging threshold for drugs, biologicals, and radiopharmaceuticals that were not new and did not have pass-through status was established at $55. For CYs 2008 and 2009, the packaging threshold for drugs, biologicals, and radiopharmaceuticals that were not new and do not have pass-through status was established at $60. The methodology used to establish the $55 threshold for CY 2007, the $60 threshold for CYs 2008 and 2009, and our approach for CY 2010 are discussed in more detail in section Start Printed Page 60485 V.B.2.b. of this final rule with comment period.
2. Criteria for Packaging Payment for Drugs, Biologicals, and Radiopharmaceuticals
a. Background
As indicated in section V.B.1. of this final rule with comment period, in accordance with section 1833(t)(16)(B) of the Act, the threshold for establishing separate APCs for payment of drugs and biologicals was set to $50 per administration during CYs 2005 and 2006. In CY 2007, we used the fourth quarter moving average Producer Price Index (PPI) levels for prescription preparations to trend the $50 threshold forward from the third quarter of CY 2005 (when the Pub. L. 108–173 mandated threshold became effective) to the third quarter of CY 2007. We then rounded the resulting dollar amount to the nearest $5 increment in order to determine the CY 2007 threshold amount of $55. Using the same methodology as that used in CY 2007 (which is discussed in more detail in the CY 2007 OPPS/ASC final rule with comment period (71 FR 68085 through 68086)), we set the packaging threshold for establishing separate APCs for drugs and biologicals at $60 for CYs 2008 and 2009.
Following the CY 2007 methodology, for the CY 2010 OPPS/ASC proposed rule we used updated fourth quarter moving average PPI levels to trend the $50 threshold forward from the third quarter of CY 2005 to the third quarter of CY 2009 and again rounded the resulting dollar amount ($65.07) to the nearest $5 increment, which yielded a figure of $65. In performing this calculation, we used the most up-to-date forecasted, quarterly PPI estimates from CMS' Office of the Actuary (OACT). As actual inflation for past quarters replaced forecasted amounts, the PPI estimates for prior quarters have been revised (compared with those used in the CY 2007 OPPS/ASC final rule with comment period) and were incorporated into our calculation. Based on the calculations described above, we proposed a packaging threshold for CY 2010 of $65. (For a more detailed discussion of the OPPS drug packaging threshold and the use of the PPI for prescription drugs, we refer readers to the CY 2007 OPPS/ASC final rule with comment period (71 FR 68085 through 68086).)
b. Cost Threshold for Packaging of Payment for HCPCS Codes that Describe Certain Drugs, Nonimplantable Biologicals, and Therapeutic Radiopharmaceuticals (“Threshold-Packaged Drugs”)
To determine their proposed CY 2010 packaging status, for the CY 2010 OPPS/ASC proposed rule we calculated the per day cost of all drugs on a HCPCS code-specific basis (with the exception of those drugs and biologicals with multiple HCPCS codes that include different dosages as described in section V.B.2.c. of the CY 2010 OPPS/ASC proposed rule (74 FR 35321) and excluding diagnostic radiopharmaceuticals, contrast agents, and implantable biologicals that we proposed to continue to package in CY 2010 as discussed in section V.B.2.d. of the CY 2010 OPPS/ASC proposed rule (74 FR 35323 through 35324) and this final rule with comment period), nonimplantable biologicals, and therapeutic radiopharmaceuticals (collectively called “threshold-packaged” drugs) that had a HCPCS code in CY 2008 and were paid (via packaged or separate payment) under the OPPS, using CY 2008 claims data processed before January 1, 2009. In order to calculate the per day costs for drugs, nonimplantable biologicals, and therapeutic radiopharmaceuticals to determine their proposed packaging status in CY 2010, we used the methodology that was described in detail in the CY 2006 OPPS proposed rule (70 FR 42723 through 42724) and finalized in the CY 2006 OPPS final rule with comment period (70 FR 68636 through 70 FR 68638).
To calculate the CY 2010 proposed rule per day costs, we used an estimated payment rate for each drug and nonimplantable biological HCPCS code of ASP+4 percent (which was the payment rate we proposed for separately payable drugs and nonimplantable biologicals in CY 2010, as discussed in more detail in section V.B.3.b. of the CY 2010 OPPS/ASC proposed rule (74 FR 35324 through 35326)). We used the manufacturer submitted ASP data from the fourth quarter of CY 2008 (data that were used for payment purposes in the physician's office setting, effective April 1, 2009) to determine the proposed rule per day cost.
As is our standard methodology, for CY 2010, we proposed to use payment rates based on the ASP data from the fourth quarter of CY 2008 for budget neutrality estimates, packaging determinations, impact analyses, and completion of Addenda A and B to the proposed rule because these were the most recent data available for use at the time of development of the proposed rule. These data were also the basis for drug payments in the physician's office setting, effective April 1, 2009. For items that did not have an ASP-based payment rate, such as therapeutic radiopharmaceuticals, we used their mean unit cost derived from the CY 2008 hospital claims data to determine their proposed per day cost. We packaged items with a per day cost less than or equal to $65 and identified items with a per day cost greater than $65 as separately payable. Consistent with our past practice, we crosswalked historical OPPS claims data from the CY 2008 HCPCS codes that were reported to the CY 2009 HCPCS codes that we displayed in Addendum B to the proposed rule for payment in CY 2010.
Comment: Several commenters supported CMS' proposal to increase the packaging threshold to $65 for CY 2010. However, the majority of commenters objected to the proposed increase to the OPPS packaging threshold.
A few commenters recommended that CMS consider either eliminating the drug packaging threshold and providing separate payment for all drugs with HCPCS codes or freezing the packaging threshold at $60 for CY 2010. Some commenters objected to the use of a packaging threshold under the OPPS when one is not used for physician's office payment and believed that eliminating the drug packaging threshold would allow for parity in drug payment between the HOPD setting and the physician's office setting. These commenters expressed concern that the packaging threshold may impede beneficiary access to lower-cost packaged drugs in the HOPD setting. In addition, some commenters believed that eliminating the packaging threshold and paying separately for all drugs in the HOPD setting would allow a more accurate calculation of the separately payable payment amount for drugs (otherwise referred to as the ASP+X percent amount). Other commenters stated that CMS should not increase the drug packaging threshold because other changes in the drug payment ratesetting methodology were proposed. These commenters requested that CMS only change one aspect of the drug payment methodology at a time to allow for greater understanding of the impact of proposed changes to drug payment.
Response: As fully discussed in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66757 through 66758) and the CY 2009 OPPS/ASC final rule with comment period (73 FR 68643), we continue to believe that unpackaging payment for all drugs, Start Printed Page 60486 biologicals, and radiopharmaceuticals is inconsistent with the concept of a prospective payment system and that such a change could create an additional reporting burden for hospitals. The OPPS and the MPFS that applies to physician's office services are fundamentally different payment systems with essential differences in their payment policies and structures. Specifically, the OPPS is a prospective payment system, based on the concept of payment for groups of services that share clinical and resource characteristics. Payment is made under the OPPS according to prospectively established payment rates that are related to the relative costs of hospital resources for services. The MPFS is a fee schedule based on the relative value of each individual component of a service. Consistent with the MPFS approach, separate payment is made for each drug provided in the physician's office, but the OPPS packages payment for certain drugs into the associated procedure payments for the APC group. Given the fundamental differences between the MPFS payment mechanism and the OPPS payment mechanism, differences in the degrees of packaged payment and separate payment between these two systems are only to be expected. In general, we do not believe that our packaging methodology under the OPPS results in limited beneficiary access to drugs because packaging is a fundamental component of a prospective payment system that accounts for the cost of certain items and services in larger payment bundles, recognizing that some clinical cases may be more costly and others less costly but that, on average, OPPS payment is appropriate for the services provided.
We note that, in CYs 2005 and 2006, the statutorily mandated drug packaging threshold was set at $50, and we continue to believe that it is appropriate to continue a modest drug packaging threshold for the CY 2010 OPPS for the reasons set forth below. As stated in the CY 2007 OPPS/ASC final rule with comment period (71 FR 68086), we believe that packaging certain items is a fundamental component of a prospective payment system, that packaging these items does not lead to beneficiary access issues and does not create a problematic site of service differential, that the packaging threshold is reasonable based on the initial establishment in law of a $50 threshold for the CY 2005 OPPS, that updating the $50 threshold is consistent with industry and government practices, and that the PPI for prescription preparations is an appropriate mechanism to gauge Part B drug inflation. Therefore, because of our continued belief that packaging is a fundamental component of a prospective payment system that contributes to important flexibility and efficiency in the delivery of high quality hospital outpatient services, we are not adopting the commenters' recommendations to pay separately for all drugs, biologicals, and radiopharmaceuticals for CY 2010 or to eliminate or to freeze the packaging threshold at $60.
Finally, we believe that our continued application of the methodology initially adopted in CY 2007 to update the drug packaging threshold does not inhibit our ability to propose additional changes to the nonpass-through drug payment methodology under the OPPS. We note that for the past several years, we have made a number of proposals to revise our drug payment methodology, while continuing to implement our established methodology for annually updating the drug packaging threshold. While we have not finalized any of these previous proposals, we have consistently applied the methodology described above to update the drug packaging threshold while examining a variety of alternatives for determining payment for separately payable drugs without pass-through status.
Comment: One commenter to the CY 2009 OPPS/ASC final rule with comment period noted that HCPCS code J3300 (Injection, triamcinolone acetonide, preservative free, 1 mg) should not be packaged as established in the final rule with comment period because the per day cost of this drug is over the CY 2009 OPPS drug packaging threshold of $60 per day.
Response: While the payment for HCPCS code J3300 was adopted on an interim final basis as packaged for CY 2009 (status indicator “N”), upon receipt of this public comment we reviewed our calculation and released a correction notice changing the status indicator to “K” for CY 2009 (74 FR 4343). In addition, we discussed this status indicator change in the April 2009 OPPS quarterly update CR (Transmittal 1702, CR 6416, dated March 13, 2009).
Comment: One commenter stated that HCPCS code J3473 (Injection, hyaluronidase, recombinant, 1 USP unit) was incorrectly assigned status indicator “N” in the CY 2010 OPPS/ASC proposed rule. The commenter argued that coding errors resulted in hospital claims data indicating that per day costs of HCPCS code J3473 is below the drug packaging threshold for CY 2010. The commenter explained that a variety of HCPCS codes and various dosage descriptors for similar products contributed to hospital coding errors, and that the product described by HCPCS code J3473 is only sold in a single use vial of 150 units, with an ASP that exceeds the CY 2010 packaging threshold. The commenter noted that this concern had been raised with the CMS HCPCS Workgroup but a request for a new HCPCS code descriptor was denied.
Response: HCPCS code J3473 expired from pass-through status on December 31, 2008, and was paid separately in CY 2009 because the estimated per day cost, using updated final rule claims data from CY 2007, showed that the per day cost of this drug exceeded the CY 2009 drug packaging threshold. For CY 2010, we proposed to package HCPCS code J3473 as the estimated per day cost did not exceed the proposed CY 2010 drug packaging threshold. The OPPS relies on hospital claims data in order to determine payment rates. For drugs and biologicals, we rely upon hospital claims data, in part, to determine the estimated per day cost we use in our annual packaging determination. In addition, the concern about discrepancies between HCPCS code descriptors for similar products is under the purview of the CMS HCPCS Workgroup, the sole creator and maintainer of HCPCS codes and their descriptors. We remind hospitals through each OPPS quarterly update CR that when billing for drugs, biologicals, and radiopharmaceuticals, they should make certain that the reported units of service of the reported HCPCS code are consistent with the quantity of the drug, biological, or radiopharmaceutical that was used in the care of the patient. Therefore, we expect that the data that we receive on hospital claims accurately reflect the services that were provided to the beneficiary.
As is our standard methodology, we used updated claims data and ASP rates to make final packaging determinations for CY 2010. For HCPCS code J3473, our CY 2008 claims data showed approximately 2,100 days and 226,800 units from 37 providers. While this drug was not commonly used in CY 2008, we have no reason to believe that the estimated per day cost of HCPCS code J3473 of approximately $57, based on our methodology described above as applied to claims from a modest number of providers, is not reflective of the per day cost to hospitals for furnishing the drug. Therefore, we have determined that the per day cost of HCPCS code J3473 does not exceed the $65 packaging threshold for drugs and Start Printed Page 60487 biologicals and payment for HCPCS code J3473 is packaged in CY 2010.
For purposes of this final rule with comment period, we again followed the CY 2007 methodology for CY 2010 and used updated fourth quarter moving average PPI levels to trend the $50 threshold forward from the third quarter of CY 2005 to the third quarter of CY 2010 and again rounded the resulting dollar amount ($66.55) to the nearest $5 increment, which yielded a figure of $65. In performing this calculation, we used the most up-to-date forecasted, quarterly PPI estimates from CMS' OACT.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to continue use of the established methodology for annually updating the OPPS packaging threshold for drugs and biologicals by the PPI for prescription drugs. The final CY 2010 drug packaging threshold is $65, calculated according to the threshold update methodology that we have applied since CY 2007.
In CY 2005 (69 FR 65779 through 65780), we implemented a policy that exempted the oral and injectable forms of 5-HT3 antiemetic products from our packaging policy, providing separate payment for these drugs regardless of their estimated per day costs through CY 2009. There are currently seven Level II HCPCS codes for 5-HT3 antiemetics that describe four different drugs, specifically dolasetron mesylate, granisetron hydrochloride, ondansetron hydrochloride, and palonosetron hydrochloride. Each of these drugs, except palonosetron hydrochloride, is available in both injectable and oral forms, so seven HCPCS codes are available to describe the four drugs in all of their forms. As of 2008, both ondansetron hydrochloride and granisetron hydrochloride were available in generic versions. We have now paid separately for all 5-HT3 antiemetics for 5 years under a policy that exempts these products from the drug packaging methodology. While we continue to believe that use of these antiemetics is an integral part of an anticancer treatment regimen and that OPPS claims data demonstrate their increasingly common hospital outpatient utilization, in the CY 2010 OPPS/ASC proposed rule (74 FR 35320), we indicated that we no longer believe that a specific exemption to our standard drug payment methodology is necessary for CY 2010 to ensure access to the most appropriate antiemetic product for Medicare beneficiaries.
We analyzed historical hospital outpatient claims data for the seven 5-HT3 antiemetic products that have been subject to this packaging exemption, and we found that HCPCS code J2405 (Injection, ondansetron hydrochloride, per 1 mg) was the dominant product used in the hospital outpatient setting both before and after the adoption of our 5-HT3 packaging exemption in CY 2005. Prior to this packaging exemption, payment for HCPCS code J2405 was packaged in CY 2004. HCPCS code J2405 was modestly costly relative to the other 5-HT3 antiemetics in CY 2004, but its per day cost still fell below the applicable packaging threshold of $50. Since CY 2005, the injectable form of ondansetron hydrochloride has experienced a significant change in its pricing structure as generic versions of the drug have become available, including a steady decline in its estimated per day cost. Notwithstanding this change in price, we have observed continued growth in its OPPS utilization. For CY 2008, HCPCS code J2405 was the least costly of the seven 5-HT3 antiemetics, with an estimated per day cost of only approximately $1 in CY 2008 (based on July 2008 ASP information), yet we observed that it constituted 88 percent of all treatment days of 5-HT3 antiemetics in the CY 2008 OPPS claims data. Using April 2009 ASP information for the CY 2010 proposed rule, we estimated a per day cost of only approximately $1 for HCPCS code J2405. For the five modestly priced 5-HT3 antiemetics, we estimated CY 2010 per day costs between approximately $7 and $50, while we estimated a per day cost for the most costly 5-HT3 antiemetic, J2469 (Injection, palonosetron hcl, 25 mcg), of $174 per day. In light of an anticipated relatively constant pricing structure for these drugs in CY 2010, combined with our experience that prescribing patterns for these 5-HT3 antiemetics are not very sensitive to changes in price, we did not believe that continuing to exempt these drugs from our standard OPPS drug packaging methodology was appropriate for CY 2010. Therefore, for CY 2010, because we proposed to no longer exempt the 5-HT3 antiemetic products from our standard packaging methodology, we proposed to package payment for all of the 5-HT3 antiemetics except palonosetron hydrochloride, consistent with their estimated per day costs from CY 2008 claims data.
At the August 2009 meeting of the APC Panel, the APC Panel recommended that when CMS changes the dollar amount of the drug packaging threshold and determines that some drugs within a single therapeutic class fall on either side of the packaging threshold, CMS consider packaging all of the drugs within that class on the basis of feedback from providers, the APC Panel, and stakeholders. Our response to this recommendation is included in our response to comments below.
Comment: The majority of commenters opposed the proposal to no longer continue to exempt the oral and injectable forms of 5-HT3 antiemetics from packaging, thereby packaging all but one 5-HT3 antiemetic. Many commenters requested that CMS continue to exempt all 5-HT3 antiemetics from the packaging methodology in order to preserve access to these products. The commenters expressed concern that hospitals may choose to only provide the separately payable antiemetic instead of the antiemetic that is most beneficial for the beneficiary. One commenter requested that CMS not finalize the CY 2010 proposal to apply the packaging threshold to 5-HT3 antiemetics until more information is available on the impact of packaging these products and to avoid unintended consequences, such as changes in prescribing practices, which may result from this policy.
However, several commenters expressed support for the proposed payment for 5-HT3 antiemetic products in the HOPD for CY 2010. These commenters stated that the majority of the products would be packaged under the proposal, and that would lead to reduced beneficiary copayments. The commenters offered their support due to the availability of lower-cost generic versions of some of the products and CMS' data analysis. The commenters also noted that the single product that would be paid separately under the proposal, HCPCS code J2469 (Injection, palonosetron hcl, 25 mcg), has unique properties that indicate separate payment would be appropriate.
Response: We continue to believe that use of these antiemetics is an integral part of an anticancer treatment regimen and that OPPS claims data demonstrate their increasingly common hospital outpatient utilization. As discussed above, our analysis for the CY 2010 OPPS/ASC proposed rule (74 FR 35320 through 35321) found that the most frequently used 5-HT3 antiemetic constituted 88 percent of all treatment days, and had an estimated per day cost of approximately $1 in CY 2008. The per day costs of other 5-HT3 antiemetics with per day costs below the CY 2010 drug packaging threshold of $65 (as discussed above) ranged from $8 to $51 per day. The single 5-HT3 antiemetic with a per day cost that exceeded the Start Printed Page 60488 CY 2010 drug packaging threshold is HCPCS code J2469.
As stated in the proposed rule (74 FR 35320), we no longer believe that a specific exemption to our standard drug payment methodology is necessary for CY 2010 to ensure access to the most appropriate antiemetic product for Medicare beneficiaries. We believe that our analysis, along with the historical stability in prescribing patterns and the availability of generic alternatives for several of these products, allows us to discontinue our policy of specifically exempting these products from the OPPS drug packaging threshold.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to apply the CY 2010 drug packaging threshold to all 5-HT3 antiemetics. We expect that packaging will encourage hospitals to use the most cost-efficient 5-HT3 antiemetic that is clinically appropriate. We also anticipate that hospitals will continue to provide care that is aligned with the best interests of the patient. We do not believe that our CY 2010 policy to apply the drug packaging threshold to 5-HT3 antiemetics will limit beneficiaries' ability to receive clinically appropriate drugs and biologicals. The final CY 2010 OPPS status indicators for 5-HT3 antiemetics are listed in Table 34 below.
| CY 2010 HCPCS code | CY 2010 long descriptor | CY 2010 SI |
|---|---|---|
| J1260 | Injection, dolasetron mesylate, 10 mg | N |
| J1626 | Injection, granisetron hydrochloride, 100 mcg | N |
| J2405 | Injection, ondansetron hydrochloride, per 1 mg | N |
| J2469 | Injection, palonosetron hcl, 25 mcg | K |
| Q0166 | Granisetron HCL, 1 mg, oral, FDA approved prescription antiemetic, for use as a complete therapeutic substitute for an IV antiemetic at the time of chemotherapy treatment, not to exceed a 24-hour dosage regimen | N |
| Q0179 | Ondansetron HCL 8 mg, oral, FDA approved prescription antiemetic, for use as a complete therapeutic substitute for an IV antiemetic at the time of chemotherapy treatment, not to exceed a 48-hour dosage regimen | N |
| Q0180 | Dolasetron mesylate, 100 mg, oral, FDA approved prescription antiemetic, for use as a complete therapeutic substitute for an IV antiemetic at the time of chemotherapy treatment, not to exceed a 24-hour dosage regimen | N |
Comment: One commenter suggested that CMS institute a packaging threshold exemption for antineoplastic agents and other anticancer therapeutic agents. The commenter believed that anticancer agents, as a class, are not appropriate for packaging because of the toxicity, side effects, interactions with other drugs, and level of patient specificity associated with these therapies. The commenter requested that CMS not apply the drug packaging threshold for anticancer agents and any product that is typically used in chemotherapy supportive care regimens. Instead, the commenter requested that CMS provide separate payment for all of these products in CY 2009.
In addition, several commenters requested that CMS apply the same principle to other groups of drugs in order to equalize payment methodologies across drugs in the same clinical category. One commenter suggested that CMS institute a similar policy for anticoagulant therapies provided in the HOPD. The commenter noted that in the group of anticoagulant therapies, the majority are packaged while one drug is paid separately. The commenter was concerned that these different payment methodologies provide hospitals an incentive to use the separately payable drug, although the commenter noted that treatments are not interchangeable and that benefits vary by patient.
Response: As we discussed in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66757) and the CY 2009 OPPS/ASC final rule with comment period (73 FR 68643), as we continue to explore the possibility of additional encounter-based or episode-based payment in future years, we may consider additional options for packaging drug payment in the future. For example, a higher drug packaging threshold could eliminate existing disparities in payment methodologies for other drug groups and provide similar methods of payment across items in a group. Nevertheless, as discussed in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68643), while we may be interested in alternative threshold methodologies for future ratesetting purposes, we realize that there are existing situations where drugs in a particular category vary in their payment treatment under the OPPS, with some drugs packaged and others separately paid.
We continue to believe the challenges associated with categorizing drugs to assess them for differences in their OPPS payment methodologies are significant, and we are not convinced that ensuring the same payment treatment for all drugs in any particular drug category is essential at this time. Therefore, we do not believe that it would be appropriate at this time to take any additional steps to ensure that all drugs in a specific category, including anticoagulants and antineoplastic agents, are all separately paid (or, alternatively, all packaged), as requested by some commenters.
While some commenters requested that we seek feedback from interested stakeholders when the packaging threshold creates a payment methodology disparity between drugs within a single therapeutic class, we note that we provide an opportunity through the annual OPPS/ASC rulemaking cycle for public comment on the proposed packaging status of drugs and biologicals for the next calendar year. Further, we regularly accept meeting requests from interested providers and stakeholders on a variety of issues, and we address APC Panel recommendations in our annual proposed and final rules. We have often received public comments related to our proposed packaging status for particular drugs and biologicals, and we expect to continue to receive public comments regarding the proposed packaging status for drugs and biologicals in the future. In this manner, we would address specific concerns about the proposed packaging status for individual drugs and biologicals in the future, including those within a single therapeutic class where some drugs may be proposed to be packaged while others are proposed to be separately payable. While we have not defined classes of drugs that may or Start Printed Page 60489 may not be affected by the packaging threshold, we are accepting the APC Panel recommendation to continue to seek feedback on the proposed packaging status of all drugs under the OPPS through the annual rulemaking process. However, implicit in the APC Panel's recommendation is that we consider packaging all drugs within a therapeutic class and, as described above, we have not defined classes of drugs for consideration in the context of proposed changes to the annual drug packaging threshold.
In summary, after consideration of the public comments we received, we are finalizing our proposed CY 2010 treatment of 5-HT3 antiemetics as follows. We are finalizing, without modification, our proposal to apply the drug packaging methodology to all 5-HT3 antiemetics for CY 2010. In addition, we are not providing any exceptions to the standard drug packaging methodology for any class of drugs, including anticoagulants and anticancer therapies, for CY 2010. Finally, we are accepting the APC Panel recommendation to continue to consider feedback from providers, the APC Panel, and stakeholders when finalizing the packaging status of drugs and biologicals.
Having specified our standard drug packaging methodology for all drugs and biologicals makes no exceptions for different drugs and biologicals in the same therapeutic class for CY 2010, we must adopt final packaging determinations for CY 2010 for each drug and biological for this final rule with comment period. Our policy during previous cycles of the OPPS has been to use updated ASP and claims data to make final determinations of the packaging status of HCPCS codes for drugs, nonimplantable biologicals, and therapeutic radiopharmaceuticals for the final rule with comment period. We note that it is also our policy to make an annual packaging determination for a HCPCS code only when we develop the OPPS/ASC final rule for the update year. Only HCPCS codes that are identified as separately payable in the final rule with comment period are subject to quarterly updates. For our calculation of per day costs of HCPCS codes for drugs and nonimplantable biologicals in the CY 2010 OPPS/ASC final rule with comment period, we proposed to use ASP data from the first quarter of CY 2009, which is the basis for calculating payment rates for drugs and biologicals in the physician's office setting using the ASP methodology, effective July 1, 2009, along with updated hospital claims data from CY 2008. We note that we also used these data for budget neutrality estimates and impact analyses for this CY 2010 OPPS/ASC final rule with comment period. Payment rates for HCPCS codes for separately payable drugs and nonimplantable biologicals included in Addenda A and B to this final rule with comment period are based on ASP data from the second quarter of CY 2009, which are the basis for calculating payment rates for drugs and biologicals in the physician's office setting using the ASP methodology, effective October 1, 2009. These rates would then be updated in the January 2010 OPPS update, based on the most recent ASP data to be used for physician's office and OPPS payment as of January 1, 2010. For items that do not currently have an ASP-based payment rate, we recalculated their mean unit cost from all of the CY 2008 claims data and updated cost report information available for the CY 2010 final rule with comment period to determine their final per day cost.
Consequently, the packaging status of some HCPCS codes for drugs, nonimplantable biologicals, and therapeutic radiopharmaceuticals in this CY 2010 OPPS/ASC final rule with comment period using the updated data may be different from the same drug HCPCS code's packaging status determined based on the data used for the proposed rule. Under such circumstances, in the CY 2010 OPPS/ASC proposed rule (74 FR 35320), we proposed to continue the established policies initially adopted for the CY 2005 OPPS (69 FR 65780) in order to more equitably pay for those drugs whose median cost fluctuates relative to the CY 2010 OPPS drug packaging threshold and the drug's payment status (packaged or separately payable) in CY 2009. Specifically, we proposed for CY 2010 to apply the following policies to these HCPCS codes for drugs, nonimplantable biologicals, and therapeutic radiopharmaceuticals whose relationship to the $65 drug packaging threshold changes based on the final updated data:
- HCPCS codes for drugs and nonimplantable biologicals that were paid separately in CY 2009 and that were proposed for separate payment in CY 2010, and then have per day costs equal to or less than $65, based on the updated ASPs and hospital claims data used for the CY 2010 final rule with comment period, would continue to receive separate payment in CY 2010.
- HCPCS codes for drugs and nonimplantable biologicals that were packaged in CY 2009 and that were proposed for separate payment in CY 2010, and then have per day costs equal to or less than $65, based on the updated ASPs and hospital claims data used for the CY 2010 final rule with comment period, would remain packaged in CY 2010.
- HCPCS codes for drugs and nonimplantable biologicals for which we proposed packaged payment in CY 2010 but then have per day costs greater than $65, based on the updated ASPs and hospital claims data used for the CY 2010 final rule with comment period, would receive separate payment in CY 2010.
We did not receive any public comments on our proposal to apply the established policies initially adopted for the CY 2005 OPPS (69 FR 65780) in order to more equitably pay for those drugs whose median cost fluctuates relative to the CY 2010 OPPS drug packaging threshold and the drug's payment status (packaged or separately payable) in CY 2009. Therefore, we are finalizing our proposal, without modification, for CY 2010.
We note that HCPCS codes J1652 (Injection, fondaparinux sodium, 0.5 mg), J2430(Injection, pamidronate disodium, per 30 mg); J7191 (Factor viii (antihemophilic factor (porcine)), per i.u.), J9165 (Injection, diethylstilbestrol diphosphate, 250 mg), and J9209 (Injection, mesna, 200 mg) were all paid separately in CY 2009 and were proposed for separate payment in CY 2010 but had final per day costs of less than the $65 drug packaging threshold, based on the updated ASPs and the CY 2008 hospital claims data available for this CY 2010 final rule with comment period. Therefore, HCPCS codes J1652, J2430, J7191, J9165 and J9209 will continue to be paid separately in CY 2010 according to the established methodology set forth above.
In addition, we proposed to provide separate payment for HCPCS codes J2670 (Injection, tolazoline HCL, up to 25 mg) and J3320 (Injection, spectinomycin dihydrochloride, up to 2 gm) in CY 2010, although their payment was packaged in CY 2009. Using updated ASPs and the CY 2008 hospital claims data available for this final rule with comment period, HCPCS codes J2670 and J3320 now have per day costs less than $65. In accordance with our established policy for such cases, for CY 2010 we will package payment for HCPCS codes J2670 and J3320.
Finally, we proposed to package HCPCS code Q2004 (Irrigation solution for treatment of bladder calculi, for example renacidin, per 500 ml) for CY 2010. Using updated ASPs and the CY 2008 hospital claims data available for this final rule with comment period, HCPCS code Q2004 now has a per day Start Printed Page 60490 cost greater than $65. In accordance with our established policy for such cases, for CY 2010 we will pay for HCPCS code Q2004 separately.
c. Packaging Determination for HCPCS Codes That Describe the Same Drug or Biological But Different Dosages
In the CY 2008 OPPS/ASC final rule with comment period (72 FR 66776), we began recognizing, for OPPS payment purposes, multiple HCPCS codes reporting different dosages for the same covered Part B drugs or biologicals in order to reduce hospitals' administrative burden by permitting them to report all HCPCS codes for drugs and biologicals. In general, prior to CY 2008, the OPPS recognized for payment only the HCPCS code that described the lowest dosage of a drug or biological. We extended this recognition to multiple HCPCS codes for several other drugs under the CY 2009 OPPS (73 FR 68665). During CYs 2008 and 2009, we applied a policy that assigned the status indicator of the previously recognized HCPCS code to the associated newly recognized code(s), reflecting the new code(s)' packaged or separately payable status. In the CY 2008 OPPS/ASC final rule with comment period (72 FR 66775), we explained that once claims data were available for these previously unrecognized HCPCS codes, we would determine the packaging status and resulting status indicator for each HCPCS code according to the general, established HCPCS code-specific methodology for determining a code's packaging status for a given update year. However, we also stated that we planned to closely follow our claims data to ensure that our annual packaging determinations for the different HCPCS codes describing the same drug or biological did not create inappropriate payment incentives for hospitals to report certain HCPCS codes instead of others.
CY 2008 is the first year of claims data for the HCPCS codes describing different dosages of the same drug or biological that were newly recognized in CY 2008. Applying our standard HCPCS code-specific packaging determination methodology as described in the CY 2010 OPPS/ASC proposed rule (74 FR 35321 through 35323), we found that our CY 2008 claims data would result in several different packaging determinations for different codes describing the same drug or biological. Furthermore, our claims data included few units and days for a number of these newly recognized HCPCS codes, resulting in our concern that these data reflected claims from only a small number of hospitals, even though the drug or biological itself may be reported by many other hospitals under the most common HCPCS code. We were concerned about proposing different packaging determinations for multiple HCPCS codes for the same drug or biological driven by different costs associated with the varying dosages of the same drug or biological and a small number of claims for the less common dosages that are not representative of the costs of all hospitals billing for the drug or biological. This is especially true when the general policy of the current CMS HCPCS Workgroup is to establish a single HCPCS code for a drug or biological, with a dosage that would allow accurate reporting of a patient dose for all anticipated clinical uses of the drug or biological.
Based on these findings from our first available claims data for the newly recognized HCPCS codes, in the CY 2010 OPPS/ASC proposed rule (74 FR 35321 through 35323) we explained that we believe that adopting our standard HCPCS code-specific packaging determinations for these codes could lead to payment incentives for hospitals to report certain HCPCS codes instead of others, particularly because we do not currently require hospitals to report all drug and biological HCPCS codes under the OPPS in consideration of our previous policy that generally recognized only the lowest dosage HCPCS code for a drug or biological for OPPS payment. Therefore, for CY 2010 we proposed to make packaging determinations on a drug-specific basis, rather than a HCPCS code-specific basis, for those HCPCS codes that describe the same drug or biological but different dosages. To identify all HCPCS codes for drugs and biologicals to which this proposed policy would apply, we first included the drugs and biologicals with multiple HCPCS codes that we newly recognized for payment in CY 2008 and CY 2009. We then reviewed all of the remaining drug and biological HCPCS codes to identify other drugs and biologicals for which longstanding OPPS policy recognized for payment multiple HCPCS codes for different dosages of the same drug or biological, so that our CY 2010 proposal would apply to the packaging determinations for these drugs and biologicals and their associated HCPCS codes. All of the drug and biological HCPCS codes that we proposed to be subject to this drug-specific packaging determination methodology were listed in Table 24 of the proposed rule (74 FR 35321 through 35323).
In order to propose a packaging determination that is consistent across all HCPCS codes that describe different dosages of the same drug or biological, we aggregated both our CY 2008 claims data and our pricing information at ASP+4 percent across all of the HCPCS codes that describe each distinct drug or biological in order to determine the mean units per day of the drug or biological in terms of the HCPCS code with the lowest dosage descriptor. We then multiplied the weighted average ASP+4 percent payment amount across all dosage levels of a specific drug or biological by the estimated units per day for all HCPCS codes that describe each drug or biological from our claims data to determine the estimated per day cost of each drug or biological at less than or equal to $65 (whereupon all HCPCS codes for the same drug or biological would be packaged) or greater than $65 (whereupon all HCPCS codes for the same drug or biological would be separately payable). The proposed packaging status of each drug and biological HCPCS code to which this methodology would apply was displayed in Table 24 of the proposed rule (74 FR 35321 through 35323).
Comment: Several commenters supported the proposal to make packaging determinations on a drug-specific basis rather than a HCPCS code-specific basis for drugs with multiple HCPCS codes describing different dosages.
Response: We continue to believe that adopting the standard HCPCS code-specific packaging determinations for these codes could lead to payment incentives for hospitals to report certain HCPCS codes for drugs instead of others. Making packaging determinations on a drug-specific basis eliminates these incentives and allows hospitals flexibility in choosing to report all HCPCS codes for different dosages of the same drug or only the lowest dosage HCPCS code.
Therefore, after consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to make a single packaging determination for a drug, rather than an individual HCPCS code, when a drug has multiple HCPCS codes describing different dosages. For CY 2010, we have aggregated both our CY 2008 claims data and our pricing information at ASP+4 percent across all of the HCPCS codes that describe each distinct drug or biological in order to determine the mean units per day of the drug or biological in terms of the HCPCS code with the lowest dosage descriptor. We then multiplied the weighted average ASP+4 percent payment amount across all dosage levels of a specific drug or biological by the estimated units per day for all HCPCS codes that Start Printed Page 60491 describe each drug or biological from our claims data to determine the estimated per day cost of each drug or biological at less than or equal to $65 (whereupon all HCPCS codes for the same drug or biological would be packaged) or greater than $65 (whereupon all HCPCS codes for the same drug or biological would be separately payable). The final CY 2010 packaging status of each drug and biological HCPCS code to which this methodology applies is displayed in Table 35 below.
We note that new HCPCS code Q2024 (Injection, bevacizumab, 0.25 mg) was implemented effective in October 2009 and represents a different dosage descriptor for the same drug described by HCPCS code J9035 (injection, bevacizumab, 10 mg). Further, HCPCS code Q2024 has been replaced with HCPCS code C9257 (Injection, bevacizumab, 0.25 mg), effective January 1, 2010. In accordance with our CY 2010 policy to make a single packaging determination for a single drug, we are applying the methodology described above to bevacizumab and are assigning the applicable bevacizumab HCPCS codes the same packaging status for CY 2010. HCPCS codes C9257 and J9035 are included in Table 35 below.
In addition, HCPCS codes J0530 (Injection, penicillin g benzathine and penicillin g procaine, up to 600,000 units); J0540 (Injection, penicillin g benzathine and penicillin g procaine, up to 1,200,000 units); and J0550 (Injection, penicillin g benzathine and penicillin g procaine, up to 2,400,000 units), have been replaced with HCPCS code J0559 (injection, penicillin G benzathine and penicillin G procaine, 2500 units) for CY 2010. While we had proposed to treat HCPCS codes J0530, J0540 and J0550 as drugs with multiple HCPCS codes and multiple dosage descriptors via the methodology finalized above, this is no longer necessary as there is a single code for this product in CY 2010. In order to make a packaging determination for new HCPCS code J0559, we used updated hospital claims data from HCPCS codes J0530, J0540 and J0550 and ASP pricing information to determine the estimated per day cost for the drug as described above. Because the estimated per day cost was less than our CY 2010 packaging threshold of $65, we assigned status indicator “N” to HCPCS code J0559 for CY 2010. We note that HCPCS codes J0530, J0540, and J0550 are not displayed in Table 35 below because there is only a single HCPCS code for the drug in CY 2010.
Finally, HCPCS codes J7502 (Cyclosporine, oral, 100 mg) and J7515 (Cyclosporine, oral, 25 mg) were proposed to be packaged in CY 2010 based on the methodology discussed above for drugs with multiple HCPCS codes with different dosage descriptors. As is our standard methodology, we use updated final rule data and updated ASP rates for purposes of this final rule with comment period to calculate per day estimates for final packaging determinations. Using this updated data and the multiple HCPCS code methodology discussed above, the per day cost of the drug described by HCPCS codes J7502 and J7515 would exceed the packaging threshold for CY 2010. Therefore, in accordance with the policy that was finalized in section V.B.2.b. above for HCPCS codes for drugs and nonimplantable biologicals for which we proposed packaged payment in CY 2010 but then have per day costs greater than $65, based on the updated ASPs and hospital claims data available for the CY 2010 final rule with comment period, HCPCS codes J7502 and J7515 are separately payable in CY 2010.
Start Printed Page 60492 Start Printed Page 60493 Start Printed Page 60494 Start Printed Page 60495d. Packaging of Payment for Diagnostic Radiopharmaceuticals, Contrast Agents, and Implantable Biologicals (“Policy-Packaged” Drugs and Devices)
Prior to CY 2008, the methodology of calculating a product's estimated per day cost and comparing it to the annual OPPS drug packaging threshold was used to determine the packaging status of drugs, biologicals, and radiopharmaceuticals under the OPPS (except for our CYs 2005 through 2009 exemption for 5-HT3 antiemetics). However, as established in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66766 through 66768), we began packaging payment for all diagnostic radiopharmaceuticals and contrast agents into the payment for the associated procedure, regardless of their per day costs. In addition, in CY 2009 we adopted a policy that packaged the payment for nonpass-through implantable biologicals into payment for the associated surgical procedure on the claim (73 FR 68633 through 68636). We refer to diagnostic radiopharmaceuticals and contrast agents collectively as “policy-packaged” drugs and to implantable biologicals as devices because we proposed to treat Start Printed Page 60496 implantable biologicals as devices for all OPPS payment purposes beginning in CY 2010.
According to our regulations at § 419.2(b), as a prospective payment system, the OPPS establishes a national payment rate that includes operating and capital-related costs that are directly related and integral to performing a procedure or furnishing a service on an outpatient basis including, but not limited to, implantable prosthetics, implantable durable medical equipment, and medical and surgical supplies. Packaging costs into a single aggregate payment for a service, encounter, or episode-of-care is a fundamental principle that distinguishes a prospective payment system from a fee schedule. In general, packaging the costs of items and services into the payment for the primary procedure or service with which they are associated encourages hospital efficiencies and also enables hospitals to manage their resources with maximum flexibility.
Prior to CY 2008, we noted that the proportion of drugs, biologicals, and radiopharmaceuticals that were separately paid under the OPPS had increased in recent years, a pattern that we also observed for procedural services under the OPPS. Our final CY 2008 policy that packaged payment for all nonpass-through diagnostic radiopharmaceuticals and contrast agents, regardless of their per day costs, contributed significantly to expanding the size of the OPPS payment bundles and is consistent with the principles of a prospective payment system.
We believe that packaging the payment for diagnostic radiopharmaceuticals and contrast agents into the payment for their associated procedures continues to be appropriate for CY 2010. As discussed in more detail in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68645 through 68649), we presented several reasons supporting our initial policy to package payment of diagnostic radiopharmaceuticals and contrast agents into their associated procedures on a claim. Specifically, we stated that we believed packaging was appropriate because: (1) The statutory requirement that we must pay separately for drugs and biologicals for which the per day cost exceeds $50 under section 1833(t)(16)(B) of the Act has expired; (2) we believe that diagnostic radiopharmaceuticals and contrast agents function effectively as supplies that enable the provision of an independent service; and (3) section 1833(t)(14)(A)(iii) of the Act requires that payment for specified covered outpatient drugs (SCODs) be set prospectively based on a measure of average hospital acquisition cost. For these reasons, we believed that our proposal to continue to treat diagnostic radiopharmaceuticals and contrast agents differently from other SCODs was appropriate for CY 2010. Therefore, in the CY 2010 OPPS/ASC proposed rule (74 FR 35323), we proposed to continue packaging payment for all contrast agents and diagnostic radiopharmaceuticals, collectively referred to as “policy-packaged” drugs, regardless of their per day costs, for CY 2010.
For more information on how we proposed to set CY 2010 payment rates for nuclear medicine procedures in which diagnostic radiopharmaceuticals are used and echocardiography services provided with and without contrast agents, we refer readers to sections II.A.2.d.(5) (74 FR 35276) and (4) (74 FR 35269), respectively, of the proposed rule and this final rule with comment period.
In CY 2009 (73 FR 68634), we began packaging the payment for all nonpass-through implantable biologicals into payment for the associated surgical procedure. Because implantable biologicals may sometimes substitute for nonbiological devices, we noted that if we were to provide separate payment for implantable biologicals without pass-through status, we would potentially be providing duplicate device payment, both through the packaged nonbiological device cost already included in the surgical procedure's payment and separate biological payment. We concluded that we saw no basis for treating implantable biological and nonbiological devices without pass-through status differently for OPPS payment purposes because both are integral to and supportive of the separately paid surgical procedures in which either may be used. Therefore, in CY 2009, we adopted a final policy to package payment for all nonpass-through implantable biologicals that are surgically inserted or implanted (through a surgical incision or a natural orifice), like our longstanding policy that packages payment for all implantable nonbiological devices without pass-through status.
For CY 2010, we continue to believe that the policy to package payment for implantable devices that are integral to the performance of separately paid procedures should also apply to payment for all implantable biologicals without pass-through status, when those biologicals function as implantable devices. Therefore, in the CY 2010 OPPS/ASC proposed rule (74 FR 35323), we proposed to continue to package payment for nonpass-through implantable biologicals that are surgically inserted or implanted (through a surgical incision or a natural orifice) into the body, referred to as devices, in CY 2010. In accordance with this proposal, two of the products with expiring pass-through status for CY 2010 are biologicals that are solely surgically implanted according to their FDA-approved indications. These products are described by HCPCS codes C9354 (Acellular pericardial tissue matrix of non-human origin (Veritas), per square centimeter) and C9355 (Collagen nerve cuff (NeuroMatrix), per 0.5 centimeter length). Like the three implantable biologicals with expiring pass-through status in CY 2009 that were discussed in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68633 through 68634), we believe that the two biologicals specified above with expiring pass-through status for CY 2010 differ from other biologicals paid under the OPPS in that they specifically function as surgically implanted devices. As a result of the proposed CY 2010 packaged payment methodology for all nonpass-through implantable biologicals, we proposed to package payment for HCPCS codes C9354 and C9355 and assign them status indicator “N” for CY 2010. In addition, any new biologicals without pass-through status that are surgically inserted or implanted (through a surgical incision or a natural orifice) would be packaged in CY 2010. Moreover, for nonpass-through biologicals that may sometimes be used as implantable devices, we continue to instruct hospitals to not bill separately for the HCPCS codes for the products when used as implantable devices. This reporting ensures that the costs of these products that may be, but are not always, used as implanted biologicals are appropriately packaged into payment for the associated implantation procedures.
Comment: Several commenters objected to CMS' proposal to package payment for all diagnostic radiopharmaceuticals and contrast agents in CY 2010. A number of commenters stated that diagnostic radiopharmaceuticals and contrast agents with per day costs over the proposed OPPS drug packaging threshold are defined as SCODs and, therefore, should be assigned separate APC payments. In particular, the commenters questioned CMS' authority to classify groups of drugs, such as diagnostic radiopharmaceuticals and contrast agents, and implement packaging and payment policies that do Start Printed Page 60497 not reflect their status as SCODs. Some commenters stressed that hospitals consider radiopharmaceuticals to be drugs, rather than supplies, and that these products are not interchangeable for patients receiving specific nuclear medicine scans. The commenters recommended that diagnostic radiopharmaceuticals should be subject to the same per day cost drug packaging threshold that applies to other drugs, in order to determine whether their payment would be packaged or made separately.
In addition, the commenters objected to the proposal to package payment for diagnostic radiopharmaceuticals and contrast agents because, as SCODs, the commenters believed these products were required by statute to be paid at average acquisition cost. The commenters explained that, when several different diagnostic radiopharmaceuticals or contrast agents may be used for a particular procedure, the costs of those diagnostic radiopharmaceuticals or contrast agents are averaged together and added to the cost for the procedure in order to determine the payment rate for the associated procedural APC. Therefore, the commenters argued that the amount added to the procedure cost through packaging, representing the cost of the diagnostic radiopharmaceutical or contrast agent, did not reflect the average acquisition cost of any one particular item but, rather, reflected the average cost of whatever items may have been used with that particular procedure.
Finally, one commenter requested clarification on when CMS treats an implantable device as a biological for payment purposes.
Response: As discussed in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66766), the CY 2009 OPPS/ASC final rule with comment period (73 FR 68645) and the CY 2010 OPPS/ASC proposed rule (74 FR 35323), we continue to believe diagnostic radiopharmaceuticals and contrast agents are different from other drugs and biologicals for several reasons. We note that the statutorily required OPPS drug packaging threshold has expired, and we continue to believe that diagnostic radiopharmaceuticals and contrast agents are always ancillary and supportive to an independent service, rather than serving themselves as the therapeutic modality. We packaged their payment in CYs 2008 and 2009 as ancillary and supportive services in order to provide incentives for greater efficiency and to provide hospitals with additional flexibility in managing their resources. In order for payment to be packaged, it is not necessary that all products be interchangeable in every case, and we recognize that in some cases hospitals may utilize higher cost products and in some cases lower cost products, taking into consideration the clinical needs of the patient and efficiency incentives. While we recognize this variability from case to case, on average under a prospective payment system we expect payment to pay appropriately for the services furnished. In the past, we have classified different groups of drugs for specific payment purposes, as evidenced by our CY 2005 through CY 2009 policy regarding 5–HT3 anti-emetics and their exemption from the drug packaging threshold. We note that we treat diagnostic radiopharmaceuticals and contrast agents as “policy-packaged” drugs because our policy is to package payment for all of the products in the category.
In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68634), we also began packaging the payment for all nonpass-through implantable biologicals into payment for the associated surgical procedure because we consider these products to always be ancillary and supportive to independent services, just like implantable nonbiological devices that are always packaged. Therefore, we currently package payment for nonpass-through implantable biologicals, also known as devices, that are surgically inserted or implanted (through a surgical incision or a natural orifice) into the body. As we stated in the CY 2010 OPPS/ASC proposed rule (74 FR 35324), we continue to believe that payment should be packaged for nonpass-through implantable biologicals for CY 2010.
Although our final CY 2009 policy that we are continuing for CY 2010, as discussed below, packages payment for all diagnostic radiopharmaceuticals, contrast agents, and nonpass-through implantable biologicals into the payment for their associated procedures, we are continuing to provide payment for these items in CY 2010 based on a proxy for average acquisition cost just as we did in CY 2009. We continue to believe that the line-item estimated cost for a diagnostic radiopharmaceutical, contrast agent, or nonpass-through implantable biological in our claims data is a reasonable approximation of average acquisition and preparation and handling costs for diagnostic radiopharmaceuticals, contrast agents, and nonpass-through implantable biologicals, respectively. As we discussed in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68645), we believe that hospitals have adapted to the CY 2006 coding changes for radiopharmaceuticals and responded to our instructions to include charges for radiopharmaceutical handling in their charges for the radiopharmaceutical products. Further, because the standard OPPS packaging methodology packages the total estimated cost of each radiopharmaceutical, contrast agent, or nonimplantable biological on each claim (including the full range of costs observed on the claims) with the cost of associated procedures for ratesetting, this packaging approach is consistent with considering the average cost for radiopharmaceuticals, contrast agents, or nonpass-through implantable biologicals, rather than the median cost. In addition, as we noted in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68646), these drugs, biologicals, or radiopharmaceuticals for which we have not established a separate APC and, therefore, for which payment would be packaged rather than separately provided under the OPPS, could be considered to not be SCODs. Similarly, drugs and biologicals with per day costs of less than $65 in CY 2010 that are packaged and for which a separate APC has not been established also would not be SCODs. This reading is consistent with our final payment policy whereby we package payment for diagnostic radiopharmaceuticals, contrast agents, and nonpass-through implantable biologicals and provide payment for these products through payment for their associated procedures.
Comment: Several commenters disagreed with the proposal to distinguish between diagnostic and therapeutic radiopharmaceuticals for payment purposes under the OPPS. The commenters noted that CMS' identification of HCPCS codes A9542 (Indium In-111 ibritumomabituxetan, diagnostic, per study dose, up to 5 millicuries) and A9544 (Iodine I–131 tositumomab, diagnostic, per study dose) as diagnostic radiopharmaceuticals was inappropriate because these radiopharmaceuticals function as dosimetric radiopharmaceuticals, and they both have higher than average costs associated with their acquisition and significant compounding costs in comparison with other nuclear medicine imaging agents. A few commenters explained that these radiopharmaceutical products are used as part of a therapeutic regimen and, therefore, should be considered therapeutic for OPPS payment purposes. Start Printed Page 60498
Response: As discussed above, and in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66641), the CY 2009 OPPS/ASC final rule with comment period (73 FR 68645) and the CY 2010 OPPS/ASC proposed rule (74 FR 35323), we classified each radiopharmaceutical into one of two groups according to whether its long descriptor contained the term “diagnostic” or “therapeutic.” HCPCS codes A9542 and A9544 both contain the term “diagnostic” in their long code descriptors. Therefore, according to our established methodology, we continue to classify them as diagnostic for the purposes of CY 2010 OPPS payment. While we understand that these items are provided in conjunction with additional supplies, imaging tests, and therapeutic radiopharmaceuticals for patients already diagnosed with cancer, we continue to believe that the purpose of administering the products described by HCPCS codes A9542 and A9544 is diagnostic in nature. As we first stated in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66641), we continue to believe that HCPCS codes A9542 and A9544 are diagnostic radiopharmaceuticals. While they are not used to diagnose disease, they are used to determine whether future therapeutic services would be beneficial to the patient and to determine how to proceed with therapy. While a group of associated services may be considered a therapeutic regimen by some commenters, HCPCS codes A9542 and A9544 are provided in conjunction with a series of nuclear medicine imaging scans. Many nuclear medicine studies using diagnostic radiopharmaceuticals are provided to patients who already have an established diagnosis. We continue to consider HCPCS codes A9542 and A9544 to be diagnostic because these items are provided for the purpose of a diagnostic imaging procedure and are used to identify the proper dose of the therapeutic agent to be provided at a later time.
Comment: Some commenters recommended using the ASP methodology to package payment for nonpass-through diagnostic radiopharmaceuticals, noting that it would be inconsistent for CMS to provide payment for diagnostic radiopharmaceuticals that have pass-through status based on the ASP methodology, and then, after the diagnostic radiopharmaceutical's pass-through payment status expires, package the costs included in historical hospital claims data, rather than using the ASP methodology to pay for the product. The commenters believed that the ASP methodology would be more reflective of actual diagnostic radiopharmaceutical costs and would not be subject to the billing inconsistencies that are present in hospital claims data. Therefore, the commenters concluded that it would be illogical to transition from an accurate methodology to estimate hospital costs (such as the ASP methodology) to a less accurate methodology (based on hospital claims data) once a product is packaged after its pass-through payment expires.
Response: While we understand the commenters' request for the continued use of ASP data for purposes of packaging costs after a diagnostic radiopharmaceutical's pass-through payment period has ended, based on their belief that ASP data are more accurate than hospital claims data, we continue to believe that hospitals have the ability to identify and set charges for any new diagnostic radiopharmaceutical product accurately during its 2 to 3 year pass-through time period while the product has the potential to be paid based on ASP. Packaging hospital costs based on hospital claims data is how the costs of all packaged items are factored into payment rates for associated procedures under the OPPS. We believe that the costs reported on claims, as determined by hospitals, are the most appropriate representation of the costs of diagnostic radiopharmaceuticals that should be packaged into payment for the associated nuclear medicine procedures.
We recognize that radiopharmaceuticals are specialized products that have unique costs associated with them. However, we believe that the costs are reflected in the charges that hospitals set for them and in the Medicare cost report where the full costs and charges associated with the services are reported. Therefore, the packaged costs of diagnostic radiopharmaceuticals are calculated like any other OPPS costs and packaged into the cost of the nuclear medicine service to which they are ancillary and supportive. This methodology is the basis for the payment of nuclear medicine procedures in the same way that other packaged costs contribute to the payment rates for the services to which they are an integral part.
Comment: Some commenters believed that packaging payment for diagnostic radiopharmaceuticals and nonpass-through implantable biologicals would undermine the clinical and resource homogeneity in the various procedural APCs, resulting in 2 times violations.
Response: As we stated in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68647), we agree that packaging the costs of ancillary and supportive services into the cost of an independent service can change the median cost for that service and could result in 2 times violations. However, we disagree that we should refrain from packaging payment for ancillary and supportive items into the payment for the service in which they are used in order to prevent the occurrence of 2 times violations. Instead, we believe that we should reconfigure APCs when necessary to resolve 2 times violations where they occur. As we discussed in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68647), because we have traditionally paid for a service package under the OPPS as represented by a HCPCS code for the major procedure that is assigned to an APC group for payment, we assess the applicability of the 2 times rule to services at the HCPCS code level, not at a more specific level based on the individual diagnostic radiopharmaceuticals or nonpass-through implantable biologicals that may be utilized in a service reported with a single HCPCS code. Furthermore, if the use of a very expensive diagnostic radiopharmaceutical in a clinical scenario causes a specific procedure to be much more expensive for the hospital than the APC payment, we consider such a case to be the natural consequence of a prospective payment system that anticipates that some cases will be more costly and others less costly than the procedure payment. This same logic would apply to situations in which a nonpass-through implantable biological is implanted in a surgical procedure and results in an increase in a procedure's cost to the hospital for an individual case. In addition, very high cost cases could be eligible for outlier payment. As we note elsewhere in this final rule with comment period, decisions about packaging and bundling payment involve a balance between ensuring some separate payment for individual services and establishing incentives for efficiency through larger units of payment. In the case of diagnostic radiopharmaceuticals and nonpass-through implantable biologicals, these products are part of the OPPS payment package for the procedures in which they are used. We refer readers to section II.A.d.(5) of this final rule with comment period for a discussion of payment for nuclear medicine procedures.
Comment: One commenter recommended that CMS provide separate payment for all diagnostic radiopharmaceuticals with a median per Start Printed Page 60499 day cost greater than $200. The commenter believes that this recommendation is most consistent with the APC Panel's recommendation to CMS at the September 2007 APC Advisory Panel meeting.
Response: At the September 2007 APC Panel meeting, the APC Panel recommended that CMS package radiopharmaceuticals with a median per day cost of less than $200 but pay separately for radiopharmaceuticals with a per day cost of $200 or more. In the CY 2008 OPPS/ASC final rule with comment period (72 FR 66638), we did not accept the APC Panel's recommendation, citing an inability to determine an empirical basis for paying separately for radiopharmaceuticals with a per day cost in excess of $200. Instead, as proposed, for CY 2008 we finalized the packaging of payment for all diagnostic radiopharmaceuticals. We continue to believe that diagnostic radiopharmaceuticals are ancillary and supportive to the nuclear medicine procedures in which they are used and that their costs should be packaged into the primary procedures with which they are associated. We do not believe it would be appropriate to set a cost threshold for packaging diagnostic radiopharmaceuticals because, regardless of their per day cost, they are always supportive of an independent procedure that is the basis for administration of the diagnostic radiopharmaceutical.
After consideration of the public comments we received, we are finalizing our CY 2010 proposals, without modification, to continue to package payment for all nonpass-through diagnostic radiopharmaceuticals, contrast agents, and implantable biologicals that are surgically inserted or implanted into the body, regardless of their per day costs. Given the inherent function of contrast agents and diagnostic radiopharmaceuticals as ancillary and supportive to the performance of an independent procedure and the similar functions of implantable biological and nonbiological devices, we continue to view the packaging of payment for contrast agents, diagnostic radiopharmaceuticals, and implantable biologicals as a logical expansion of packaging payment for drugs and biologicals. In addition, as we initially established in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66768), we are finalizing our proposal to continue to identify diagnostic radiopharmaceuticals specifically as those Level II HCPCS codes that include the term “diagnostic” along with a radiopharmaceutical in their long code descriptors, and therapeutic radiopharmaceuticals as those Level II HCPCS codes that include the terms “therapeutic” along with a radiopharmaceutical in their long code descriptors. For more information on how we set CY 2010 payment rates for nuclear medicine procedures in which diagnostic radiopharmaceuticals are used and echocardiography services provided with and without contrast agents, we refer readers to section II.A.2.d (5) and (4), respectively, of this final rule with comment period.
3. Payment for Drugs and Biologicals Without Pass-Through Status That Are Not Packaged
a. Payment for Specified Covered Outpatient Drugs (SCODs) and Other Separately Payable and Packaged Drugs and Biologicals
Section 1833(t)(14) of the Act defines certain separately payable radiopharmaceuticals, drugs, and biologicals and mandates specific payments for these items. Under section 1833(t)(14)(B)(i) of the Act, a “specified covered outpatient drug” is a covered outpatient drug, as defined in section 1927(k)(2) of the Act, for which a separate APC has been established and that either is a radiopharmaceutical agent or is a drug or biological for which payment was made on a pass-through basis on or before December 31, 2002.
Under section 1833(t)(14)(B)(ii) of the Act, certain drugs and biologicals are designated as exceptions and are not included in the definition of “specified covered outpatient drugs,” known as SCODs. These exceptions are—
- A drug or biological for which payment is first made on or after January 1, 2003, under the transitional pass-through payment provision in section 1833(t)(6) of the Act.
- A drug or biological for which a temporary HCPCS code has not been assigned.
- During CYs 2004 and 2005, an orphan drug (as designated by the Secretary).
Section 1833(t)(14)(A)(iii) of the Act requires that payment for SCODs in CY 2006 and subsequent years be equal to the average acquisition cost for the drug for that year as determined by the Secretary, subject to any adjustment for overhead costs and taking into account the hospital acquisition cost survey data collected by the Government Accountability Office (GAO) in CYs 2004 and 2005. If hospital acquisition cost data are not available, the law requires that payment be equal to payment rates established under the methodology described in section 1842(o), section 1847A, or section 1847B of the Act, as calculated and adjusted by the Secretary as necessary.
Section 1833(t)(14)(E) of the Act provides for an adjustment in OPPS payment rates for overhead and related expenses, such as pharmacy services and handling costs. Section 1833(t)(14)(E)(i) of the Act required MedPAC to study pharmacy overhead and to make recommendations to the Secretary regarding whether, and if so how, a payment adjustment should be made to compensate hospitals for them. Section 1833(t)(14)(E)(ii) of the Act authorizes the Secretary to adjust the weights for ambulatory procedure classifications for SCODs to take into account the findings of the MedPAC study.
In the CY 2006 OPPS proposed rule (70 FR 42728), we discussed the June 2005 report by MedPAC regarding pharmacy overhead costs in HOPDs and summarized the findings of that study:
- Handling costs for drugs, biologicals, and radiopharmaceuticals administered in the HOPD are not insignificant;
- Little information is available about the magnitude of pharmacy overhead costs;
- Hospitals set charges for drugs, biologicals, and radiopharmaceuticals at levels that reflect their respective handling costs; and
- Hospitals vary considerably in their likelihood of providing services which utilize drugs, biologicals, or radiopharmaceuticals with different handling costs.
As a result of these findings, MedPAC developed seven drug categories for pharmacy and nuclear medicine handling costs based on the estimated level of hospital resources used to prepare the products (70 FR 42729). Associated with these categories were two recommendations for accurate payment of pharmacy overhead under the OPPS.
1. CMS should establish separate, budget neutral payments to cover the costs hospitals incur for handling separately payable drugs, biologicals, and radiopharmaceuticals.
2. CMS should define a set of handling fee APCs that group drugs, biologicals, and radiopharmaceuticals based on attributes of the products that affect handling costs; CMS should instruct hospitals to submit charges for these APCs and base payment rates for the handling fee APCs on submitted charges reduced to costs.
In response to the MedPAC findings, in the CY 2006 OPPS proposed rule (70 FR 42729), we discussed our belief that, because of the varied handling resources Start Printed Page 60500 required to prepare different forms of drugs, it would be impossible to exclusively and appropriately assign a drug to a certain overhead category that would apply to all hospital outpatient uses of the drug. Therefore, our CY 2006 OPPS proposal included a proposal to establish three distinct Level II HCPCS C-codes and three corresponding APCs for drug handling categories to differentiate overhead costs for drugs and biologicals (70 FR 42730). We also proposed: (1) To combine several overhead categories recommended by MedPAC; (2) to establish three drug handling categories, as we believed that larger groups would minimize the number of drugs that may fit into more than one category and would lessen any undesirable payment policy incentives to utilize particular forms of drugs or specific preparation methods; (3) to collect hospital charges for these HCPCS C-codes for 2 years; and (4) to ultimately base payment for the corresponding drug handling APCs on CY 2006 claims data available for the CY 2008 OPPS.
In the CY 2006 OPPS final rule with comment period (70 FR 68659 through 68665), we discussed the public comments we received on our proposal regarding pharmacy overhead. The overwhelming majority of commenters did not support our proposal and urged us not to finalize this policy, as it would be administratively burdensome for hospitals to establish charges for HCPCS codes for pharmacy overhead and to report them. Therefore, we did not finalize this proposal for CY 2006. Instead, we established payment for separately payable drugs and biologicals at ASP+6 percent, which we calculated by comparing the estimated aggregate cost of separately payable drugs and biologicals in our claims data to the estimated aggregate ASP dollars for separately payable drugs and biologicals, using the ASP as a proxy for average acquisition cost (70 FR 68642). Hereinafter, we refer to this methodology as our standard drug payment methodology. We concluded that payment for drugs and biologicals and pharmacy overhead at a combined ASP+6 percent rate would serve as the best proxy for the combined acquisition and overhead costs of each of these products.
In the CY 2007 OPPS/ASC final rule with comment period (71 FR 68091), we finalized our proposed policy to provide a single payment of ASP+6 percent for the hospital's acquisition cost for the drug or biological and all associated pharmacy overhead and handling costs. The ASP+6 percent rate that we finalized was higher than the equivalent average ASP-based amount calculated from claims of ASP+4 percent according to our standard drug payment methodology, but we adopted payment at ASP+6 percent for stability while we continued to examine the issue of the costs of pharmacy overhead in the HOPD.
In the CY 2008 OPPS/ASC proposed rule (72 FR 42735), in response to ongoing discussions with interested parties, we proposed to continue our methodology of providing a combined payment rate for drug and biological acquisition and pharmacy overhead costs. We also proposed to instruct hospitals to remove the pharmacy overhead charge for both packaged and separately payable drugs and biologicals from the charge for the drug or biological and report the pharmacy overhead charge on an uncoded revenue code line on the claim. We believed that this would provide us with an avenue for collecting pharmacy handling cost data specific to drugs in order to package the overhead costs of these items into the associated procedures, most likely drug administration services. Similar to the public response to our CY 2006 pharmacy overhead proposal, the overwhelming majority of commenters did not support our CY 2008 proposal and urged us to not finalize this policy (72 FR 66761). At its September 2007 meeting, the APC Panel recommended that hospitals not be required to separately report charges for pharmacy overhead and handling and that payment for overhead be included as part of drug payment. The APC Panel also recommended that CMS continue to evaluate alternative methods to standardize the capture of pharmacy overhead costs in a manner that is simple to implement at the organizational level (72 FR 66761). Because of concerns expressed by the APC Panel and public commenters, we did not finalize the proposal to instruct hospitals to separately report pharmacy overhead charges for CY 2008. Instead, in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66763), we finalized a policy of providing payment for separately payable drugs and biologicals and their pharmacy overhead at ASP+5 percent as a transition from their CY 2007 payment of ASP+6 percent to payment based on the equivalent average ASP-based payment rate calculated from hospital claims according to our standard drug payment methodology, which was ASP+3 percent for the CY 2008 OPPS/ASC final rule with comment period. Hospitals continued to include charges for pharmacy overhead costs in the line-item charges for the associated drugs reported on claims.
For CY 2009, we proposed to pay separately payable drugs and biologicals at ASP+4 percent, including both SCODs and other drugs without CY 2009 OPPS pass-through status, based on our standard drug payment methodology, and we also proposed to split the “Drugs Charged to Patients” cost center into two cost centers: One for drugs with high pharmacy overhead costs and one for drugs with low pharmacy overhead costs (73 FR 41492). We noted that we expected that CCRs from the proposed new cost centers would be available in 2 to 3 years to refine OPPS drug cost estimates by accounting for differential hospital markup practices for drugs with high and low overhead costs. After consideration of the public comments received and the APC Panel recommendations, we finalized a CY 2009 policy (73 FR 68659) to provide payment for separately payable nonpass-through drugs and biologicals based on costs calculated from hospital claims at a 1-year transitional rate of ASP+4 percent, in the context of an equivalent average ASP-based payment rate of ASP+2 percent calculated according to our standard drug payment methodology from the final rule claims and cost report data. We did not finalize our proposal to split the single standard “Drugs Charged to Patients” cost center into two cost centers largely due to concerns raised to us by hospitals about the associated administrative burden. Instead, we indicated in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68659) that we would continue to explore other potential approaches to improve our drug cost estimation methodology, thereby increasing payment accuracy for separately payable drugs and biologicals.
In response to the CMS proposals for the CY 2008 and CY 2009 OPPS, a group of pharmacy stakeholders (hereinafter referred to as the pharmacy stakeholders), including some cancer hospitals, some pharmaceutical manufacturers, and some hospital and professional associations, commented that CMS should pay an acquisition cost of ASP+6 percent for separately payable drugs, should substitute ASP+6 percent for the packaged cost of all packaged drugs and biologicals on procedure claims, and should redistribute the difference between the aggregate estimated packaged drug cost in claims and payment for all drugs, including packaged drugs at ASP+6 percent, as separate pharmacy overhead payments for separately payable drugs. They Start Printed Page 60501 indicated that this approach would preserve the aggregate drug cost observed in the claims data, while significantly increasing payment accuracy for individual drugs and procedures using packaged drugs. Their suggested approach would provide a separate overhead payment for each separately payable drug or biological at one of three different levels, depending on the pharmacy stakeholders' assessment of the complexity of pharmacy handling associated with each specific drug or biological (73 FR 68651 through 68652). Each separately payable drug or biological HCPCS code would be assigned to one of the three overhead categories, and the separate pharmacy overhead payment applicable to the category would be made when each of the separately payable drugs or biologicals was paid.
At the February 2009 APC Panel meeting, the APC Panel recommended that CMS pay for the acquisition cost of all separately payable drugs at no less than ASP+6 percent. The APC Panel also recommended that CMS package payment at ASP+6 percent on claims for all drugs that are not separately payable and use the difference between these rates and CMS' cost derived from charges to create a pool to provide more appropriate payment for pharmacy service costs and that CMS pay for pharmacy services costs using this pool, applying a tiered approach to payments based on some objective criteria related to the pharmacy resources required for groups of drugs. The APC Panel further recommended that, if CMS does not implement the drug payment recommendations specified above, CMS should exclude data from hospitals that participate in the 340B Federal drug pricing program from its ratesetting calculations for drugs and CMS should pay 340B hospitals in the same manner as it pays non-340B hospitals. Hospitals that participate in the 340B program are generally hospitals that serve a disproportionate share of low-income patients and receive disproportionate share payments under the IPPS. These facilities may acquire outpatient drugs and biologicals at prices that are substantially below ASP because the 340B program requires drug manufacturers to provide outpatient drugs to eligible entities at a reduced price and these reduced price sales are not included in the ASP submissions of manufacturers to Medicare. Public presenters at the February 2009 APC Panel meeting emphasized that the purpose of the 340B Federal drug pricing program is to ensure access to drugs for low-income patients by supplementing the higher cost of providing care to low-income patients born by hospitals serving a disproportionate share of these patients. The agenda, recommendations, and report from the February 2009 APC Panel meeting are posted on the CMS Web site at: http://www.cms.hhs.gov/FACA. We respond to these APC Panel recommendations in our discussion of the proposed CY 2010 policy that follows.
b. Payment Policy
Section 1833(t)(14)(A)(iii) of the Act, as described above, continues to be applicable to determining payments for SCODs for CY 2010. This provision requires that payment for SCODs be equal to the average acquisition cost for the drug for that year as determined by the Secretary, subject to any adjustment for overhead costs and taking into account the hospital acquisition cost survey data collected by the GAO in CYs 2004 and 2005. If hospital acquisition cost data are not available, the law requires that payment be equal to payment rates established under the methodology described in section 1842(o), section 1847A, or section 1847B of the Act, as calculated and adjusted by the Secretary as necessary. In addition, section 1833(t)(14)(E)(ii) of the Act authorizes the Secretary to adjust APC weights to take into account the 2005 MedPAC report relating to overhead and related expenses, such as pharmacy services and handling costs. Since CY 2006, when we first adopted our standard methodology of paying for separately payable drugs and biologicals based on the equivalent average ASP-based payment rate calculated from claims and cost report data, we have applied this methodology to payment for all separately payable drugs and biologicals without pass-through status, both SCODs and other drugs and biologicals that do not meet the statutory definition of SCODs. We have seen no reason to distinguish SCODs from these other separately payable drugs and biologicals, and under our standard drug payment methodology, we have used ASP data and costs estimated from charges on hospital claims data as a proxy for the average hospital acquisition cost that the statute requires for payment of SCODs and to provide payment for the associated pharmacy overhead cost.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35332), we proposed to redistribute between one-third and one-half of the difference between the aggregate claims cost for coded packaged drugs and biologicals with an ASP and ASP dollars for those products, which resulted in our proposal to pay for the acquisition and pharmacy overhead costs of separately payable drugs and biologicals that did not have pass-through payment status at ASP+4 percent. Based on the rationale described in the CY 2010 OPPS/ASC proposed rule (74 FR 35326 through 35333), we believed that approximately $150 million of the estimated $395 million total in pharmacy overhead cost included in our claims data for coded packaged drugs and biologicals with an ASP above the aggregate ASP dollars of these packaged products should be attributed to separately payable drugs and biologicals to provide an adjustment for the pharmacy overhead costs of these separately payable products. As a result, we also proposed to reduce the cost of these packaged drugs and biologicals that is included in the payment for procedural APCs to offset the $150 million adjustment to payment for separately payable drugs and biologicals. In addition, we proposed that any redistribution of pharmacy overhead cost that may arise from CY 2010 final rule data would occur only from coded packaged drugs and biologicals with an ASP to separately payable drugs and biologicals, thereby maintaining the estimated total cost of drugs and biologicals.
Using our CY 2010 proposed rule data, and applying our longstanding methodology for calculating the total cost of separately payable drugs and biologicals in our claims compared to the ASP dollars for the same drugs and biologicals, without applying the proposed overhead cost redistribution, we determined that the estimated aggregate cost of separately payable drugs and biologicals (status indicators “K” and “G”), including acquisition and pharmacy overhead costs, was equivalent to ASP–2 percent. Therefore, under our standard drug payment methodology, we would pay for separately payable drugs and biologicals at ASP–2 percent for CY 2010, their equivalent average ASP-based payment rate. We also determined that the estimated aggregate cost of coded packaged drugs and biologicals with an ASP (status indicator “N”), including acquisition and pharmacy overhead costs, was equivalent to ASP+247 percent. We found that the estimated aggregate cost for all coded drugs and biologicals (status indicators “N,” “K,” and “G), including acquisition and pharmacy overhead costs, was equivalent to ASP+13 percent. For a detailed explanation of our standard process for these calculations, we refer Start Printed Page 60502 readers to the CY 2006 OPPS proposed rule (70 FR 42725).
| Total ASP dollars for drugs and biologicals in claims data (in millions)* | Total cost of drugs and biologicals in claims data (in millions)** | Ratio of cost to ASP | ASP+X percent | |
|---|---|---|---|---|
| Coded Packaged Drugs and Biologicals with an ASP | $160 | $555 | 3.47 | ASP+247 |
| Separately Payable Drugs and Biologicals | 2,589 | 2,539 | 0.98 | ASP–2 |
| All Coded Drugs and Biologicals | 2,749 | 3,094 | 1.13 | ASP+13 |
| *Total April 2009 ASP dollars (ASP multiplied by drug or biological units in CY 2008 claims) for drugs and biologicals with a HCPCS code and ASP information. | ||||
| **Total cost in the CY 2008 claims data for drugs and biologicals with a HCPCS code and April 2009 ASP information. | ||||
In the proposed rule, we recognized that there may be concern over whether the actual full cost (acquisition and pharmacy overhead) of separately payable drugs and biologicals could be 2 percent less than ASP for these products (74 FR 35327), although we did not have ASP information specifically for their sales to hospitals. Similarly, we acknowledged that a full cost (acquisition and pharmacy overhead) of ASP+247 percent for coded packaged drugs and biologicals with an ASP seemed relatively high. When we subtracted the total ASP dollars for packaged drugs and biologicals with a reported ASP amount in the CY 2008 claims data ($160 million), our proxy for their acquisition cost, from the total cost of packaged drugs and biologicals in the same claims ($555 million), we found that the difference, which we viewed as the pharmacy overhead cost currently attributed to packaged drugs and biologicals was $395 million. While we had no way of assessing whether this current distribution of overhead cost to coded packaged drugs and biologicals with an ASP was appropriate, we acknowledged that the established method of converting billed charges to costs had the potential to “compress” the calculated costs to some degree. Further, we recognized that the attribution of pharmacy overhead costs to packaged or separately payable drugs and biologicals through our standard drug payment methodology of a combined payment for acquisition and pharmacy overhead costs depends, in part, on the treatment of all drugs and biologicals each year under our annual drug packaging threshold. Changes to the packaging threshold may result in changes to payment for the overhead cost of drugs and biologicals that do not reflect actual changes in hospital pharmacy overhead cost for those products. For these reasons, we believed that some portion, but not all, of the $395 million in total overhead cost that is associated with coded packaged drugs and biologicals with an ASP based on our standard drug payment methodology should, at least for CY 2010, be attributed to separately payable drugs and biologicals. Although we believed that for CY 2010 it would be prudent to redistribute some pharmacy overhead cost between coded packaged drugs and biologicals with an ASP at ASP+247 percent and separately payable drugs and biologicals at ASP–2 percent that would result from our standard drug payment methodology, the amount of overhead cost redistribution that would be appropriate between the packaged and separately payable drugs and biologicals in a payment system that is fundamentally based on averages was not fully evident. Pharmacy overhead cost includes, but is not limited to, some costs of indirect overhead that are shared by all hospital items and services, such as administrative and general costs, capital costs, staff benefits, and other facility costs. With regard to these indirect overhead costs, the amount of indirect overhead cost that is attributable to an inexpensive (typically packaged) drug is the same in dollar value as the amount of indirect overhead cost that is attributable to an extremely costly drug (typically separately payable). Hence, the indirect overhead costs that are common to all drugs and biologicals have no relationship to the cost of an individual drug or biological, or to the complexity of the handling, preparation, or storage of that individual drug or biological. Therefore, we believed that the indirect overhead cost alone for an inexpensive drug or biological could be far in excess of the ASP for that inexpensive product.
Layered on these indirect overhead costs are the pharmacy overhead direct costs of staff, supplies, and equipment that are directly attributable only to the storage, handling, preparation, and distribution of drugs and biologicals and which do vary, sometimes considerably, depending upon the drug being furnished. As we describe above, in its June 2005 Report to Congress, MedPAC found that drugs can be categorized into seven different categories based on the handling costs (that is, the direct costs) incurred (70 FR 42729). Similarly, the pharmacy stakeholders, whose suggested approach the APC Panel recommended that we accept for CY 2010, identified three categories of pharmacy overhead complexity with variable costs, to which they assigned individual drugs and biologicals for purposes of implementing their recommended redistribution of the difference between aggregate dollars for all drugs and biologicals at ASP+6 percent and aggregate cost for all drugs and biologicals in the claims data as additional pharmacy overhead payments.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35328), we acknowledged that the observed combined payment for acquisition and pharmacy overhead costs of ASP–2 percent for separately payable drugs and biologicals may be too low and ASP+247 percent for coded packaged drugs and biologicals with an ASP in the CY 2010 claims data may be too high. However, we stated our belief that the pharmacy stakeholders' recommendation to set packaged drug and biologicals dollars to ASP+6 percent was inappropriate given our understanding that an equal allocation of indirect overhead costs among packaged and separately payable drugs and biologicals would lead to a higher observed ASP+X percent than ASP+6 percent for packaged drugs and biologicals. As discussed above, the indirect overhead costs that are common to all drugs and biologicals have no relationship to the cost of an individual drug or biological, or to the complexity Start Printed Page 60503 of the handling, preparation, or storage of that individual drug or biological. Therefore, we stated our belief that the indirect overhead cost alone for an inexpensive drug or biological which would be packaged could be far in excess of the ASP for that inexpensive product. In contrast, we would expect that the indirect overhead cost alone for an expensive drug or biological which would be separately paid could be far less than the ASP for that expensive product.
Therefore, as discussed in the proposed rule, we believed that some middle ground would represent the most accurate redistribution of pharmacy overhead cost. We stated that the assumption that approximately one-third to one-half of the total pharmacy overhead cost currently associated with coded packaged drugs and biologicals with an ASP was a function of both charge compression and our choice of an annual drug packaging threshold offered a more appropriate allocation of drug and biological cost to separately payable drugs and biologicals. One-third of the $395 million of pharmacy overhead cost associated with coded packaged drugs and biologicals with an ASP was $132 million, whereas one-half was $198 million. Within the one-third to one-half parameters, we proposed that reallocating $150 million in drug and biological cost observed in the claims data from coded packaged drugs and biologicals with an ASP to separately payable drugs and biologicals for CY 2010 would more appropriately distribute pharmacy overhead cost among packaged and separately payable drugs and biologicals than either of the two other options, that is, paying for separately payable drugs and biologicals at ASP–2 percent according to our standard drug payment methodology or adopting the pharmacy stakeholders' recommendation. We stated that if we attributed $150 million in additional cost to the payment for the drugs and biologicals for which we proposed to pay separately for the CY 2010 OPPS, we determined a payment rate for separately payable drugs and biologicals of ASP+4 percent as displayed in Table 26 of the proposed rule (74 FR 35328) that is reprinted below as Table 37. Thus, we proposed a pharmacy overhead adjustment for separately payable drugs and biologicals in CY 2010 that would result in their payment at ASP+4 percent. We proposed to accomplish this adjustment by redistributing one-third to one-half of the pharmacy overhead cost of coded packaged drugs and biologicals with an ASP ($150 million), which represented a reduction in cost of coded packaged drug and biologicals with an ASP in the CY 2010 proposed rule claims data of 27 percent.
| Total ASP dollars for drugs and biologicals in claims data (in millions)* | Total cost of drugs and biologicals in claims Data After Adjustment (in millions)** | Ratio of cost to ASP (column C/column B) | ASP+X percent | |
|---|---|---|---|---|
| Coded Packaged Drugs and Biologicals with an ASP | $160 | $405 | 2.53 | ASP+153 |
| Separately Payable Drugs and Biologicals | 2,589 | 2,689 | 1.04 | ASP+4 |
| All Coded Drugs and Biologicals | 2,749 | 3,094 | 1.13 | ASP+13 |
| *Total April 2009 ASP dollars (ASP multiplied by drug or biological units in CY 2008 claims) for drugs and biologicals with a HCPCS code and ASP information. | ||||
| **Total cost in the CY 2008 claims data for drugs and biologicals with a HCPCS code and April 2009 ASP information. | ||||
Comment: Many commenters agreed with CMS that it was unlikely that the full cost (acquisition and pharmacy overhead) of separately payable drugs and biologicals could be 2 percent less than ASP for these products, and that the full cost (acquisition and pharmacy overhead) of packaged drugs could be 247 percent of ASP.
Response: We continue to find that the results of our standard drug payment methodology are unlikely to accurately reflect the full cost of acquisition and pharmacy overhead for separately payable drugs and biologicals and packaged drugs and biologicals due to hospital charging practices and our use of an annual drug packaging threshold. Using our CY 2010 final rule data, and applying our longstanding methodology for calculating the total cost of separately payable drugs and biologicals in our claims compared to the ASP dollars for the same drugs and biologicals and without applying the proposed overhead cost redistribution, we determined that the estimated aggregate cost of separately payable drugs and biologicals (status indicators “K” and “G”), including acquisition and pharmacy overhead costs, is equivalent to ASP–3 percent (compared to ASP–2 percent as presented in the proposed rule). Therefore, under our standard drug payment methodology, we would pay for separately payable drugs and biologicals at ASP–3 percent for CY 2010, their equivalent average ASP-based payment rate. We also determined that the estimated aggregate cost of coded packaged drugs and biologicals with an ASP (status indicator “N”), including acquisition and pharmacy overhead costs, is equivalent to ASP+259 percent (compared to ASP+247 percent as presented in the proposed rule). We found that the estimated aggregate cost for all coded drugs and biologicals (status indicators “N,” “K,” and “G), including acquisition and pharmacy overhead costs, is equivalent to ASP+11 percent (compared to ASP+13 percent as presented in the proposed rule). These values are shown in Table 38 below. Start Printed Page 60504
| ASP+X for all coded drugs and biologicals with an ASP | ASP+X for coded packaged drugs and biologicals with an ASP | ASP+X for separately payable drugs and biologicals | |
|---|---|---|---|
| CY 2010 Proposed Rule* | ASP+13 | ASP+247 | ASP–2 |
| CY 2010 Final Rule** | ASP+11 | ASP+258 | ASP–3 |
| *Based on CY 2010 proposed rule claims data and April 2009 ASPs. | |||
| **Based on CY 2010 final rule claims data and July 2009 ASPs. | |||
Comment: Some commenters agreed with CMS' assertion that packaged drugs and biologicals typically have an aggregate absolute pharmacy overhead cost that exceeds the acquisition cost of the packaged drugs and biologicals. One commenter claimed that ASP+6 percent would be insufficient to accurately account for the acquisition and pharmacy overhead costs of packaged drugs. In addition, a few commenters recommended that CMS modify the pharmacy stakeholders' approach by packaging the cost of drugs and biologicals at ASP plus 100 percent, rather than the stakeholder's recommended amount of ASP+6 percent.
Response: We continue to be concerned with a methodology that would package the cost of all packaged drugs and biologicals at ASP+6 percent. As stated in our proposal, we have no data specific to the overhead costs of these drugs and biologicals and, therefore, we cannot determine with any certainty an ASP+X value relating specifically to their costs for acquisition and pharmacy overhead. While we appreciate the recommendation of the commenters to package payment for the acquisition and pharmacy overhead costs of inexpensive drugs below the drug packaging threshold at ASP plus 100 percent, we cannot verify that using ASP plus 100 percent would result in an accurate estimate of acquisition and pharmacy overhead costs of packaged drugs and biologicals.
Comment: One commenter noted that a key CMS assumption behind the standard methodology for calculating the ASP+X percent payment rate, and the redistribution methodology by implication, is that the average overhead cost for drugs and biologicals ultimately must appear in the revenue producing cost center 5600 “Drugs Charged to Patients,” along with the acquisition cost of drugs and biologicals. The commenter acknowledged that CMS' standard drug payment methodology relies on appropriate allocation of pharmacy overhead cost in order to derive an accurate payment amount for separately payable versus packaged drugs and biologicals. The commenter specifically cited weak cost reporting instructions for the “Drugs Charged to Patients” cost center and cost center 1600 “Pharmacy” and questioned whether pharmacy overhead cost adequately and appropriately appears in the “Drugs Charged to Patients” cost center. Specifically, the commenter noted that costs accumulated in the “Drugs Charged to Patients” cost center remain in that cost center, but asserted that costs accumulated in the 1600 “Pharmacy” cost center would be allocated across revenue producing cost centers on the basis of costed requisitions. The commenter asked a series of specific questions about how costs are accumulated in both the “Pharmacy” and “Drugs Charged to Patients” cost centers, including (1) what costs hospitals actually report in the “Pharmacy” cost center versus the “Drugs Charged to Patients” cost center; (2) which revenue producing cost centers have costed requisitions that would receive “Pharmacy” cost center overhead costs, that is, do hospitals account for contrast agent costs under radiology revenue producing cost centers; (3) how much of “Pharmacy” cost center overhead costs is allocated to “Drugs Charged to Patients;” and (4) when would hospitals not account for the cost of a drug in the “Drugs Charged to Patients” cost center.
Response: We acknowledge that the CCR for the “Drugs Charged to Patients” cost center reflects the average acquisition cost for drugs and biologicals reported in that cost center, as well as the average pharmacy overhead cost for those drugs and biologicals. In addition, use of this CCR to estimate costs from charges on claims has the potential to “compress” the pharmacy overhead costs for expensive drugs and biologicals, where hospitals differentially distribute pharmacy overhead among their charges for drugs and biologicals by marking up the charges for expensive drugs and biologicals proportionally less than inexpensive drugs and biologicals. We have stated that combining this compression with a packaging threshold may lead us to underestimate the pharmacy overhead costs of separately payable drugs and biologicals and overestimate the overhead costs of packaged drugs and biologicals.
At a minimum, the CCR for the cost center “Drugs Charged to Patients” that CMS uses to estimate costs from charges for drugs and biologicals in our claims data should consist of charges for all drugs and biologicals separately charged to patients and the related costs for those separately chargeable drugs and biologicals. A hospital would not include the charge and cost for a drug or biological in the “Drugs Charged to Patients” cost center if the hospital did not separately charge the drug or biological to a patient. The identification of costs for the “Drugs Charged to Patients” cost center can occur in several ways.
First, we generally believe that the indirect costs that are common to all drugs and biologicals, including administrative and general costs, capital costs, staff benefits, and other facility costs, and the more direct costs of handling, preparation, and storage are accumulated as total pharmacy operation costs in cost center 1600 “Pharmacy.” Second, hospitals can choose to treat the acquisition cost of their drugs and biologicals in several ways. Frequently, hospitals accumulate and report the acquisition costs of drugs and biologicals (costed requisitions) directly in the most appropriate revenue producing cost center on Worksheet A of the cost report. We expect that, the majority of the time, hospitals accrue the largest acquisition cost of drugs and biologicals in the “Drugs Charged to Patients” cost center, which is specific to these items. However, hospitals may also account for the acquisition cost of unique drugs and biologicals, such as contrast agents, in other revenue producing cost centers such as the radiology cost centers, when the Start Printed Page 60505 contrast agents are not separately charged to the patient. Assignment of acquisition cost to a different revenue producing cost center can only occur if the drug or biological was not separately charged to a patient. Although the commenter suggested that contrast agents may appear in other cost centers, our claims data demonstrate a significant volume of contrast agents reported under a pharmacy revenue code. Therefore, we believe that hospitals largely are charging patients specifically for contrast agents and accounting for these costs and charges in the “Drugs Charged to Patients” cost center. The hospital would then allocate the total overhead costs from the “Pharmacy” general services cost center to all revenue producing cost centers that have costed requisitions for drugs and biologicals on Worksheet B–1. In this circumstance, a large proportion of the total cost of the “Pharmacy” cost center would be allocated to the “Drugs Charged to Patients” cost center, assuming a concentration of costed requisitions for drugs and biologicals in the “Drugs Charged to Patients” cost center. The total pharmacy cost being allocated is an aggregation that commingles the overhead costs of a variety of drugs and biologicals. The resulting CCR for the “Drugs Charged to Patients” cost center should reflect both the average acquisition cost of drugs and biologicals, including those that are expensive and inexpensive, in that cost center and the average pharmacy overhead cost apportioned to that cost center.
Hospitals also may include the acquisition cost of drugs and biologicals directly in the “Pharmacy” general services cost center and reclassify this cost to revenue producing cost centers, including “Drugs Charged to Patients,” before allocating the total cost of the “Pharmacy” cost center, which would have an effect similar to directly reporting the cost of drugs in the revenue producing cost centers on Worksheet A, as discussed above. In this situation, overhead cost from the “Pharmacy” cost center would be allocated to each of the revenue producing cost centers on the basis of costed requisitions. Some hospitals include the acquisition cost of drugs and biologicals directly in the “Pharmacy” cost center but do not reclassify this cost to the appropriate revenue producing cost center on Worksheet A, and instead allocate those costs on Worksheet B–1 together with the overhead cost of the pharmacy using costed requisitions. Regardless of which method described above that the provider uses, the resulting CCR for the “Drugs Charged to Patients” cost center should reflect the average acquisition cost for drugs and biologicals in that cost center and the average pharmacy overhead apportioned to that cost center. Our redistribution methodology acknowledges that relying on a single CCR has the potential to “compress” overhead costs and that combining this compression with a packaging threshold leads us to underestimate the overhead costs of separately payable drugs and biologicals and overestimate the overhead costs of packaged drugs and biologicals.
As we discussed in our proposal, we did not propose to redistribute pharmacy overhead cost from packaged to separately payable drugs and biologicals utilizing a methodology that would provide a separate pharmacy overhead payment for each separately payable drug and biological based on its pharmacy complexity. The OPPS is a prospective payment system that provides payment for groups of services and we believe that it is important, at a minimum, to maintain the current size of the OPPS payment bundles, in order to encourage efficiency in the hospital outpatient setting. As we stated in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66613), we believe it is important that the OPPS create incentives for hospitals to provide only necessary, high quality care and to provide that care as efficiently as possible. We have considered in recent years how we could increase packaging under the OPPS in a manner that would create incentives for efficiency while providing hospitals with flexibility to provide care in the most appropriate way for each Medicare beneficiary. Hospitals have repeatedly explained that they consider the acquisition and pharmacy overhead costs of drugs in setting their charges for drugs, and we have continued to provide a single payment for the acquisition and pharmacy overhead costs of separately payable drugs and biologicals under the OPPS consistent with this hospital charging practice. While we have worked to develop, and are now implementing, a refined payment methodology for drugs and biologicals for the CY 2010 OPPS that we believe will pay more accurately for the pharmacy overhead cost of packaged and separately payable drugs and biologicals, we do not believe it would be appropriate to unbundle the current single combined payment for the acquisition and overhead costs of a separately payable drug into two distinct payments, a drug payment and a pharmacy overhead payment. Furthermore, we note that section 1833(t)(14)(E)(ii) of the Act specifically authorizes the Secretary to adjust the APC payment weights for SCODs to take into account the recommendations of MedPAC on pharmacy overhead costs. We believe our CY 2010 approach that will adjust the APC payment for separately payable drugs and biologicals to more accurately pay for their associated pharmacy overhead cost, rather than provide a separate payment for a drug's pharmacy overhead cost each time the product is separately paid, is consistent with this statutory provision. Therefore, as we proposed, we are continuing to make a single bundled payment for the acquisition and pharmacy overhead costs of separately payable drugs and biologicals under the CY 2010 OPPS, an approach we believe both continues to encourage hospital efficiencies in the provision of drugs and biologicals to Medicare beneficiaries in the hospital outpatient setting and improves payment accuracy for the acquisition and pharmacy overhead costs of drugs and biologicals.
To confirm the portion of the $395 million in estimated pharmacy overhead cost associated with coded packaged drugs and biologicals with an ASP that should be attributable to separately payable drugs and biologicals for the proposed rule, we used information from a variety of sources in order to corroborate the appropriateness of our policy to redistribute between one-third and one-half of the difference ($150 million) between the aggregate claims cost for packaged drugs and biologicals and ASP dollars for the same drugs and biologicals to separately payable drugs and biologicals. In the CY 2010 OPPS/ASC proposed rule (74 FR 35330 through 35331), we presented two separate analyses which confirmed that our proposed redistribution of $150 million in pharmacy overhead cost associated with the cost of packaged drugs and biologicals was appropriate.
We began the analytic exercise with three fundamental assumptions. The first assumption was that the hospital acquisition cost of separately payable drugs and biologicals, on average, is not less than 100 percent of ASP. We believed that this assumption was valid because we have been told that hospitals pay a range of prices for the same drug or biological. Some hospitals may be able to take advantage of volume and group purchasing to achieve significant discounts for certain drugs and biologicals, but other hospitals may pay more than average for drugs and biologicals because of their low volume Start Printed Page 60506 usage or a hospital's remote geographic location. Further, hospitals often serve as community care resources so they must provide drugs and biologicals to meet the needs of all of the patients who present to their facilities for care. The amounts and nature of those drugs and biologicals may vary significantly and unpredictably over time, particularly for smaller hospitals, due to changing availability of other care settings in their communities, such as physicians' offices, or due to emergencies, and this variability may constrain hospitals' ability to purchase all necessary quantities of certain drugs and biologicals based on best price contractual agreements negotiated in advance. Hence, we believed that the ASP was likely a fair estimate of hospitals' average acquisition cost of drugs and biologicals in general, excluding direct and indirect overhead costs.
The second assumption was that coded packaged drugs and biologicals with an ASP, as a group, typically have an aggregate absolute pharmacy overhead cost (direct and indirect) that exceeds the acquisition cost of the packaged drugs and biologicals, as measured by ASP. We believed that this assumption was appropriate because packaged drugs and biologicals carry the same absolute amount of indirect overhead cost per drug or biological administered as separately payable drugs and biologicals and because many packaged drugs and biologicals have extremely low ASPs but some of the same direct costs (for example, recordkeeping, storage, safety precautions, and disposal requirements) as separately payable drugs and biologicals. Our proposed rule claims data showed that the weighted average ASP for the coded drugs and biologicals with an ASP that we proposed to package for CY 2010 was approximately $7 per day per packaged drug or biological, and we believed that it was a reasonable assumption that the full pharmacy overhead cost for a drug or biological (direct and indirect) equals or exceeds that amount.
Our final assumption was that, on average, the pharmacy overhead cost of separately payable drugs and biologicals, as a group, was not greater than the acquisition cost of the separately payable drugs and biologicals. We believed that this assumption is appropriate because separately payable drugs and biologicals carry the same absolute amount of indirect pharmacy overhead cost per drug or biological administered as packaged drugs and biologicals. While we have been told by MedPAC and the pharmacy stakeholders that separately payable drugs and biologicals generally have direct pharmacy overhead costs that are significantly higher than the direct overhead costs of packaged drugs and biologicals, we do not believe that they exceed the acquisition cost of separately payable drugs and biologicals. The weighted average ASP for the drugs and biologicals in our proposed rule claims data that we proposed for separate payment for CY 2010 was approximately $954 per day per separately payable drug or biological. We believed that the full pharmacy overhead cost for a separately payable drug or biological would not, on average, exceed the weighted average per day ASP. Hence, we believed these last two assumptions about the relationship of ASP to full pharmacy overhead cost (direct and indirect) for packaged and separately payable drugs and biologicals were appropriate for purposes of these analyses.
Having made these assumptions, for the proposed rule, we reduced the $395 million in estimated pharmacy overhead cost that exceeded the ASP dollars for coded packaged drugs and biologicals with an ASP (their average acquisition cost) by $50 million. Fifty million dollars in additional cost was necessary to raise the estimated cost calculated for separately payable drugs and biologicals from hospital claims data from 98 percent of ASP to 100 percent of ASP, in order to reach our estimate of the average hospital acquisition cost of separately payable drugs and biologicals of ASP. This left $345 million in estimated residual pharmacy overhead cost that continued to be associated with packaged drugs and biologicals. We stated our belief that a portion of this cost was associated with coded packaged drugs and biologicals with an ASP in our claims data, both due to charge compression and our choice of an annual drug packaging threshold, and would continue to be less accurately associated with packaged drugs and biologicals were we not to engage in further redistribution of that portion of this residual pharmacy overhead cost of packaged drugs and biologicals.
We then performed two analyses using information provided by the MedPAC Report (June 2005 Report to Congress) and by the pharmacy stakeholders (February 2009 presentation to the APC Panel and other meetings with CMS) that we applied to our proposed rule claims data to estimate the amount of residual pharmacy overhead cost associated with packaged drugs and biologicals that should more accurately be attributed to separately payable drugs and biologicals. To perform these analyses, we used proposed rule claims data only for those drugs and biologicals described by HCPCS codes that met the following criteria:
- The proposed CY 2010 OPPS status indicator for the HCPCS code was “G” for pass-through drugs and biologicals (excluding pass-through radiopharmaceuticals), “K” for separately payable drugs and biologicals that do not have pass-through status, or “N” for packaged drugs and biologicals, where the packaged status of these nonpass-through drugs and biologicals was determined by an estimate of cost per day based on ASP+4 percent;
- April 2009 pricing information based on the ASP methodology (other than mean cost from claims data) was available for the HCPCS code, and we would use the ASP methodology to pay for the HCPCS code if it had a status indicator of “K” or “G”; and
- CY 2008 OPPS claims data included claims for the HCPCS code or an equivalent predecessor code.
We first converted six of the seven categories that MedPAC recommended for reporting pharmacy overhead costs to three CMS categories (low, medium, and high), as we had proposed for the CY 2006 OPPS (70 FR 42729 through 42730); the seventh MedPAC category was not pertinent for this exercise because it is for the overhead cost attributable to radiopharmaceuticals. The CMS categories are defined as: Low (Orals); medium (Injection/Sterile Preparation; Single IV Solution/Sterile Preparation; Compounded Reconstituted IV Preparations); and high (Specialty IV or Agents requiring special handling in order to preserve their therapeutic value; Cytotoxic Agents in all formulations requiring personal protective equipment). We then derived a relative overhead weight for each of the three CMS categories by averaging the overhead weights for the six pertinent MedPAC categories. These averages were not weighted. The derived relative overhead weights for the CMS categories were as follows: Low = 1.00 (corresponding to MedPAC Category 1); medium = 3.61 (corresponding to MedPAC Categories 1, 2, and 3); and high = 11.11 (corresponding to MedPAC categories 5 and 6).
We also calculated a relative overhead weight for each of the three categories of pharmacy overhead complexity that were provided by the pharmacy stakeholders, using the different fixed dollar amounts that these stakeholders recommended that CMS pay for pharmacy overhead costs if we were to Start Printed Page 60507 make such payments for “all drugs” (packaged and separately payable). The pharmacy stakeholders' categories are defined as: Low (Dispense without manipulation: e.g., oral drugs, pre-filled syringes); medium (Injectable drug with one step manipulation: e.g., simple injections); and high (Multiple step injectable products and chemotherapy that require safety considerations). The pharmacy stakeholders' relative overhead weights were as follows: Low = 1; medium = 2.67; and high = 5.50.
Using the pharmacy stakeholders' overhead categories (low, medium, and high) and incorporating the pharmacy stakeholders' assignments of specific drugs and biologicals to levels of pharmacy complexity that they previously provided to CMS, we then assigned the remaining HCPCS codes for drugs and biologicals (approximately 50 percent of all drug and biological HCPCS codes with an associated ASP) based on our understanding of the characteristics of the categories. Similarly, we assigned all drug and biological HCPCS codes to the CMS categories created from the MedPAC groups for the derived relative overhead weights based on the definitions of those categories. Although the subsequent analytic processes were identical, we performed these analyses separately using the derived CMS overhead category weights (results are in Table 39) and using the pharmacy stakeholders' overhead category weights (results are in Table 40).
Specifically, for the proposed rule we assigned the overhead weights to each drug and biological in the set of drugs and biologicals qualifying for this exercise. We then calculated a per unit overhead cost by dividing the total relative weight for all drugs and biologicals in this exercise (low, medium, and high) into the residual pharmacy overhead cost from packaged drugs and biologicals of $345 million. Using the relative weights for each scenario, we estimated the exact per unit pharmacy overhead cost reallocation for each low, medium, and high pharmacy overhead category. We then added this payment amount to ASP for each drug and biological and reassessed the amount of total claims cost for separately payable and packaged drugs and biologicals and calculated our standard ratio of aggregate claims cost to aggregate ASP dollars for separately payable and packaged drugs and biologicals. The results of these analyses are reprinted in Tables 39 and 40 below.
| Total ASP dollars for drugs and biologicals in claims data (in millions)* | Total cost of drugs and biologicals in claims data after adjustment (in millions)** | Ratio of cost to ASP (column C/ column B) | ASP+X Percent | |
|---|---|---|---|---|
| Coded Packaged Drugs and Biologicals with an ASP | $160 | $390 | 2.44 | ASP+144 |
| Separately Payable Drugs and Biologicals | 2,589 | 2,704 | 1.04 | ASP+4 |
| All Coded Drugs and Biologicals | 2,749 | 3,094 | 1.13 | ASP+13 |
| * Total April 2009 ASP dollars (ASP multiplied by drug or biological units in CY 2008 claims) for drugs and biologicals with a HCPCS code and ASP information. | ||||
| ** Total cost in the CY 2008 claims data after adjustment for drugs and biologicals with a HCPCS code and April 2009 ASP information. | ||||
| Total ASP dollars for drugs and biologicals in claims data (in millions)* | Total cost of drugs and biologicals in claims data after adjustment (in millions)** | Ratio of cost to ASP (column C/ column B) | ASP+X Percent | |
|---|---|---|---|---|
| Coded Packaged Drugs and Biologicals with an ASP | $160 | $402 | 2.51 | ASP+151 |
| Separately Payable Drugs and Biologicals | 2,589 | 2,692 | 1.04 | ASP+4 |
| All Coded Drugs and Biologicals | 2,749 | 3,094 | 1.13 | ASP+13 |
| * Total April 2009 ASP dollars (ASP multiplied by drug units in CY 2008 claims) for drugs with a HCPCS code and ASP information. | ||||
| ** Total cost in the CY 2008 claims data after adjustment for drugs with a HCPCS code and April 2009 ASP information. | ||||
As shown in Tables 39 and 40, the ratio of adjusted cost in the claims data for separately payable drugs and biologicals to ASP increased compared to the value derived from our standard methodology and declined for packaged drugs and biologicals with an associated ASP compared to the value calculated according to our standard drug payment methodology as shown in Table 41. Specifically, for the proposed rule under our standard methodology without adjustment of the pharmacy overhead cost currently attributed to packaged drugs and biologicals with an associated ASP, we would have made packaged payment at ASP+247 percent. Using the CMS overhead weights, this value declined to ASP+144 percent and using the pharmacy stakeholders' overhead weights, it declined to ASP+151 percent.
Under our standard drug payment methodology, without adjustment of the pharmacy overhead cost currently attributed to separately payable drugs and biologicals, we estimated for the proposed rule that separately payable drugs and biologicals would be paid at ASP–2 percent. Assuming a base average acquisition cost for all drugs and biologicals of ASP and using the CMS overhead weights to redistribute Start Printed Page 60508 the residual $345 million in pharmacy overhead cost associated with coded packaged drugs and biologicals with an ASP in the claims data, this value increased to ASP+4 percent, and using the pharmacy stakeholders' overhead weights to redistribute the residual $345 million in pharmacy overhead cost, this value also increased to ASP+4 percent.
Based on these analyses, for the proposed rule, we estimated that we would redistribute $165 million in pharmacy overhead cost from coded packaged drugs and biologicals with an ASP to separately payable drugs and biologicals by setting the average acquisition cost for all drugs and biologicals to ASP and using the CMS overhead weights, and we would redistribute $153 million in pharmacy overhead cost from coded packaged drugs and biologicals with an ASP to separately payable drugs and biologicals by setting the average acquisition cost for all drugs and biologicals to ASP and using the pharmacy stakeholders' overhead weights. These observed outcomes were consistent with our CY 2010 proposal to redistribute between one-third and one-half of the $395 million of pharmacy overhead cost currently associated with packaged drugs and biologicals with an ASP to separately payable drugs and biologicals. These values were also consistent with the $150 million we proposed to redistribute from the cost of coded packaged drugs and biologicals with an ASP to separately payable drugs and biologicals for CY 2010, which would represent a reduction in the cost of packaged drugs and biologicals of 27 percent.
After we performed these analyses but prior to display of the CY 2010 OPPS/ASC proposed rule, the pharmacy stakeholders provided us with updated assignments of CY 2009 drug HCPCS codes to their recommended levels of pharmacy complexity. We then assigned the remaining HCPCS codes for drugs and biologicals that the pharmacy stakeholders had not assigned based on our understanding of the characteristics of their categories. We recalibrated our model to incorporate the updated information. We observed no substantive changes in our findings, with the revised overhead category assignments redistributing $159 million from packaged to separately payable drugs and biologicals and resulting in an ASP+X percentage of ASP+4 percent for separately payable drugs and biologicals and ASP+148 percent for packaged drugs and biologicals with an ASP.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35331), we indicated that these analyses based on our synthesis of existing data and information from a variety of sources supported the appropriateness of a redistribution of the magnitude we proposed for CY 2010. We believed that our analyses of the claims data using the CMS relative overhead weights derived from the 2005 MedPAC pharmacy overhead study and using the pharmacy overhead category payments, levels of complexity, and assignments of drugs provided by the pharmacy stakeholders (where available), confirmed that payment for separately payable drugs and biologicals at ASP+4 percent would represent a reasonable aggregate adjustment for the pharmacy overhead cost of these separately payable drugs and biologicals, compared to the payment that would result from the standard drug payment methodology. We stated our belief that payment for separately payable drugs at ASP+4 percent would ensure that hospitals are paid appropriately for the average hospital acquisition cost and the pharmacy overhead cost that our analyses show would be appropriately redistributed from the estimated cost of overhead associated with drugs and biologicals with an ASP that we proposed to package for CY 2010.
Our proposal for CY 2010 relied upon the premise of providing a pharmacy overhead adjustment to payment for separately payable drugs by redistributing calculated pharmacy overhead cost from coded packaged drugs and biologicals with an ASP to separately payable drugs and biologicals. Therefore, regardless of the payment level that the CY 2010 OPPS/ASC final rule with comment period claims and cost report data and July 2009 ASP data ultimately suggested, we believed that any redistributed amount of pharmacy overhead cost should be removed from the estimated cost of pharmacy overhead associated with coded packaged drugs and biologicals with an ASP. We proposed to redistribute pharmacy overhead cost within the estimated total amount of acquisition and overhead cost for all drugs and biologicals with an ASP that has been reported to us by hospitals by making a pharmacy overhead adjustment to payment for separately payable drugs and biologicals that is based upon a partial redistribution of the pharmacy overhead cost of coded packaged drugs and biologicals with an ASP. As described previously in this section, we proposed that any redistribution of pharmacy overhead cost that may arise from CY 2010 final rule data would occur only from some drugs and biologicals to other drugs and biologicals, thereby maintaining the estimated total cost of drugs and biologicals in our claims data (no redistribution of cost would occur from other services to drugs and biologicals or vice versa). While there is some evidence that relatively more pharmacy overhead cost should be associated with separately payable drugs and biologicals and less pharmacy overhead cost should be associated with packaged drugs and biologicals in order to improve payment accuracy, we concluded that the recent RTI report on the OPPS' hospital-specific CCR methodology (“Refining Cost to Charge Ratios for Calculating APC and DRG Relative Payment Weights,” July 2008 final report), the June 2005 MedPAC study of hospital outpatient pharmacy overhead costs, and our claims analyses discussed in the proposed rule presented no evidence that the total cost of drugs and biologicals (including acquisition and overhead costs) is understated in the claims data that we use to model the upcoming prospective payment year in relation to the costs of other services paid under the OPPS. Therefore, to improve the distribution of pharmacy overhead cost within the total estimated cost for all drugs and biologicals, without adversely affecting the relativity of payment weights for all services paid under the OPPS, we reasoned that it would be most appropriate to redistribute pharmacy overhead cost only within the total estimated cost of coded packaged and separately payable drugs and biologicals. By redistributing pharmacy overhead cost only within the total estimated cost of packaged and separately payable drugs and biologicals, we would maintain a constant total cost of drugs and biologicals under the OPPS as reported to us by hospitals, without redistributing cost from other OPPS services to the cost of drugs and biologicals under the budget neutral OPPS.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35332), we indicated that while we agree conceptually with the APC Panel that a redistribution of pharmacy overhead cost in our claims data from coded packaged drugs and biologicals with an ASP to separately payable drugs and biologicals is appropriate, we did not accept the APC Panel's February 2009 recommendation that CMS pay for the acquisition cost of all separately payable drugs at no less than ASP+6 percent because, as we discussed previously in this section, our analyses of claims data indicated that appropriate payment for the acquisition Start Printed Page 60509 and pharmacy overhead costs of separately payable drugs would be ASP+4 percent. We also did not accept the APC Panel's February 2009 recommendation that CMS package the cost of packaged drugs at ASP+6 percent, use the difference between this cost and CMS' cost derived from charges to provide more appropriate payment for pharmacy services costs, and pay for pharmacy services using this amount by applying a tiered approach to payments based on criteria related to the pharmacy resources required for groups of drugs. We believed that the recommendation to package the cost of packaged drugs at ASP+6 percent would underpay for the pharmacy overhead cost of packaged drugs, which we expected would be higher in relation to ASP than the pharmacy overhead cost of separately payable drugs. Further, as discussed earlier in this section, because the OPPS is a prospective payment system that relies on payment for groups of services to encourage hospital efficiencies, we did not believe payment for pharmacy overhead costs that is separate from the OPPS payment for the acquisition costs of drugs would be appropriate.
The APC Panel further recommended that, if CMS did not adopt a methodology consistent with their recommendations summarized above, CMS should exclude data from hospitals that participate in the 340B program from its ratesetting calculations for drugs and that CMS should pay 340B hospitals in the same manner as it pays non-340B hospitals. In the proposed rule, we did not accept the APC Panel's recommendation that CMS propose to exclude data from hospitals that participate in the 340B program from its ratesetting calculations for drugs. For CY 2010, we note that we proposed a drug payment methodology that partially resembled the methodology recommended by the APC Panel because the proposal incorporated a redistribution of pharmacy overhead cost from coded packaged drugs and biologicals with an ASP to separately payable drugs and biologicals. However, excluding data from hospitals that participate in the 340B program from our ASP+X calculation, but paying those hospitals at that derived payment amount, would effectively redistribute payment to drugs and biologicals from payment for other services under the OPPS, and we did not believe this redistribution would be appropriate. In our CY 2010 proposal, we did accept the APC Panel's February 2009 recommendation that CMS propose to pay 340B hospitals in the same manner as non-340B hospitals are paid. Commenters on the CY 2009 OPPS/ASC final rule with comment period were generally opposed to differential payment for hospitals based on their 340B participation status, and we did not believe it would be appropriate to exclude claims from this subset of hospitals in the context of our CY 2010 proposal to pay all hospitals at the same rate for separately payable drugs and biologicals.
Moreover, as discussed above, while we did not propose to adopt the APC Panel's specific recommended methodology to redistribute pharmacy overhead cost that would otherwise be paid through payment for packaged drugs and biologicals, our proposed CY 2010 pharmacy adjustment methodology that would result in the payment of separately payable drugs and biologicals at ASP+4 percent incorporated a more limited redistribution of pharmacy overhead cost for coded packaged drugs and biologicals with an ASP (ASP is necessary to calculate an overhead amount) that would preserve the aggregate drug cost in the claims, a result consistent with the APC Panel's recommendations. Therefore, we believed that it would be appropriate to propose to pay 340B hospitals at the same rates that we are proposing to pay non-340B hospitals, and we proposed to include the claims and cost report data for 340B hospitals in the data we had used for our analyses in order to calculate the payment rates for drugs and biologicals and other services for the CY 2010 OPPS.
In conclusion, we proposed for CY 2010 to redistribute between one-third and one-half of the difference between the aggregate claims cost for coded packaged drugs and biologicals with an ASP and ASP dollars for those products, which resulted in proposed payment for the acquisition and pharmacy overhead costs of separately payable drugs and biologicals that do not have pass-through payment status of ASP+4 percent. This payment amount reflected an APC drug payment adjustment for pharmacy overhead cost. To accomplish this payment adjustment, we also proposed to reduce the cost of coded packaged drugs and biologicals with an ASP that was incorporated into the payment for procedural APCs by the amount of pharmacy overhead cost that was redistributed from these packaged drugs and biologicals to the payment for separately payable drugs and biologicals. The proposal was based on the proposed redistribution of $150 million (through a 27 percent reduction in the cost of coded packaged drug and biologicals with an ASP), between one-third and one-half of the pharmacy overhead cost (the cost above ASP) of coded packaged drugs and biologicals with an ASP in hospital outpatient claims, to the cost of separately payable drugs and biologicals, preserving the aggregate cost of all drugs and biologicals observed in the most recent claims and cost report data available for the proposed rule. We further proposed that the claims data for 340B hospitals be included in the calculation of payment for drugs and biologicals under the CY 2010 OPPS, and that 340B hospitals would be paid the same amounts for separately payable drugs and biologicals as hospitals that do not participate in the 340B program. Finally, we proposed that, in accordance with our standard drug payment methodology, the estimated payments for separately payable drugs and biologicals would be taken into account in the calculation of the weight scaler that would apply to the relative weights for all procedural services (but would not apply to separately payable drugs and biologicals) paid under the OPPS, as required by section 1833(t)(14)(H) of the Act.
At the August 2009 meeting of the APC Panel, the APC Panel recommended that CMS pay for all separately payable drugs at a rate of ASP+6 percent. The APC Panel recommended that CMS redistribute costs from packaged drugs to separately payable drugs as outlined in the CY 2010 OPPS/ASC proposed rule. Further, the APC Panel recommended that CMS analyze the impact on different classes of hospitals of payment at ASP+6 percent for separately payable drugs compared with CY 2009 payment at ASP+4 percent. In addition, the APC Panel requested that CMS provide an impact analysis of payment for separately payable drugs at ASP+6 percent on payment rates for other services that use packaged drugs compared with CY 2009 payment at ASP+4 percent. Finally, the APC Panel recommended that CMS and stakeholders continue to refine their analysis of payment for drugs, biologicals, and radiopharmaceuticals to assess the infrastructure costs associated with the preparation and handling of these products. Our responses to these recommendations are included in our responses to comments below.
Comment: Several commenters, including MedPAC, generally agreed with CMS' proposal to redistribute pharmacy overhead cost from packaged to separately payable drugs and biologicals. The commenters appreciated that the proposed Start Printed Page 60510 methodology would not pose an administrative burden to hospitals.
However, many commenters disagreed with CMS' calculation of the total estimated pharmacy overhead cost of $395 million in the claims data associated with packaged drugs that resulted in the proposed redistribution of $150 million, which was between one-half and one-third of this overhead cost. The commenters stated that CMS' estimate of $395 million was too low to represent the aggregate pharmacy overhead cost of all packaged drugs and biologicals, resulting in an underestimate of how much overhead cost should be redistributed to separately payable drugs and biologicals and, therefore, a proposed payment rate for separately payable drugs and biologicals that was too low. They explained that, although CMS allows flexibility in hospital charging practices to account for drug and biological cost on hospital claims, CMS' proposed rule calculation did not take hospital charging practices for packaged drugs and biologicals into account. Specifically, in CMS' estimation of the cost of packaged drugs and biologicals, the commenters pointed out that CMS omitted costs from claims data for drugs and biologicals that either do not have a HCPCS code or do not have a reported ASP, including those costs reported under a pharmacy revenue code line without a drug or biological HCPCS code due to hospital choice or claims processing requirements. The commenters argued that these uncoded packaged drug and biological costs represent a substantial portion of aggregate packaged drug and biological cost under the OPPS.
Some commenters estimated the additional packaged pharmacy overhead cost attributable to these uncoded drugs and biologicals to be nearly $560 million. The commenters asserted that hospitals mark up the costs of drugs and biologicals reported on claims under pharmacy revenue code lines without HCPCS codes similarly to packaged drugs and biologicals reported with HCPCS codes. Several commenters provided analyses to support their contention that the costs of uncoded pharmacy revenue code lines reflect mostly packaged drug and biological costs, and that when hospitals do not report packaged drugs and biologicals with HCPCS codes, they report uncoded pharmacy revenue code lines instead for those drugs and biologicals. The commenters concluded that a significant percentage of the uncoded costs reported under pharmacy revenue code lines is pharmacy overhead cost disproportionately attributed to packaged drugs and biologicals due to the tendency of our established methodology of converting billed charges to costs to “compress” the calculated costs to some degree and recognizing that our choice of an annual drug packaging threshold contributes to the magnitude of the ASP+X percent payment rate resulting from our standard drug payment methodology.
In order to address these concerns, the commenters recommended that CMS redistribute the pharmacy overhead cost attributed to uncoded cost reported under pharmacy revenue code lines to the cost of separately payable drugs and biologicals. Some commenters argued that, because they believe the costs on these uncoded pharmacy revenue code lines largely are for packaged drugs and biologicals with HCPCS codes, CMS could accurately assume the same proportional amount of ASP and mark up as for packaged drugs and biologicals with a HCPCS code and derive a simulated pharmacy overhead amount. Therefore, they suggested that one-third to one-half of residual pharmacy overhead cost associated with these uncoded pharmacy revenue code lines, which they estimate to total approximately $560 million, should be redistributed to the cost of separately payable drugs and biologicals.
In addition, several commenters expressed concern that hospitals may not be billing packaged drugs and biologicals with HCPCS codes appropriately, resulting in uncoded costs reported under pharmacy revenue code lines, and that this contributed to the low estimate of pharmacy overhead costs included in the proposed rule. The commenters stated that a review of the OPPS claims data found variations in how hospitals are reporting drugs and biologicals with HCPCS codes under pharmacy revenue codes. The commenters stated that some hospitals are inappropriately assigning costs for drugs and biologicals with HCPCS codes to revenue code 0250 (Pharmacy (also see 063x, an extension of 025x); General Classification), rather than revenue code 0636 (Pharmacy—Extension of 025x; Drugs Requiring Detailed coding (a)). They speculated about a variety of reasons why more HCPCS-coding for packaged drug and biological cost was not available to CMS for proposed rule estimate purposes: (1) Hospitals may have reported their packaged drugs with revenue code 0250 and the associated charges and units with no HCPCS codes because HCPCS codes are not required to be reported for packaged drugs and biologicals; (2) the associated HCPCS code may not have printed on the claim because of provider billing system settings; or (3) Medicare contractors may have instructed hospitals not to report HCPCS codes under revenue code 0250. As a result, the commenters believed that CMS' derived pharmacy overhead cost estimate for packaged drugs and biologicals based only on the cost of packaged drugs and biologicals with a HCPCS code and an ASP in the proposed rule were inaccurately low. In order to provide complete drug information for future years, they requested that CMS instruct hospitals to bill for drugs and biologicals with HCPCS codes under revenue code 0636.
Finally, some commenters expressed frustration that CMS did not provide information on the assignment of every drug and biological HCPCS code with a status indicator of “K,” “G,” or “N” to one of three categories of pharmacy overhead complexity in the analyses that CMS presented to validate the proposed redistribution of pharmacy overhead cost from packaged drugs and biologicals with an ASP to separately payable drugs and biologicals. In light of this omission, the commenters recommended that CMS redistribute the larger one-half portion of the one-third to one-half of the proposed pharmacy overhead cost to accurately account for all pharmacy costs represented in the HOPD.
Response: We proposed to reallocate approximately $150 million in pharmacy overhead cost from coded packaged drugs and biologicals with an ASP to separately payable drugs and biologicals, representing a middle ground between the one-third to one-half of the total pharmacy overhead cost associated with this set of packaged drugs and biologicals. We agree with the commenters that we did not include uncoded drug and biological costs reported under pharmacy revenue code lines in our proposed rule estimate of the pharmacy overhead costs of packaged drugs and biologicals. We also agree with the commenters that costs on uncoded pharmacy revenue code lines represent OPPS drug and biological cost. The commenters suggested that we assume the same relationship between total claim cost and ASP for the uncoded drug and biological costs in our claims data as we observe for coded packaged drugs and biologicals with an ASP, and then redistribute one-third of the assumed, associated pharmacy overhead cost to the cost of separately payable drugs and biologicals. We were interested to review the analyses provided by some commenters that used statistical techniques to compare the uncoded drug and biological costs to the costs of packaged drugs and biologicals with HCPCS codes but, at this time, we Start Printed Page 60511 cannot be certain that the assumptions suggested by the commenters would represent an accurate portion of the uncoded drug and biological cost attributable to acquisition cost versus pharmacy overhead cost. In the proposed rule, we stated that a premise of our redistribution model was our assumption that the associated aggregate ASP for packaged drugs and biologicals was a proxy for acquisition cost for this group of drugs and biologicals (74 FR 35327). Our proposed methodology identified the difference between this proxy for acquisition cost and the cost of the same coded packaged drugs and biologicals with an ASP in our claims data as pharmacy overhead cost, and it was one-third to one-half of that pharmacy overhead cost ($150 million) that we specifically proposed to redistribute from coded packaged drugs and biologicals with an ASP to the cost of separately payable drugs and biologicals.
As shown in Table 41, we determined that the estimated aggregate cost of separately payable drugs and biologicals, including acquisition and pharmacy overhead costs, is equivalent to ASP–3 percent for this final rule with comment period. A redistribution of $150 million from the pharmacy overhead cost of coded packaged drugs and biologicals with an ASP (one-third of that pharmacy overhead cost from final rule data) to the cost of separately payable drugs and biologicals would result in payment for separately payable drugs and biologicals at ASP+2 percent for CY 2010. If we were to assume the same relationship between total claim cost and ASP for the uncoded drug and biological cost in our claims data as we observe for coded packaged drugs and biologicals with an ASP as recommended by some commenters, and if we were then to redistribute one-third of the assumed, associated pharmacy overhead cost ($150 million) of uncoded drug and biological cost to the cost of separately payable drugs and biologicals, the result would be payment for separately payable drugs and biologicals at ASP+7 percent. The total cost redistribution to separately payable drugs and biologicals in this case would be $300 million, $150 million from the cost of coded packaged drugs and biologicals with an ASP and $150 million from the cost of uncoded packaged drugs and biologicals.
We understand that our proposal for a redistribution of any drug and biological cost from packaged to separately payable drugs and biologicals already is not our usual OPPS cost estimation methodology, which uses the estimated cost from claims and cost report data as reported to us by hospitals for an item or service to calculate a relative weight for that service, or in the case of drugs and biologicals, an ASP+X percent under our standard drug payment methodology. We made this redistribution proposal because we were concerned that by not redistributing pharmacy overhead cost from packaged to separately payable drugs and biologicals, an underpayment of separately payable drugs and biologicals at ASP–2 percent (ASP–3 percent based on final rule claims data) could result. We remain concerned that the redistribution of $150 million from the pharmacy overhead cost of coded packaged drugs and biologicals with an ASP to payment for separately payable drugs and biologicals, which would provide payment at ASP+2 percent, also could result in underpayment of separately payable drugs and biologicals. We are also troubled, however, that payment for separately payable drug and biologicals at ASP+7 percent resulting from an assumption that the uncoded drug and biological cost resembles the coded packaged cost of drugs and biologicals with an ASP could result in a potential payment overestimation. As noted above, we cannot be certain that we know what portion of the uncoded drug and biological cost is acquisition cost versus pharmacy overhead cost. Therefore, we are not willing to make even broader assumptions about the magnitude of ASP for uncoded drug and biological cost in claims or layer any other assumptions on the proposed methodology that would further significantly redistribute costs as reported to us by hospitals within the framework of the OPPS ratesetting methodology.
While we are not making sweeping assumptions that this uncoded packaged drug and biological cost includes a pharmacy overhead amount comparable to that of coded packaged drugs and biologicals with an ASP, we do acknowledge that there must be some pharmacy overhead cost associated with these uncoded packaged drugs and biologicals that was not accounted for in our initial estimate of the pharmacy overhead cost of packaged drugs and biologicals because we expect that hospitals would have attributed some pharmacy overhead cost to these products through their mark-up practices. Therefore, while we further examine the issue of pharmacy overhead costs and while hospitals examine administrative changes that could result in their submission of more accurate data to us as described below, we believe that the adoption of a transitional payment rate of ASP+4 percent based on a pharmacy overhead adjustment methodology for CY 2010 would base OPPS payment upon the best available proxy for the average acquisition and pharmacy overhead costs of separately payable drugs and biologicals. We note that payment for separately payable drugs and biologicals at ASP+4 percent falls within the range of ASP–3 percent, that would result from no pharmacy overhead cost redistribution from packaged to separately payable drugs and biologicals, to ASP+7 percent, that would result from redistribution of pharmacy overhead cost based on expansive assumptions about the nature of uncoded packaged drug and biological cost. We proposed payment for separately payable drugs and biologicals at ASP+4 percent for CY 2010, and our final CY 2010 transitional payment rate is consistent with this amount. We are confident that ASP+4 percent will provide appropriate payment for separately payable drugs and biologicals in CY 2010, noting that this payment is consistent with our payment in CY 2009. We are not aware of any current access problems for Medicare beneficiaries to drugs and biologicals in the HOPD based on our CY 2009 OPPS payment for separately payable drugs and biologicals at this rate.
Specifically, for CY 2010, to acknowledge the uncoded drug and biological cost without making significant further assumptions about the amount of pharmacy overhead cost associated with the drugs and biologicals captured by this cost and to pay separately payable drugs and biologicals at ASP+4 percent, we believe it currently would be appropriate to reallocate $50 million of the total uncoded drug and biological cost in order to represent the pharmacy overhead cost of uncoded packaged drugs and biologicals that should be appropriately associated with the cost of separately payable drugs and biologicals. We believe that our proposal to reallocate $150 million of cost from coded packaged drugs and biologicals with an ASP, or one-third of the pharmacy overhead cost of these products based upon the claims data available for this CY 2010 final rule, to separately payable drugs and biologicals continues to be appropriate. The commenters generally supported the one-third to one-half redistribution estimate. While some commenters requested a reallocation of one-half of Start Printed Page 60512 the pharmacy overhead cost of packaged drugs and biologicals to separately payable drugs and biologicals, as already discussed, we do not believe there is a compelling reason to reallocate that amount. We note that the reallocation of $50 million or 8 percent of the total cost of uncoded packaged drugs and biologicals assumes that whatever pharmacy overhead cost is not accurately associated with uncoded packaged drugs and biologicals, it would not be less than 8 percent of total uncoded drug and biological cost. This is intentionally a conservative estimate, as compared with the case of coded packaged drugs and biologicals with an ASP and for which we have a specific pharmacy overhead cost estimate in relationship to their known ASPs where the reallocation of $150 million constitutes 24 percent of the total cost of the coded packaged drugs and biologicals with an ASP. As stated earlier, we are unwilling to make sweeping assumptions that uncoded packaged drug and biological cost includes a pharmacy overhead amount comparable to that of coded packaged drugs and biologicals with an ASP. We are confident that this conservative estimate of $50 million for redistribution from the cost of uncoded packaged drugs and biologicals to separately payable drugs and biologicals, as opposed to the $150 million redistribution that could result from broad assumptions about the ASPs of these uncoded drugs and biologicals, is an appropriate amount for CY 2010 in light of our uncertainty about the relationship between ASP and pharmacy overhead cost for the uncoded drugs and biologicals.
In summary, with a redistribution of a total of $200 million, $150 million from the pharmacy overhead cost of coded packaged drugs and biologicals with an ASP as we proposed and $50 million from the cost of uncoded packaged drugs and biologicals for which we cannot estimate a more specific pharmacy overhead cost at this time, to separately payable drugs and biologicals, the final CY 2010 transitional payment rate for separately payable drugs and biologicals is ASP+4 percent based on the final pharmacy overhead adjustment methodology.
In response to commenters' frustration that we did not provide information on our assignment of every drug or biological HCPCS code to one of three categories of pharmacy overhead complexity in the analyses that we presented to validate the proposed redistribution methodology, we did not base our proposed redistribution amount on these analyses. We explicitly made a proposal to redistribute $150 million in estimated pharmacy overhead cost associated with coded packaged drugs and biologicals with an ASP, between one-third and one-half of the estimated pharmacy overhead cost. Although we did not provide the precise assignment of drugs and biologicals to the various categories, we did describe each set of pharmacy overhead complexity categories in the proposed rule and our methodology for redistributing pharmacy overhead cost under each scenario. In addition, we posted a clarification to this discussion for the public replicating our models on August 6, 2009 during the comment period.
Table 41 displays the final pharmacy overhead adjustment methodology for separately payable and packaged drugs and biologicals under the CY 2010 OPPS.
| Total ASP dollars for drugs and biologicals in claims data (in millions)* | Total cost of drugs and biologicals in claims data after adjustment (in millions)** | Ratio of cost to ASP (column C/ column B) | ASP+X Percent | |
|---|---|---|---|---|
| Uncoded Packaged Drugs and Biologicals | Unknown | $606 | N/A | N/A |
| Coded Packaged Drugs and Biologicals with an ASP | 172 | 466 | 2.71 | ASP+171 |
| Separately Payable Drugs and Biologicals with an ASP | 2,972 | 3,039 | 1.04 | ASP+4 |
| All Coded Drugs and Biologicals with an ASP | 3,144 | 3,505 | 1.11 | ASP+11 |
| * Total July 2009 ASP dollars (ASP multiplied by drug or biological units in CY 2008 claims) for drugs and biologicals with a HCPCS code and ASP information. | ||||
| ** Total cost in the CY 2008 claims data for drugs and biologicals. | ||||
We note that hospitals currently have a variety of ways to bill for drugs and biologicals that are not separately paid. They may report the charges for the HCPCS code separately on a line, and if the HCPCS code has a status indicator of “N,” no separate payment is made for the drug or biological, but the reported charge information is available to use for future ratesetting. Provided that information for the ASP pricing methodology was available for the drug or biological HCPCS code, we included drug or biological cost estimated from charges for claims described by this scenario in our estimation of total pharmacy overhead costs of coded packaged drugs and biologicals with an ASP for CY 2010 because we could identify these drugs and biologicals, estimate their cost from charges in CY 2008 claims data, and use their ASP pricing information. Another option available to hospitals billing for packaged drugs and biologicals is to incorporate the charge for the drug or biological in the charge for the procedure. We are unable to identify the cost estimated from charges as drug or biological cost because the procedures are not reported under a pharmacy revenue code line and, therefore, these packaged drug and biological costs were not included in our estimate of the total pharmacy overhead cost of packaged drugs and biologicals. The final way for hospitals to bill for packaged drugs and biologicals is to include charges for these items under a pharmacy revenue code line, specifically revenue code 0250, without a HCPCS code, and it is an additional $50 million from this uncoded cost of packaged drugs and biologicals that we have redistributed to the cost of separately payable drugs and biologicals in our final CY 2010 pharmacy overhead adjustment payment methodology for drugs and biologicals.
We have adopted this pharmacy overhead adjustment payment methodology for CY 2010 only after 3 distinct attempts over the 4 prior years to garner more accuracy in both the Start Printed Page 60513 claim drug or biological charge and Medicare hospital cost report data as submitted to us by hospitals in an effort to show consideration for the significant hospital administrative burden that the commenters cited in response to each proposal that, in turn, precluded further refinement of our data collection efforts. In light of our commitment to using hospital data as reported to us by hospitals to set OPPS payment rates, we believe that it would be inappropriate to assume that the costs reported under uncoded pharmacy revenue code lines are for the same drugs and biologicals, with the same ASPs, as the costs of packaged drugs and biologicals reported with HCPCS codes. We acknowledge that the pharmacy overhead cost associated with drug and biological costs reported under uncoded pharmacy revenue code lines were not included in the proposed rule estimate of total pharmacy overhead cost of coded packaged drugs and biologicals with an ASP. In response to the concerns of commenters, we have considered only a small percentage of this uncoded drug and biological cost to be misallocated pharmacy overhead cost that is appropriate for redistribution in our final CY 2010 methodology. We cannot be certain that the amount of uncoded pharmacy overhead cost is as high as some commenters suggested, that hospitals mark up these uncoded drugs and biologicals in the same way as packaged drugs and biologicals with HCPCS codes, or that significant volume for these uncoded drugs and biologicals might not warrant allocating a greater percentage of fixed pharmacy overhead cost to these drugs and biologicals. If hospitals truly desire significantly greater OPPS payment accuracy for separately payable drugs and biologicals, it is clear that hospitals will need to assume some burden in submitting more accurate data to us. In addition, we will continue to examine the issue of pharmacy overhead costs as we work to refine our transitional payment methodology for separately payable drugs and biologicals for future years.
CMS' longstanding policy is to refrain from instructing hospitals on charging practices for services under most revenue codes. We believe that this allows hospital flexibility in billing systems and provides the necessary autonomy for hospitals to manage the many variations that are possible when creating a hospital chargemaster for multiple payers. While we do not require hospitals to use revenue code 0636 (Pharmacy-Extension of 025x; Drugs Requiring Detailed coding (a)) when billing for drugs and biologicals that have HCPCS codes, whether they are separately payable or packaged, we believe that a practice of billing all drugs and biologicals with HCPCS codes under revenue code 0636 would be consistent with NUBC billing guidelines and would provide us with the most complete and detailed information for ratesetting. We note that we make packaging determinations for drugs annually based on cost information reported under HCPCS codes, so the OPPS ratesetting is best served when hospitals report charges for all items and services that have HCPCS codes under those HCPCS codes, whether or not payment for the items and services is packaged or not. As already discussed, it is our standard ratesetting methodology to rely on hospital cost and charge information as it is reported to us through the claims data. More complete data from hospitals on which drugs were provided for a specific episode would help improve payment accuracy for separately payable drugs in the future, and we encourage hospitals to change their reporting practices if they are not already reporting HCPCS codes for all drugs furnished, if specific codes are available.
Comment: Several commenters objected to the proposed redistribution methodology as it decreased payments for procedural APCs with high packaged drug costs included in their payment rates.
One commenter disagreed with the proposed redistribution methodology and its effect on the imaging procedure APCs. The commenter argued that because all contrast agents without pass-through status are packaged, regardless of an individual agent's relationship to the annual drug packaging threshold, imaging procedure APCs should be exempt from the proposed pharmacy cost redistribution methodology. If imaging procedures are not exempted from the redistribution, the commenter contended that these procedures would be disproportionately affected because the spectrum of contrast costs are currently represented as packaged costs within the imaging procedure APCs.
Similarly, another commenter requested that CMS exempt nuclear medicine procedures from the redistribution methodology. Again, the commenter stated that as all diagnostic radiopharmaceuticals are packaged, regardless of their estimated per day costs, their overhead costs are all represented in the nuclear medicine APCs and a redistribution would disproportionately affect these services.
Response: We agree that packaging all contrast agents into associated imaging procedures results in the inclusion of payment for both expensive and relatively inexpensive contrast agents in the payment for the associated imaging procedures. While the commenters contended that this policy thereby incorporates all contrast agents with different hospital mark up practices in a single packaged payment methodology and, therefore, should not be subject to the cost redistribution, we believe that contrast agents are contributing to the overall charge compression for all drugs and biologicals that is the specific target of our redistribution methodology. When examining CY 2008 claims data for the final rule, we observed that hospitals typically billed costs for contrast agents under a pharmacy revenue code (025X (Pharmacy), 026X (IV Therapy), or 063X (Pharmacy—Extension of 025X)). We believe that in almost all cases, hospitals capture the costs and charges for pharmacy revenue codes in the cost center 5600 “Drugs Charged to Patients,” and this is the cost center that we use to estimate costs from charges for the pharmacy revenue codes in our claims data each year. We make the revenue code-to-cost center crosswalk that we use to match Medicare hospital cost report information with claims data continually available for inspection and comment on the CMS Web site at: http://www.cms.hhs.gov/HospitalOutpatientPPS. The proposed methodology of redistributing pharmacy overhead cost from packaged drugs and biologicals to separately payable drugs and biologicals was a proposal to address charge compression observed within this specific cost center that captures the vast majority of costs and charges for drugs and biologicals billed on hospital outpatient claims. Therefore, as most hospitals billing contrast agents with pharmacy revenue codes are associating the contrast agent costs with the cost center 5600 “Drugs Charged to Patients,” we believe it is appropriate to redistribute cost from contrast agents to separately payable drugs and biologicals under our final CY 2010 pharmacy overhead cost redistribution methodology.
The commenter also suggested that it would be inappropriate to redistribute cost from contrast agents because, as discussed in V.B.2.d. of this final rule with comment period, it has been OPPS policy to package payment for all contrast agents since CY 2008. The proposed methodology for redistributing pharmacy overhead cost from packaged drugs and biologicals to separately payable drugs and biologicals was not only a proposal to address charge Start Printed Page 60514 compression, but specifically a proposal to address charge compression in light of our adoption of a specific drug packaging threshold, which is $65 for CY 2010. The argument that it would, therefore, be inappropriate to redistribute cost from contrast agents could have merit if there was a sizable amount of aggregate cost for contrast agents with per day costs greater than the drug packaging threshold of $65. In that case, it could be argued that the compression in cost estimates for expensive contrast agents (those with per day costs greater than the $65 packaging threshold) created by estimating costs for those agents by applying the CCR for the single cost center 5600 “Drugs Charged to Patients” to expensive contrast agents' charges would be offset by the overestimation of costs for inexpensive contrast agents (those with per day costs less than the $65 packaging threshold) created by application of the same single CCR to inexpensive contrast agents' charges, assuming that hospitals apply a lower markup to expensive contrast agents and a higher markup to inexpensive contrast agents. If the mix of expensive and inexpensive contrast agents resembled the mix of expensive and inexpensive drugs generally captured in the cost center 5600 “Drugs Charged to Patients,” the use of a single CCR would accurately estimate total cost of contrast agents in aggregate. Because all contrast agents not receiving pass-through payment are packaged, packaging an accurate aggregate cost estimate for contrast agents could argue against redistributing cost from packaged contrast agents to separately payable drugs and biologicals. However, in our CY 2010 final rule claims data, we observed only 3 percent of total contrast agent cost associated with those contrast agents that have per day costs above $65.
In conclusion, both because contrast agents are billed under pharmacy revenue codes and accounted for in the cost center 5600 “Drugs Charged to Patients” and because the per day cost of almost all contrast agents falls under the CY 2010 packaging threshold of $65, we believe the estimated cost of contrast agents contains a disproportionate amount of pharmacy overhead cost and that it is appropriate to include them in our final CY 2010 redistribution methodology.
While we believe that contrast agents are commonly billed under pharmacy revenue codes and that hospitals largely account for the cost of contrast agents under the cost center 5600 on their Medicare hospital cost report, we did not observe that hospitals apply the same practice for diagnostic radiopharmaceuticals. After reviewing our claims data, we found that the majority of diagnostic radiopharmaceuticals are billed under revenue code 0343 (Nuclear Medicine; Diagnostic Radiopharmaceuticals). As specified in our revenue code-to-cost center crosswalk, we believe hospitals largely account for the costs and charges associated with revenue code 0343 in a nonstandard cost center for Diagnostic Nuclear Medicine or the cost center 4100 “Radiology-Diagnostic.” Because the redistribution of pharmacy overhead cost from packaged drugs and biologicals to separately payable drugs and biologicals is intended to specifically address charge compression in the pharmacy cost center, in light of the above information, we excluded the costs of both diagnostic and therapeutic radiopharmaceuticals from our estimate of total drug and biological cost in the claims data for the final CY 2010 redistribution methodology. As a result, the final payment rates for nuclear medicine procedures that incorporate the costs of packaged diagnostic radiopharmaceuticals are not impacted by the final redistribution methodology.
Comment: Several commenters cited methodological concerns about the approach CMS used to calculate the proposed equivalent average ASP-based payment amount for separately payable drugs and biologicals. In addition, the commenters expressed concern that CMS' cost estimation methodology is very sensitive to changes in the underlying data and assumptions. Citing these concerns, some commenters requested payment at ASP+6 percent for parity with physician's office payment rates for drugs, arguing that hospital costs for acquisition and associated pharmacy overhead would be at least as high, if not significantly greater, than the physician's office costs.
Some commenters noted that the statute requires drug cost surveys for payment purposes for SCODs under the OPPS, and that the most recent survey available is outdated as it was performed in CY 2004 by the GAO. The commenters stated that the statute specifically requires survey data as the basis for hospital acquisition costs in order to provide a more appropriate payment methodology for drugs and biologicals, instead of costs calculated from claims data. They concluded that, by not performing a survey and by not paying for drugs and biologicals at the physician's office rate, CMS is not in compliance with the statute. The commenters acknowledged that drug cost surveys are difficult to perform. However, they asserted that, in order to comply with the requirements of the statute, either a survey should be performed or payment should be made at ASP+6 percent. Other commenters cited the methodological concerns that are described below regarding the proposed proxy for average acquisition cost based upon claims data, and stated that until CMS is able to adequately address these concerns, CMS should implement payment at ASP+6 percent pursuant to section 1833(t)(14)(A)(iii)(I).
The commenters' first methodological concern is that CMS compared cost estimates from different points in time to develop payment rates for drugs and biologicals. Specifically, several commenters noted that for the proposed rule, CMS used ASP data from the fourth quarter of CY 2008, which is the basis for calculating payment rates for drugs and biologicals in the physician's office setting using the ASP methodology effective April 1, 2009, along with hospital claims data from CY 2008 to determine the relative ASP amount for CY 2010 under CMS' proposed drug payment methodology. The commenters requested that CMS use an alternative ASP file for the final rule calculation of ASP+X to better align ASP data with hospital claims and cost report data. The commenters stated that CMS compared hospital claims data from throughout CY 2008 with costs estimated from charges that include pharmacy overhead cost, with ASP data as a proxy for acquisition cost representing drug sales in the fourth quarter of CY 2008, well after the time hospitals would have purchased most of their drugs for administration in CY 2008. As an alternative, the commenters requested that CMS use an earlier ASP file that is more representative of the costs to hospitals when they purchase drugs for the claims year. Specifically, some commenters requested that CMS use the July 1, 2008 ASP file that represents sales from the first quarter of CY 2008 when comparing CY 2008 hospital claims data to ASP data to determine an ASP+X amount.
Second, many commenters reiterated concerns that when CMS applies a single CCR to adjust charges to costs for these drugs and biologicals, charge compression leads to misallocation of the pharmacy overhead costs associated with high and low cost drugs and biologicals during ratesetting. The commenters noted that hospitals disproportionately mark up their charges for low cost drugs and biologicals to account for pharmacy overhead costs. They indicated that while the aggregate charges for inexpensive and expensive drugs may include the total pharmacy overhead Start Printed Page 60515 costs of the hospital, the charges for individual drugs and biologicals do not represent the specific acquisition and pharmacy overhead costs of that particular drug or biological. The commenters explained that hospitals apply proportionately smaller markups to higher cost items and proportionately larger markups to lower cost items. The commenters argued that by using only separately payable drugs in the calculation of the equivalent average ASP-based amount, the pharmacy overhead costs associated with these separately payable drugs that are disproportionately included in the charges for packaged drugs are not factored into the calculation, resulting in an artificially low ASP add-on percentage. Therefore, some commenters suggested using the costs of both packaged drugs and separately payable drugs when calculating the equivalent average ASP-based payment amount for separately payable drugs, as they argued that this would provide a more accurate ASP-based payment amount for separately payable drugs. As an alternative, the commenters recommended that CMS eliminate the drug packaging threshold and provide separate payment for all Part B drugs under the OPPS at an ASP+X percent amount calculated from the cost of all drugs with HCPCS codes.
Finally, several commenters noted that CMS included, in the calculation of the costs of separately payable drugs and biologicals, OPPS claims data from hospitals that receive Federal discounts on drug prices under the 340B drug pricing program. The commenters pointed out that hospital participation in the 340B program had grown substantially over the past few years, and they believed that the costs from these hospitals now constituted a significant proportion of hospital drug costs on CY 2008 OPPS claims. The commenters stated that including 340B hospital claims data when comparing aggregate hospital costs based on claims data to ASP rates contributed to an artificially low equivalent average ASP-based payment rate because ASP data specifically exclude drugs sales under the 340B program.
Response: As discussed above, the provision in section 1833(t)(14)(A)(iii) of the Act continues to be applicable to determining payments for SCODs for CY 2010. This provision requires that payment for SCODs be equal to the average acquisition cost for the drug for that year (which, at the option of the Secretary, may vary by hospital group) as determined by the Secretary, subject to any adjustment for overhead costs and taking into account the hospital acquisition cost survey data under section 1833(t)(14)(D) of the Act, or if hospital acquisition cost data are not available, then the average price for the drug in the year established under section 1842(o), 1847A, or 1847B of the Act, as the case may be, as calculated and adjusted by the Secretary as necessary for purposes of section 1833(t)(14)of the Act. In the CY 2006 OPPS final rule with comment period (70 FR 68640), we compared hospital drug cost data that were available to us at the time, specifically: (1) data from the GAO survey; (2) hospital claims data from CY 2004; and (3) ASP information. In addition, we discussed our methodology for comparing these data that represented different timeframes from 2004 to 2006. As a result of our analysis comparing these three sources, we concluded that, on average, the costs from hospital claims data representing SCODs were roughly equivalent to payment at ASP+6 percent. Therefore, we finalized a policy that used our hospital claims data as a proxy for average hospital acquisition cost and provided payment for separately payable drugs that do not have pass-through status at ASP+6 percent for CY 2006 (70 FR 68639 through 68642). While the commenters are correct that the statute allows for the use of the methodology described in section 1842(o), section 1847A or section 1847B of the Act, as calculated and adjusted by the Secretary as necessary, this is only when hospital acquisition cost data are not available. We believe that we have established both our hospital claims data and ASP data as an appropriate proxy for average hospital acquisition cost, taking the GAO survey information into account for the base year (70 FR 68641). Many of the drugs and biologicals covered under the OPPS are provided a majority of the time in the hospitals setting, and the ASP information we collect would be an adequate proxy for hospital acquisition cost. Further, as already discussed, the commenters have not disputed the accuracy of the total drug and biological cost estimated in our claims data, only the estimated cost of separately payable drugs and biologicals. While we have not yet performed hospital drug acquisition cost surveys similar to the GAO survey, we note that the statute only calls for “periodic” surveys. Therefore, we disagree that we are not complying with the statute by not performing a survey and not paying at the physician's office rate. We note, however, that we are considering the possibility of such a survey at some point in the future. Therefore, we do not believe that it is appropriate at this time to provide payment at anything other than average acquisition cost, with a redistribution for pharmacy overhead, based on the drug and biological costs observed in hospital claims data and pricing information observed in ASP data. We disagree with the commenters who believe that our redistribution methodology is not an appropriate proxy for average hospital acquisition cost, with an adjustment for pharmacy overhead cost. We have no basis for providing payment for separately payable drugs at the physician's office rate in the face of an appropriate proxy for average hospital acquisition cost, as described in detail below.
In response to the commenters who claimed that hospital costs for drug acquisition and associated pharmacy overhead would be at least as high, if not significantly greater, than the physician's office costs, we have no information that would confirm this statement. ASP information is only available for all sales of drugs and biologicals, so we cannot compare hospital and physician's office acquisition costs for individual drugs and biologicals or in aggregate. While our final CY 2010 pharmacy overhead adjustment methodology for payment of separately payable drugs and biologicals relies on the assumption that ASP is a fair estimate of hospitals' average acquisition cost of drugs and biologicals in general, we expect that drug acquisition costs could vary across settings and clinical cases. In some cases hospital drug acquisition costs could be lower than the costs to physicians' offices, based on high volume purchasing agreements, and in other cases hospital acquisition costs could be greater, based on their need for emergency purchases outside of negotiated contracts with preestablished best rates. We also expect that pharmacy overhead costs could vary across hospital and physician's office settings, based on the drugs and biologicals administered in those settings. Many hospitals provide a range of drugs and biologicals, including those with high and low pharmacy overhead costs, whereas physicians' offices may be more likely to provide drugs and biologicals with typically high (or low) pharmacy overhead costs. This unknown variability in drug acquisition and pharmacy overhead costs across settings means that we cannot conclude that the acquisition and pharmacy overhead costs of drugs and biologicals in the HOPD are greater or less than the physician's office costs. Finally, the ASP-based payment rate for drugs Start Printed Page 60516 furnished in physicians' offices is specified in the statute as ASP+6 percent, whereas the OPPS payment is based on average hospital acquisition cost and associated pharmacy overhead cost. Therefore, we do not believe that comparisons of OPPS drug payment rates with physician's office payment rates suggesting that parity is necessary are applicable to determining appropriate payment rates for separately payable drugs and biologicals under the OPPS.
For our calculation of per day costs of HCPCS codes for drugs and nonimplantable biologicals in the CY 2010 OPPS/ASC final rule with comment period, we proposed to use ASP data from the first quarter of CY 2009, which is the basis for calculating payment rates for drugs and biologicals in the physician's office setting using the ASP methodology effective July 1, 2009, along with updated hospital claims data from CY 2008 (74 FR 35320). We also proposed to use these data for budget neutrality estimates and impact analyses for this CY 2010 OPPS/ASC final rule with comment period. Payment rates for HCPCS codes for separately payable drugs and nonimplantable biologicals included in Addenda A and B to this final rule with comment period are based on ASP data from the second quarter of CY 2009, which are the basis for calculating payment rates for drugs and biologicals in the physician's office setting using the ASP methodology effective October 1, 2009.
Since implementing the ASP+X methodology in CY 2006, we have used the most recently available data to establish our relative ASP payment rate for the upcoming year, consistent with our overall policy of updating the OPPS of using the most recent claims and cost report data. For the CY 2010 final rule, this results in using July 2009 ASP payment rates (based on first quarter CY 2009 sales), CY 2008 hospital claims data, and the most recently available hospital cost reports (in the majority of cases cost reports beginning in CY 2007). As we have noted in previous years, the relative ASP+X amount is likely to change from the proposed rule to the final rule as a result of updated ASP data, hospital claims data, and updated hospital cost reports. If we were to introduce significant error into our ASP+X percent calculation by not aligning all pricing and cost data to a single period of time, we would consider changing the ASP data that we use. However, we believe that if we were to use an ASP file from CY 2008, which commenters claim would more accurately represent hospital costs associated with procuring drugs and biologicals for that claims year, we would need to offset any increases in the relative ASP amount resulting from the use of a different ASP file with a deflation adjustment for each hospital's CCRs for cost center 5600 “Drugs Charged to Patients” in order to simulate costs from claim charges in the claim year. We make comparable CCR deflation estimates when we set our fixed dollar eligibility threshold for outlier payments described in section II.F. of this final rule with comment period. Because over recent years hospital charges have typically grown faster than costs, we would expect such an adjustment to reduce estimated costs in our claims data. Therefore, we are continuing our current policy of using the most recently available claims, cost report, and ASP data when performing our ASP+X calculation under the final CY 2010 redistribution methodology in order to set payment rates for separately payable drugs and biologicals.
For CY 2010, we again attempted to address the issue of charge compression by proposing a methodology that reallocates pharmacy overhead costs from packaged drugs and biologicals to separately payable drugs and biologicals. We have made several proposals in the past to identify pharmacy overhead costs and address charge compression in the pharmacy revenue center. For the CY 2006 OPPS, we proposed to establish three distinct Level II HCPCS C-codes and three corresponding APCs for drug handling categories to differentiate overhead costs for drugs and biologicals (70 FR 42730). In the CY 2008 OPPS/ASC proposed rule (72 FR 42735), we proposed to instruct hospitals to remove the pharmacy overhead charge for both packaged and separately payable drugs and biologicals from the charge for the drug or biological and report the pharmacy overhead charge on an uncoded revenue code line on the claim. We believed that this would provide us with an avenue for collecting pharmacy handling cost data specific to drugs in order to package the overhead costs of these items into the associated procedures, most likely drug administration services. For CY 2009, we proposed to split the “Drugs Charged to Patients” cost center into two cost centers: one for drugs with high pharmacy overhead costs and one for drugs with low pharmacy overhead costs (73 FR 41492). We noted that we expected that CCRs from the proposed new cost centers would be available in 2 to 3 years to refine OPPS drug cost estimates by accounting for differential hospital markup practices for drugs with high and low overhead costs. However, we did not finalize any of these proposals due to concerns from the hospital community that these proposals would create an overwhelming burden on hospitals and staff. We have once again proposed to address the issue of charge compression, in this case without requiring any changes to current hospital reporting practices.
It has been our policy, since CY 2006, to only use separately payable drugs and biologicals in the calculation of the equivalent average ASP-based payment amount under the OPPS. We do not include packaged drugs and biologicals in this standard analysis because cost data for these items are already accounted for within the APC ratesetting process through the median cost calculation methodology discussed in section II.A.2. of this final rule with comment period. To include the costs of coded packaged drugs and biologicals in both our APC ratesetting process (for associated procedures present on the same claim) and in our ratesetting process to establish an equivalent average ASP-based payment amount for separately payable drugs and biologicals would give these data disproportionate emphasis in the OPPS system by skewing our analyses, as the costs of these packaged items would be, in effect, counted twice. Accordingly, we are not adopting the suggestion from commenters that we include all packaged and separately payable drugs and biologicals when establishing an equivalent average ASP-based rate to provide payment for the hospital acquisition and pharmacy handling costs of drugs and biologicals. However, we remind commenters that because the costs of packaged drugs and biologicals, including their pharmacy overhead costs, are packaged into the payments for the procedures in which they are administered, the OPPS provides payment for both the drugs and the associated pharmacy overhead costs through the applicable procedural APC payments. Furthermore, we disagree with the commenters who recommend that we should pay separately for all drugs and biologicals with HCPCS codes. We continue to believe that packaging is a fundamental component of a prospective payment system that contributes to important flexibility and efficiency in the delivery of high quality hospital outpatient services and, therefore, we believe it is appropriate to maintain a modest drug packaging threshold that packages the costs of Start Printed Page 60517 inexpensive drugs into payment for the associated procedures.
Moreover, we continue to believe that excluding data from hospitals that participate in the 340B program from our ASP+X calculation, and paying those hospitals at that derived payment amount, would inappropriately redistribute payment to drugs and biologicals from payment for other services under the OPPS. The ASP-equivalent cost of drugs under the OPPS that would be calculated only from claims data for non-340B hospitals would likely be higher than the cost of all drugs from our aggregate claims for all hospitals. To set drug payment rates for all hospitals based on a subset of hospital cost data, determined only from claims data for non-340B hospitals would increase the final APC payment weights for drugs in a manner that does not reflect the drug costs of all hospitals, although all hospitals, including 340B hospitals, would be paid at these rates for drugs. Furthermore, as a consequence of the statutory requirement for budget neutrality, increasing the payment weights for drugs by excluding 340B hospital claims would reduce the relative payment weights for other services in a manner that does not reflect the procedural costs of all hospitals relative to the drug costs of all hospitals, thereby distorting the relativity of payment weights for services based on hospital costs. Many commenters on the CY 2009 OPPS/ASC final rule with comment period were generally opposed to differential payment for hospitals based on their 340B participation status, and we do not believe it would be appropriate to exclude claims from this subset of hospitals in the context of a CY 2010 drug and biological payment policy that pays all hospitals at the same rate for separately payable drugs and biologicals.
Therefore, for CY 2010, we are finalizing our proposed payment for separately payable drugs and biologicals, with modification. We are redistributing $200 million from the cost of packaged drugs and biologicals to separately payable drugs and biologicals. The $200 million consists of $150 million (one-third of the pharmacy overhead cost) from the coded packaged drug and biological cost for drugs and biologicals with an ASP and $50 million from the packaged drug and biological cost for drugs and biologicals without an ASP. To model this policy for the CY 2010 final rule with comment period, we reduced the cost of coded packaged drugs and biologicals with an ASP by 24 percent (based on final rule data; reduction was 27 percent based on proposed rule data) and the cost of packaged drugs and biologicals without a HCPCS code or an ASP by 8 percent when we calculated the median cost of the CY 2010 procedural APCs. This redistribution results in payment for separately payable drugs and biologicals at a transitional rate of ASP+4 percent for CY 2010 under a pharmacy overhead adjustment methodology. We are, therefore, not accepting the August 2009 recommendation of the APC Panel to pay for separately payable drugs and biologicals at ASP+6 percent. Furthermore, because we are finalizing payment of separately payable drugs at APS+4 percent, we are not accepting the August 2009 APC Panel recommendations to analyze the impact of ASP+6 percent payment on different classes or for services that use packaged drugs compared with payment at ASP+4 percent. We are accepting the recommendation of the APC Panel to continue to refine our analyses of payment for drugs, biologicals, and radiopharmaceuticals and continue to welcome information and analyses from the public regarding pharmacy overhead costs.
Comment: One commenter requested that CMS create a HCPCS J-code for tositumomab, currently provided under a radioimmunotherapy regimen and billed as part of HCPCS code G3001 (Administration and supply of tositumomab, 450 mg). The commenter argued that because tositumomab is listed in compendia, is approved by the FDA as part of the BEXXAR® regimen, and has its own National Drug Code (NDC) number, it should be recognized as a drug and, therefore, be paid as other drugs are paid under the OPPS methodology, instead of having a payment rate determined by hospital claims data. The commenter suggested that a payment rate could be established using the ASP methodology.
Response: We have consistently noted that unlabeled tositumomab is not approved as either a drug or a radiopharmaceutical, but it is a supply that is required as part of the radioimmunotherapy treatment regimen (November 18, 2008 OPPS/ASC final rule with comment period (73 FR 68658); November 27, 2007 OPPS/ASC final rule with comment period for CY 2008 (72 FR 66765); November 10, 2005 OPPS final rule with comment period for CY 2006 (70 FR 68654); November 7, 2003 OPPS final rule with comment period for CY 2004 (68 FR 63443)). We do not make separate payment for supplies used in services provided under the OPPS. Payments for necessary supplies are packaged into payments for the separately payable services provided by the hospital. Specifically, administration of unlabeled tositumomab is a complete service that qualifies for separate payment under its own clinical APC. This complete service is currently described by HCPCS code G3001. Therefore, we do not agree with the commenter's recommendation that we should assign a separate HCPCS code to the supply of unlabeled tositumomab. Rather, we will continue to make separate payment for the administration of tositumomab, and payment for the supply of unlabeled tositumomab is packaged into the administration payment.
We note that separately payable drug and biological payment rates listed in Addenda A and B to this final rule with comment period, which illustrate the final CY 2010 payment of ASP+4 percent for separately payable nonpass-through drugs and nonimplantable biologicals and ASP+6 percent for pass-through drugs and biologicals, reflect either ASP information that is the basis for calculating payment rates for drugs and biologicals in the physician's office setting effective October 1, 2009 or mean unit cost from CY 2008 claims data and updated cost report information available for this final rule with comment period. In general, these published payment rates are not reflective of actual January 2010 payment rates. This is because payment rates for drugs and biologicals with ASP information for January 2010 will be determined through the standard quarterly process where ASP data submitted by manufacturers for the third quarter of 2009 (July 1, 2009 through September 30, 2009) are used to set the payment rates that are released for the quarter beginning in January 2010 near the end of December 2009. In addition, payment rates for drugs and biologicals for which there was no ASP information available for October 2009 payment and, therefore, these products would be paid based on mean unit cost in CY 2010 based on available information at the time of this final rule with comment period, may have ASP information available for payment for the quarter beginning in January 2010. These drugs and biologicals would then be priced based on their newly available ASP information. Finally, there may be drugs and biologicals that have ASP information available for this final rule with comment period (reflecting October 2009 ASP data) that do not have ASP information available for the quarter beginning in January 2010. These drugs and biologicals would then be paid based on mean unit cost data derived from CY 2008 hospital claims. Start Printed Page 60518 Therefore, the payment rates listed in Addenda A and B to this final rule with comment period are not for January 2010 payment purposes and are only illustrative of the CY 2010 OPPS payment methodology using the most recently available information at the time of this final rule with comment period.
4. Payment for Blood Clotting Factors
For CY 2009, we are providing payment for blood clotting factors under the OPPS at ASP+4 percent, plus an additional payment for the furnishing fee. We note that when blood clotting factors are provided in physicians' offices under Medicare Part B and in other Medicare settings, a furnishing fee is also applied to the payment. The CY 2009 updated furnishing fee is $0.164 per unit.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35333), for CY 2010, we proposed to pay for blood clotting factors at ASP+4 percent, consistent with our proposed payment policy for other nonpass-through separately payable drugs and biologicals, and to continue our policy for payment of the furnishing fee using an updated amount. Because the furnishing fee update is based on the percentage increase in the Consumer Price Index (CPI) for medical care for the 12-month period ending with June of the previous year and the Bureau of Labor Statistics releases the applicable CPI data after the MPFS and OPPS/ASC proposed rules are published, we were not able to include the actual updated furnishing fee in this proposed rule and we also are not able to include the actual updated furnishing fee in this final rule with comment period. Therefore, in accordance with our policy as finalized in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66765), we will announce the actual figure for the percent change in the applicable CPI and the updated furnishing fee calculated based on that figure through applicable program instructions and posting on the CMS Web site at: http://www.cms.hhs.gov/McrPartBDrugAvgSalesPrice/.
Comment: One commenter expressed support for CMS' proposal to continue to apply the furnishing fee for blood clotting factors provided in the OPD. The commenter stated that the furnishing fee helps ensure patient access to blood clotting factors by increasing the payment rate for these items. Another commenter disagreed with CMS' proposed payment rate of ASP+4 percent for blood clotting factors in CY 2010, even with the furnishing fee add-on. The commenters stated that ASP+4 percent was inadequate for all drugs and biologicals, and is especially inappropriate for blood clotting factors. Finally, one commenter supported the payment of blood clotting factors at the same rate that applies to other nonpass-through separately payable drugs and biologicals in the HOPD.
Response: We continue to believe that applying the furnishing fee for blood clotting factors is appropriate for CY 2010. However, we see no compelling reason to provide payment for blood clotting factors under a different methodology for OPPS purposes at this time. We believe that the payment rate of ASP+4 percent that we are finalizing for payment of all nonpass-through separately payable drugs and biologicals in CY 2010 is appropriate. In addition, we believe that it continues to be appropriate to pay a furnishing fee for blood clotting factors under the OPPS, as is done in the physician's office setting and the inpatient hospital setting.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to provide payment for blood clotting factors under the same methodology as other separately payable drugs and biologicals under the OPPS and to continue paying an updated furnishing fee.
5. Payment for Therapeutic Radiopharmaceuticals
a. Background
Section 303(h) of Public Law 108–173 exempted radiopharmaceuticals from ASP pricing in the physician's office setting. Beginning in the CY 2005 OPPS final rule with comment period, we have exempted radiopharmaceutical manufacturers from reporting ASP data for payment purposes under the OPPS. (For more information, we refer readers to the CY 2005 OPPS final rule with comment period (69 FR 65811) and the CY 2006 OPPS final rule with comment period (70 FR 68655).) Consequently, we did not have ASP data for radiopharmaceuticals for consideration for previous years' OPPS ratesetting. In accordance with section 1833(t)(14)(B)(i)(I) of the Act, we have classified radiopharmaceuticals under the OPPS as SCODs. As such, we have paid for radiopharmaceuticals at average acquisition cost as determined by the Secretary and subject to any adjustment for overhead costs. Radiopharmaceuticals also are subject to the policies affecting all similarly classified OPPS drugs and biologicals, such as pass-through payment for diagnostic and therapeutic radiopharmaceuticals and individual packaging determinations for therapeutic radiopharmaceuticals, discussed earlier in this proposed rule.
For CYs 2006 and 2007, we used mean unit cost data from hospital claims to determine each radiopharmaceutical's packaging status and implemented a temporary policy to pay for separately payable radiopharmaceuticals based on the hospital's charge for each radiopharmaceutical adjusted to cost using the hospital's overall CCR. In addition, in the CY 2006 OPPS final rule with comment period (70 FR 68654), we instructed hospitals to include charges for radiopharmaceutical handling in their charges for the radiopharmaceutical products so these costs would be reflected in the CY 2008 ratesetting process. The methodology of providing separate radiopharmaceutical payment based on charges adjusted to cost through application of an individual hospital's overall CCR for CYs 2006 and 2007 was finalized as an interim proxy for average acquisition cost because of the unique circumstances associated with providing radiopharmaceutical products to Medicare beneficiaries. The single OPPS payment represented Medicare payment for both the acquisition cost of the radiopharmaceutical and its associated handling costs.
During the CY 2006 and CY 2007 rulemaking processes, we encouraged hospitals and radiopharmaceutical stakeholders to assist us in developing a viable long-term prospective payment methodology for these products under the OPPS. As reiterated in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66766), we were pleased to note that we had many discussions with interested parties regarding the availability and limitations of radiopharmaceutical cost data.
In considering payment options for therapeutic radiopharmaceuticals for CY 2008, we examined several alternatives that we discussed in the CY 2008 OPPS/ASC proposed rule (72 FR 42738 through 42739) and CY 2008 OPPS/ASC final rule with comment period (72 FR 66769 through 66770). After considering the options and the public comments received, we finalized a CY 2008 methodology to provide prospective payment for therapeutic radiopharmaceuticals (defined as those Level II HCPCS codes that include the term “therapeutic” along with a radiopharmaceutical in their long code descriptors) using mean costs derived from the CY 2006 claims data, where the costs were determined using our standard methodology of applying hospital-specific departmental CCRs to Start Printed Page 60519 radiopharmaceutical charges, defaulting to hospital-specific overall CCRs only if appropriate departmental CCRs were unavailable (72 FR 66772). In addition, we finalized a policy to package payment for all diagnostic radiopharmaceuticals (defined as those Level II HCPCS codes that include the term “diagnostic” along with a radiopharmaceutical in their long code descriptors) for CY 2008. As discussed in the CY 2008 OPPS/ASC proposed rule (72 FR 42739), we believed that adopting prospective payment for therapeutic radiopharmaceuticals based on historical hospital claims data was appropriate because it served as our most accurate available proxy for the average hospital acquisition cost of separately payable therapeutic radiopharmaceuticals. In addition, we noted that we have found that our general prospective payment methodology based on historical hospital claims data results in more consistent, predictable, and equitable payment amounts across hospitals and likely provides incentives to hospitals for efficiently and economically providing these outpatient services.
Prior to the implementation of the final CY 2008 methodology of providing a prospective payment for therapeutic radiopharmaceuticals, section 106(b) of Public Law 110–173 was enacted on December 29, 2007 specifying payment for therapeutic radiopharmaceuticals based on individual hospital charges adjusted to cost. Therefore, hospitals continued to receive payment for therapeutic radiopharmaceuticals by applying the hospital-specific overall CCR to each hospital's charge for a therapeutic radiopharmaceutical from January 1, 2008, through June 30, 2008. As we stated in the CY 2009 OPPS/ASC proposed rule (73 FR 41493), thereafter, the OPPS would provide payment for separately payable therapeutic radiopharmaceuticals on a prospective basis, with payment rates based upon mean costs from hospital claims data as set forth in the CY 2008 OPPS/ASC final rule with comment period, unless otherwise required by law.
Following issuance of the CY 2009 OPPS/ASC proposed rule, section 142 of Public Law 110–275 amended section 1833(t)(16)(C) of the Act, as amended by section 106(a) of Public Law 110–173, to further extend the payment period for therapeutic radiopharmaceuticals based on hospital's charges adjusted to cost through December 31, 2009. Therefore, we are continuing to pay hospitals for therapeutic radiopharmaceuticals at charges adjusted to cost through the end of CY 2009.
b. Payment Policy
Since the start of the temporary cost-based payment methodology for radiopharmaceuticals in CY 2006, we have met with several interested parties on a number of occasions regarding payment under the OPPS for radiopharmaceuticals and have received numerous different suggestions from these stakeholders regarding payment methodologies that we could employ for future use under the OPPS.
In the CY 2008 OPPS/ASC final rule with comment period (72 FR 66771), we solicited comments requesting interested parties to provide information related to whether the existing ASP methodology could be used to establish payment for specific therapeutic radiopharmaceuticals under the OPPS. Similar to the recommendations we received during the CY 2008 OPPS/ASC proposed rule comment period (72 FR 66770), we received several suggestions regarding the establishment of an OPPS-specific methodology for radiopharmaceutical payment that would be similar to the ASP methodology, without following the established ASP procedures referenced at section 1847A of the Act and implemented through rulemaking. Some commenters recommended using external data submitted by a variety of sources other than manufacturers. Along this line, commenters suggested gathering information from nuclear pharmacies using methodologies with a variety of names such as Nuclear Pharmacy Calculated Invoiced Price (Averaged) (CIP) and Calculated Pharmacy Sales Price (CPSP). Other commenters recommended that CMS base payment for certain radiopharmaceuticals on manufacturer-reported ASP.
As noted in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66771), a ratesetting approach based on external data would be administratively burdensome for us because we would be required to collect, process, and review external information to ensure that it was valid, reliable, and representative of a diverse group of hospitals so that it could be used to establish rates for all hospitals. However, we specifically requested additional comments regarding the use of the existing ASP reporting structure for therapeutic radiopharmaceuticals as this established methodology was already used for payment of other drugs provided in the hospital outpatient setting (72 FR 66771). While we received several recommendations from commenters on the CY 2008 OPPS/ASC final rule with comment period regarding payment of therapeutic radiopharmaceuticals based on estimated costs provided by manufacturers or other parties, we believe that the use of external data for payment of therapeutic radiopharmaceuticals should only be adopted if those external data are subject to the same well-established regulatory framework as the ASP data currently used for payment of separately payable drugs and biologicals under the OPPS. We have previously indicated that nondevice external data used for setting payment rates should be representative of a diverse group of hospitals both by location and type, and should also identify the relevant data sources. We do not believe that external therapeutic radiopharmaceutical cost data voluntarily provided outside of the established ASP methodology, either by manufacturers or nuclear pharmacies, would generally satisfy these criteria that are minimum standards for setting OPPS payment rates.
We received public comments on the CY 2008 OPPS/ASC final rule with comment period from certain radiopharmaceutical manufacturers who indicated that the standard ASP methodology could be used for payment of certain therapeutic radiopharmaceutical products. Specifically, these manufacturers expressed interest in providing ASP data for their therapeutic radiopharmaceutical products as a basis for payment under the OPPS.
In the CY 2009 OPPS/ASC proposed rule (73 FR 41495), we proposed to allow manufacturers to submit ASP information for any separately payable therapeutic radiopharmaceutical for payment purposes under the OPPS. If ASP information was not submitted or appropriately certified by the manufacturer for a given calendar year quarter, then for that quarter we proposed to provide prospective payment based on the therapeutic radiopharmaceuticals mean cost from hospital claims data. However, as stated above, section 142 of Public Law 110–275 amended section 1833(t)(16)(C) of the Act, as amended by section 106(a) of Public Law 110–173, to further extend the payment period for therapeutic radiopharmaceuticals based on hospital's charges adjusted to cost through December 31, 2009, so we did not finalize this proposal. We note that, in response to our proposed therapeutic radiopharmaceutical payment methodology for CY 2009, we received a number of public comments that were supportive of the proposal for future years.
At the February 2009 meeting of the APC Panel, the APC Panel Start Printed Page 60520 recommended that CMS use the ASP methodology to pay for therapeutic radiopharmaceuticals and, where ASP data are not available, to pay based on mean costs from claims data for CY 2010. We accepted this recommendation, and for CY 2010, we proposed to allow manufacturers to submit ASP information for any separately payable therapeutic radiopharmaceutical in order for therapeutic radiopharmaceuticals to be paid based on ASP beginning in CY 2010 under the OPPS (74 FR 35334 through 35336). Similar to our CY 2009 proposal, for CY 2010, we did not propose to compel manufacturers to submit ASP information. Furthermore, as discussed in the CY 2009 OPPS/ASC proposed rule (73 FR 41495), we stated that the ASP data submitted would need to be provided for a patient-specific dose, or patient-ready form, of the therapeutic radiopharmaceutical in order to properly calculate the ASP amount for a given HCPCS code. In addition, in those instances where there is more than one manufacturer of a particular therapeutic radiopharmaceutical, we noted that all manufacturers would need to submit ASP information in order for payment to be made on an ASP basis. We specifically requested public comment on the development of a crosswalk, similar to the NDC/HCPCS crosswalk for separately payable drugs and biologicals posted on the CMS Web site at: http://www.cms.hhs.gov/McrPartBDrugAvgSalesPrice/01a1_2009aspfiles.asp#TopOfPage, for use for therapeutic radiopharmaceuticals.
Comment: Several commenters requested guidance on how costs associated with the manufacturing, compounding, and preparation of therapeutic radiopharmaceuticals should be reported when submitting ASP. The commenters stated that there are several activities that may take place at a variety of locations in order to prepare a radiopharmaceutical for patient administration. These services range in complexity from activities typical to any hospital pharmacy, such as drawing up a therapeutic radiopharmaceutical into a syringe and ensuring proper disposal of wasted product, to more complex processing such as preparing the therapeutic radiopharmaceutical itself by radiolabeling a cold kit (nonradioactive compound or complex that is combined with a radioisotope and results in a radiopharmaceutical) supplied by the manufacturer using a radioisotope supplied by the manufacturer or another source. As CMS requested ASP information from separately payable radiopharmaceutical manufacturers in the form of a patient-specific dose, or patient-ready form, several commenters argued that the ASP amount reported to CMS should reflect all costs associated with these additional activities or items, such as the radioisotope and radiolabeling processes, needed to provide a patient-ready dose of a radiopharmaceutical. Several commenters pointed out that, based on current business practices, a single manufacturer might be unable to report ASP data for a therapeutic radiopharmaceutical described by a HCPCS code that incorporated all of these costs, and others were concerned that CMS intended for the manufacturer to collect and report the costs of the activities of other entities, such as freestanding radiopharmacies, that would not typically be reflected in the radiopharmaceutical manufacturer's sales price.
Response: In the following response, we discuss our expectations for manufacturer submission of ASP data to CMS to set OPPS payment rates for separately payable therapeutic radiopharmaceuticals. This methodology also would apply to manufacturers submitting ASP data for diagnostic and therapeutic radiopharmaceuticals with pass-through status. As discussed in section V.A. of this final rule with comment period, we would use any submitted ASP information for separately payable diagnostic and therapeutic radiopharmaceuticals with pass-through status to establish a payment rate of ASP+6 percent, consistent with our policy for pass-through payment of drugs and biologicals. We note that ASP submissions for radiopharmaceutical payment under the OPPS would need to meet all of the existing regulatory and subregulatory requirements of the ASP reporting process under sections 1847A and 1927(b)(3) of the Act, except as otherwise specified in this final rule with comment period.
For CY 2010, when reporting an ASP for a separately payable radiopharmaceutical, we expect that the ASP data reported by a manufacturer would be representative of the item(s) sold by the manufacturer. We used the term “patient-ready” in our proposed rule to ensure that ASP data submitted for OPPS payment purposes for separately payable radiopharmaceuticals reflect the costs of all the component materials of the finished radiopharmaceutical product. We expect that the ASP data would represent the sales price of all of the component materials of the finished radiopharmaceutical product sold by the manufacturer in terms that reflect the applicable HCPCS code descriptor, such as “treatment dose” or “millicurie.” We understand that manufacturers of separately payable radiopharmaceuticals produce radiopharmaceuticals that require a variety of processing steps in order to finalize the product for administration to a beneficiary. For example, some radiopharmaceuticals are the combined product of a cold kit produced by one manufacturer, which is then radiolabeled with a radioisotope provided by a freestanding or hospital nuclear pharmacy. At a minimum, to be used for separate OPPS radiopharmaceutical payment, the ASP data reported by a manufacturer must represent sales of all of the component materials associated with the radiopharmaceutical. In the context of radiopharmaceuticals used in the HOPD, we would expect that the component materials would include at least the cold kit and the radioisotope needed to radiolabel the cold kit in order to make the radiopharmaceutical.
With regard to additional processing steps, we believe manufacturers of radiopharmaceuticals could include in their calculations of ASP for OPPS payment purposes in addition to the prices for the component materials, the portion of the sales price attributable to the production of the manufactured product as it is sold by the manufacturer reporting ASP data. Radiopharmaceuticals are unique in that they require a radioisotope in addition to the cold kit and, at a minimum, they require radiolabeling the cold kit in order to produce a final radiopharmaceutical product. We note that manufacturers have the discretion to determine the form of the final product that is sold, and that the manufacturing process may include processing of the component materials in a variety of ways. To the extent that the price includes processing steps that are a service performed by the manufacturer to produce a radiopharmaceutical, we believe that ASP data submitted for purposes of calculating OPPS payment for radiopharmaceuticals can appropriately capture those additional processing costs.
However, we do not believe that all processing steps that may be needed to prepare the separately payable radiopharmaceutical for administration to the beneficiary must be included in the submitted ASP data in order for the OPPS to use manufacturer-reported ASP data as the basis for Start Printed Page 60521 radiopharmaceutical payment. We expect that the costs of any further processing of the radiopharmaceutical component materials after the manufacturer's sales, which could include radiolabeling when a manufacturer only sells the component materials or could consist of additional preparation besides radiolabeling, would not be included in the ASP data submitted by the manufacturer. However, these processing costs would be paid under the OPPS through the single ASP+4 percent payment rate for nonpass-through, separately payable therapeutic radiopharmaceuticals, in the same way that the OPPS currently pays for the acquisition and pharmacy overhead costs of separately payable drugs and biologicals through this single payment. We do not believe that it would be appropriate to include in the ASP for OPPS purposes the radiopharmaceutical processing services performed in a freestanding radiopharmacy or hospital pharmacy to prepare a final product or its component materials for patient administration after the manufactured product is sold by the manufacturer reporting ASP. In this case, the combined OPPS ASP+4 percent CY 2010 payment for the acquisition and pharmacy overhead and handling costs of the separately payable therapeutic radiopharmaceuticals would pay for these additional processing activities.
To be sufficient for purposes of calculating the OPPS payment, all radiopharmaceutical ASP submissions must meet the existing regulatory and subregulatory requirements of the ASP submission process under sections 1847A and 1927(b)(3) of the Act, except as otherwise specified in this final rule with comment period. In particular, we believe the “bona fide service fee” test in the ASP regulations is instructive here, and we would expect manufacturers to apply the “bona fide service fee” test to determine whether service fees it pays to another entity are “bona fide service fees.” We believe the “bona fide service fee” test can be used in the OPPS ASP context to determine whether a fee that the manufacturer pays to a radiopharmacy for performing a service on behalf of the radiopharmaceutical manufacturer could be excluded from the ASP calculation—that is, it would not be considered a price concession that otherwise would reduce the ASP. The definition of a “bona fide service fee” is included in the ASP regulations (§ 414.802), which defines these fees as “fees paid by a manufacturer to an entity, that represent fair market value for a bona fide, itemized service actually performed on behalf of the manufacturer that the manufacturer would otherwise perform (or contract for) in the absence of the service arrangement, and that are not passed on in whole or in part to a client or customer of an entity whether or not the entity takes title to the drug.” In the context of the ASP calculation under section 1847A of the Act and its implementing regulations, “bona fide service fees” are not considered price concessions that must be deducted from the ASP. Similarly, we believe that for OPPS purposes, fees that are paid by the manufacturer that meet the “bona fide service fee” test would not reduce the ASP. Thus, a radiopharmaceutical manufacturer could contract with an entity, consistent with these regulations, to perform certain steps in the radiopharmaceutical manufacturing process that the manufacturer would otherwise perform itself in order to make the final radiopharmaceutical product, and the fees paid to the entity could qualify as a “bona fide service fee” that would be included in the ASP calculation for OPPS purposes. In light of the necessity of radiolabeling to the production of radiopharmaceuticals, we further believe that for OPPS purposes, the manufacturer's purchase of the radioisotope and payment for radiolabeling the cold kit could be factored into the manufacturer's price for the finished product, and if the fees the manufacturer paid meet the “bona fide service fee” test, they would not need to be netted against the price for purposes of calculating the manufacturer's ASP for the therapeutic radiopharmaceutical. Thus, in effect, for OPPS purposes, if a manufacturer chooses to contract for or purchase these items or services, fees for these bona fide services could be included in the manufacturer-reported ASP. We fully expect that the manufacturer would comply with the ASP regulations and, in particular, would factor these fees into the ASP only if the prices of the services performed by the radiopharmacy are fair market value, and the fees are not passed on to any purchasers of the separately payable radiopharmaceutical.
In summary, a patient-specific dose or patient-ready form in the context of OPPS ASP submission for radiopharmaceutical payment means that the ASP reflecting manufacturer sales must represent sales of all of the component materials for the radiopharmaceutical, including a minimum of a cold kit and a radioisotope, and be reported in terms that reflect the applicable HCPCS code descriptor, such as “treatment dose” or “millicurie.” The ASP would not necessarily take into account the preparation of the final form of the therapeutic radiopharmaceutical for patient administration, including radiolabeling, which may be conducted by the manufacturer, freestanding radiopharmacy, hospital pharmacy, or other entity. With respect to the latter, fees paid by the manufacturer for these services would be excluded from the ASP calculation (that is, would not be considered price concessions that reduce the ASP), only if they are “bona fide service fees” as defined in the regulations governing ASP. Thus, if the manufacturer pays a “bona fide service” fee for the services of the freestanding radiopharmacy, hospital pharmacy, or other entity, and reflects that fee in its price for the radiopharmaceutical, the amount of the “bona fide service fee” would be taken into account in the reported ASP data. However, manufacturers are not required to pay for the preparation of a radiopharmaceutical (including radiolabeling) in a freestanding radiopharmacy, hospital pharmacy, or other entity after sale of all of the component materials, and in that case, the cost of those services would not be reflected in the ASP data submitted to CMS. Manufacturers should submit ASP data for a separately payable radiopharmaceutical that incorporates prices for sales of all of the component materials by the manufacturer. Any additional costs associated with the preparation of the radiopharmaceutical for administration to a beneficiary after the manufacturer's sale of the component materials and any processing that the manufacturer conducts would be paid through the single OPPS ASP+4 percent payment for the acquisition and pharmacy overhead and handling costs of the nonpass-through, separately payable therapeutic radiopharmaceutical.
Comment: Several commenters expressed support for CMS's proposal to make prospective payment for therapeutic radiopharmaceuticals using the ASP methodology in CY 2010 to pay the same ASP+4 percent payment rate that CMS proposed for separately payable nonpass-through drugs and nonimplantable biologicals. A few commenters suggested that CMS consider alternatives to the percentage-based add-on to ASP inherent in the single combined payment for acquisition and handling costs of ASP+4 percent to better account for the more intensive handling that radiopharmaceuticals require. The Start Printed Page 60522 commenters recommended a variety of options, including a fixed add-on payment comparable to the complexity of handling involved, a consignment ASP method that would account for the costs associated with the handling of a radiopharmaceutical, the preparation of an invoice that would give a standard drug percentage for handling charges, or the establishment of some other separate payment mechanism to capture the costs of radiolabeling. One commenter suggested that CMS could create a Level II HCPCS code for hospitals to report their charges for radiolabeling conducted by a radiopharmacy, and hospital cost information developed from these charges could be used to establish a separate payment for radiolabeling services.
Response: We appreciate the commenters' support for our proposal to make prospective payment for nonpass-through, separately payable therapeutic radiopharmaceuticals at the same ASP+4 percent payment rate for a “patient-ready” dose of a radiopharmaceutical that we establish for separately payable drugs and nonimplantable biologicals. In our response to the previous comment, we established our interpretation of “patient-ready” for purposes of the OPPS to mean the ASP, reported in terms that reflect the applicable HCPCS code descriptor, for all component materials of the radiopharmaceutical and any additional processing, including radiolabeling, that is reflected in the price the manufacturer charges for the radiopharmaceutical so long as the fees paid for such additional processing meet the “bona fide service fee” test under the regulations implementing section 1847A of the Act. We explicitly note that because radiopharmaceuticals uniquely require radiolabeling of their component materials, we believe that for purposes of OPPS ASP reporting, radiolabeling could constitute a bona fide service on behalf of the manufacturer, and the fees for which could meet the “bona fide service fee” test. Given our position on radiolabeling, we similarly believe that significant processing costs associated with handling a radiopharmaceutical may be reflected in the prices used to calculate the manufacturer's ASP data for OPPS purposes. As noted above, the combined single payment for nonpass-through, separately payable therapeutic radiopharmaceutical acquisition and overhead costs embodied in the ASP+4 percent payment rate for CY 2010 would address any other processing after the sale by the manufacturer, and we believe this payment is sufficient for these additional handling costs borne by the hospital. Under this interpretation of “patient-ready” dose, we do not believe that making an additional payment for more intensive handling costs is necessary.
We also do not believe that establishing a separate Level II HCPCS code to exclusively capture radiopharmaceutical handling costs is an appropriate approach when we have not adopted such an approach to capture the pharmacy overhead costs of other drugs and biologicals, which also may be substantial in some cases. We have heard from hospitals previously on the issue of separately reporting charges for pharmacy handling costs of drugs. In the CY 2007 OPPS/ASC final rule with comment period (71 FR 68090), we discussed our efforts to create a set of Level II HCPCS codes that hospitals would be able to use to indicate the relative resource levels of pharmacy handling involved in preparing a reported drug, biological, or radiopharmaceutical for administration. This methodology would have allowed us to begin collecting data on pharmacy overhead costs for possible use in future ratesetting calculations, yet we did not finalize this proposal due to the overwhelming response from the hospital community citing the tremendous administrative burden separately reporting these pharmacy handling codes and charges would have placed on hospital resources. We continue to believe that hospitals would likely view such an approach for radiopharmaceuticals alone as burdensome.
Comment: Several commenters responded to CMS' request for public comments on the development of a crosswalk similar to the NDC/HCPCS crosswalk for separately payable drugs and biologicals. These commenters support a NDC/HCPCS “crosswalk” to allow ASP to be utilized.
Response: We appreciate the commenter's support for implementing a “crosswalk” for use for separately payable radiopharmaceuticals. We believe that an NDC/HCPCS crosswalk for nonpass-through, separately payable therapeutic radiopharmaceuticals and pass-through diagnostic and therapeutic radiopharmaceuticals that is similar to the crosswalk for separately payable drugs and biologicals is appropriate, and we will, therefore, work to develop and implement the appropriate NDC/HCPCS crosswalk for separately payable radiopharmaceuticals.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35335), we stated that we continue to believe that the use of ASP information for OPPS payment would provide an opportunity to improve payment accuracy for separately payable radiopharmaceuticals by applying an established methodology that has already been successfully implemented under the OPPS for other separately payable drugs and biologicals. As is the case with other drugs and biologicals subject to ASP reporting under section 1847A of the Act, we stated that in order for a separately payable radiopharmaceutical to receive OPPS payment based on ASP beginning January 1, 2010, we would need to receive ASP information from the manufacturer no later than November 2, 2009 that would reflect separately payable radiopharmaceutical sales in the third quarter of CY 2009 (July 1, 2009 through September 30, 2009). Our normal deadline for January submission is November 1, but because November 1 falls on a Sunday, the ASP submission deadline for January 2010 payment is November 2, 2009. We stated that these data would not be available for publication in the CY 2010 OPPS/ASC final rule with comment period but would be included in the January 2010 OPPS quarterly release that would update the payment rates for separately payable drugs, biologicals, and therapeutic radiopharmaceuticals based on the most recent ASP data, consistent with our customary practice over the past 4 years when we have used the ASP methodology for payment of separately payable drugs and biologicals under the OPPS. In addition, we proposed to receive information from radiopharmaceutical manufacturers that would allow us to calculate a unit dose cost estimate based on the applicable HCPCS code for the separately payable radiopharmaceutical.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35335), we acknowledged that we realized that not all therapeutic radiopharmaceutical manufacturers may be willing or able to submit ASP information for a variety of reasons. We proposed to provide payment at the OPPS ASP rate if ASP information is available for a given calendar year quarter or, if ASP information is not available, we proposed to provide payment based on the most recent hospital mean unit cost data that we have available. We indicated our belief that both methodologies represent an appropriate and adequate proxy for average hospital acquisition cost and associated handling costs for these products. Therefore, if ASP information for the appropriate period of sales related to payment in any CY 2010 quarter was not available, we proposed to rely on the CY 2008 mean unit cost Start Printed Page 60523 data derived from hospital claims to set the payment rates for therapeutic radiopharmaceuticals. We noted that this is not the usual OPPS process that relies on alternative data sources, such as WAC or AWP, when ASP information is temporarily unavailable, prior to defaulting to the mean unit cost from hospital claims data. We proposed a methodology specific to nonpass-through, separately payable therapeutic radiopharmaceuticals where we would immediately default to the mean unit cost from hospital claims data if sufficient ASP data were not available because we did not propose to require therapeutic radiopharmaceutical manufacturers to report ASP data at this time. We indicated that we did not believe that WAC or AWP is an appropriate proxy to provide OPPS payment for average therapeutic radiopharmaceutical acquisition cost and associated handling costs when manufacturers are not required to submit ASP data. Payment based on WAC or AWP under the established OPPS ASP methodology for payment of separately payable drugs and biologicals is usually temporary for a calendar quarter until a manufacturer is able to submit the required ASP data in accordance with the quarterly ASP submission timeframes for reporting under section 1847A of the Act. However, we were concerned that because ASP reporting for OPPS payment of separately payable therapeutic radiopharmaceuticals would not be required for CY 2010, a manufacturer's choice to not submit ASP could result in payment for a separately payable therapeutic radiopharmaceutical based on WAC or AWP for a full year, a result which we believed would be inappropriate. Therefore, for separately payable therapeutic radiopharmaceutical payment under the OPPS, we proposed that the OPPS ASP methodology would pay based on ASP, with payment based on mean unit cost from OPPS claims data if ASP data were not available for a calendar quarter.
Recognizing that we may need to utilize mean unit cost data to pay for nonpass-through, separately payable therapeutic radiopharmaceuticals in CY 2010 if ASP data are not submitted, for the CY 2010 proposed rule we evaluated the mean unit cost information in the CY 2010 claims data for all therapeutic radiopharmaceuticals. We noticed that we had numerous claims with service units greater than one for HCPCS code A9543 (Yttrium Y-90 ibritumomab tiuxetan, therapeutic, per treatment dose, up to 40 millicuries) and A9545 (Iodine I-131 tositumomab, therapeutic, per treatment dose), when the long descriptors for these therapeutic radiopharmaceuticals clearly indicate “per treatment dose” and, therefore, we expected the service units on every claim to be one. In contrast, the other six therapeutic radiopharmaceuticals that would be separately payable in CY 2010 all include “per millicurie” in their HCPCS code descriptors, so reporting multiple service units for those items could be appropriate. We did not believe that hospitals billing more than one unit of HCPCS code A9543 or A9545 on a claim were correctly reporting these products and, therefore, we believed that these claims were incorrectly coded. Although we do not normally examine hospital reporting patterns for individual services, pricing an individual item, such as a therapeutic radiopharmaceutical with low volume, may argue for more aggressive trimming to remove inaccurate claims. Therefore, we removed all claims with units greater than one for these two therapeutic radiopharmaceuticals before estimating their mean unit costs. Because we did not have ASP data for therapeutic radiopharmaceuticals that were used for payment in April 2009, the proposed payment rates included in Addenda A and B to the proposed rule were based on mean costs from historical hospital claims data available for the proposed rule, subject to the additional trimming of incorrectly coded claims for HCPCS codes A9543 and A9545 as described above.
Similar to the ASP process already in place for separately payable drugs and biologicals under the OPPS, we proposed to update ASP data for therapeutic radiopharmaceuticals through our quarterly process as updates become available. In addition, we proposed to assess the availability of ASP data for therapeutic radiopharmaceuticals quarterly, and if ASP data become available midyear, we proposed to transition at the next available quarter to ASP-based payment. For example, if ASP data are not available for the quarter beginning January 2010 (that is, ASP information reflective of third quarter CY 2009 sales are not submitted in November 2009), then the next opportunity to begin payment based on ASP data for a therapeutic radiopharmaceutical would be April 2010 if ASP data reflective of fourth quarter CY 2009 sales were submitted in February 2010.
Comment: Many commenters agreed with CMS' proposal to permit, but not require, radiopharmaceutical manufacturers to submit ASP data. One commenter encouraged CMS to obtain data from all therapeutic radiopharmaceutical manufacturers across all therapeutic radiopharmaceuticals, not just a few. Several commenters expressed concern over the proposed immediate collection of ASP data from manufacturers for the January 2010 OPPS quarterly update. They stated that manufacturers would need an adequate amount of time to submit ASP data for the third quarter of CY 2009 (July 1, 2009 through September 30, 2009).
A few commenters recommended that CMS establish a transition period of 6 months or longer to provide more time for manufacturers to compile and submit ASP data. Another commenter recommended that CMS accept therapeutic radiopharmaceutical ASP data 30 days after finalizing and publishing CMS' CY 2010 OPPS/ASC final rule. This would extend the deadline for which ASP data would be submitted for the January 2010 OPPS quarterly update from the usual November 2, 2009 ASP submission deadline to November 30, 2009.
During a transition period, several commenters recommended that CMS continue its current policy of paying for therapeutic radiopharmaceuticals at charges adjusted to cost, as opposed to the proposed default of mean unit cost derived from claims data. A number of commenters requested open dialogue with CMS on what a manufacturer would need to submit to accurately report ASP for a “patient-ready” radiopharmaceutical dose.
Response: We appreciate the commenters' support for our proposal to pay for nonpass-through, separately payable therapeutic radiopharmaceuticals in CY 2010 using the ASP methodology. We proposed to allow manufacturers to submit ASP information for any nonpass-through, separately payable therapeutic radiopharmaceutical in order to establish an ASP+4 percent payment rate under the OPPS for the therapeutic radiopharmaceutical beginning in CY 2010. Consistent with our authority to collect data in order to determine payment amounts, we intend to collect ASP data for separately payable nonpass-through and pass-through therapeutic radiopharmaceuticals (and diagnostic radiopharmaceuticals with pass-through status), adopting the same submission requirements as we do for drugs and biologicals under section 1847A of the Act and the corresponding regulations, except as otherwise specified in this final rule with comment period for specific OPPS purposes. As we stated in the CY 2010 Start Printed Page 60524 OPPS/ASC proposed rule (74 CR 35335), we continue to believe that the use of ASP information for OPPS payment would provide an opportunity to improve payment accuracy for separately payable radiopharmaceuticals by applying an established methodology that has already been successfully implemented under the OPPS to set prospective payment rates for other separately payable drugs and biologicals.
In recognizing the potential burden involved in reporting ASP data and our belief in the accuracy of prospective payment rates based on claims data, we did not propose to require manufacturers to submit ASP information. Although one commenter suggested that we collect ASP from all manufacturers of therapeutic radiopharmaceuticals, we continue to believe that the challenges involved in reporting ASP for radiopharmaceuticals, given the variety of manufacturing processes, are significant in some cases and, therefore, that payment based on mean unit cost from historical hospital claims data offers the best proxy for average hospital acquisition cost and associated handling costs for a radiopharmaceutical in the absence of ASP. We continue to believe that we should allow, but not require, manufacturers to submit ASP information for therapeutic radiopharmaceuticals. If ASP information is unavailable for a therapeutic radiopharmaceutical, meaning if a manufacturer is not willing or not able to submit ASP information, we will provide payment based on the mean unit cost of the product that is applicable to payment rates for the year the nonpass-through therapeutic radiopharmaceutical is administered. We continue to believe that both methodologies represent an appropriate proxy for average hospital acquisition cost and associated handling costs for nonpass-through, separately payable therapeutic radiopharmaceuticals. We expect manufacturers to submit ASP data for all component materials and any “bona fide service fees” that are reflected in the price for additional processing to produce the separately payable radiopharmaceutical, in an aggregated form in per millicurie or per dosage unit that matches the HCPCS code descriptor for that radiopharmaceutical. We note that the separately payable, nonpass-through therapeutic radiopharmaceutical payment rates listed in Addenda A and B to this final rule with comment period are the mean unit costs from CY 2008 hospital claims data, subject to the additional trimming of incorrectly coded claims for HCPCS codes A9543 and A9545 as proposed and described above, that we would use for payment of a separately payable radiopharmaceutical if ASP information from the manufacturer were not submitted for the product for the applicable OPPS payment quarter.
For CY 2010, we are not implementing a transition period of payment at charges adjusted to cost for therapeutic radiopharmaceuticals. We note that section 1833(t)(16)(C) of the Act continues the payment period for therapeutic radiopharmaceuticals based on a hospital's charges adjusted to cost through December 31, 2009, and this requirement expires beginning CY 2010. We believe it would not be consistent with the statutory expiration of the charges-adjusted-to-cost payment methodology to continue payment using this approach for any portion of CY 2010. For manufacturers that cannot initially submit ASP data, we believe that mean cost payment for a nonpass-through, separately payable therapeutic radiopharmaceutical provides our best proxy estimate of average hospital acquisition cost and associated handling costs and implements prospective payment. In examining the CY 2008 claims data, aggregate therapeutic radiopharmaceutical payment at mean unit cost would be comparable to payment at charges adjusted to cost in CY 2010 assuming no charge inflation between CY 2008 and CY 2010, and we observe deflation in per unit charges for some therapeutic radiopharmaceuticals between CY 2007 and CY 2008 claims. Finally, because we proposed to update payment based on ASP submissions on a quarterly basis, manufacturers would not need to wait one year to be paid based on ASP but could work toward submitting ASP data for April 2010 payment if they were unable to provide data for January 2010 payment.
We recognize that the timeframe for submitting ASP information by November 2, 2009, to begin ASP-based payment on January 1, 2010 is extremely close to the display date of this final rule with comment period. While we expect that most manufacturers interested in reporting ASP for their therapeutic radiopharmaceutical already have begun the process of compiling that data given that we have proposed ASP-based payment under the OPPS for nonpass-through, separately payable therapeutic radiopharmaceuticals for 2 years in a row, we understand that manufacturers will not have had the opportunity to consider our discussion in this final rule with comment period that clarifies the term “patient-ready” in their preparation of ASP data for OPPS purposes. As suggested by the commenters, we recognize that some manufacturers may need to discuss with us how to report ASP for a “patient-ready” dose of their particular radiopharmaceutical. We encourage manufacturers with questions regarding their submissions to contact us, especially if they intend to submit by November 2, 2009. Manufacturers can contact us immediately by sending an email to the OPPS mailbox: OutpatientPPS@cms.hhs.gov. We will be monitoring this mailbox closely. We will provide any assistance that we can within the confines of the ASP quarterly production schedule to facilitate accurate and timely reporting of ASP and payment based on ASP as early as possible. To further our commitment to helping manufacturers submit ASP data in a timely fashion, we also intend to post guidance on the definition of “patient-ready” dose for reporting radiopharmaceutical ASP for OPPS use, and on how manufacturers should compile and submit ASP data for that dose on the CMS Web site at: http://www.cms.hhs.gov/HospitalOutpatientPPS/05_OPPSGuidance.asp#TopOfPage, at about the time that this final rule with comment period goes in display at the Federal Register .
Comment: One commenter expressed concern over CMS proposal to pay for therapeutic radiopharmaceuticals using the ASP methodology for CY 2010. The commenter stated that the methodology established in the proposal, to pay for therapeutic radiopharmaceuticals under the ASP methodology and if ASP is unavailable to make payment based upon the most recent hospital mean unit cost data that CMS has available, would not provide accurate data and, therefore, would not pay accurately for therapeutic radiopharmaceuticals. The commenter was skeptical that manufacturers would submit ASP data in a timely and accurate manner, because the commenter believed manufacturers have little incentive to do so. The commenter recommended that CMS base payment on hospital invoice data in order to provide accurate payment.
Response: We disagree with the commenter and continue to believe that providing payment for therapeutic radiopharmaceuticals based on ASP or mean unit cost if ASP information is not available would provide appropriate payment for these products. We acknowledge in the proposed rule (74 FR 35335) that some manufacturers may be unable or unwilling to submit ASP Start Printed Page 60525 data for the CY 2010 January OPPS quarterly update and we, therefore, proposed to make payment based on the most recent hospital mean unit cost data that we have available for therapeutic radiopharmaceuticals if ASP is not available. Many other commenters, including radiopharmaceutical manufacturers, stated that manufacturers have a significant incentive to submit ASP information because they believe payment based on the default of mean unit cost would not be most reflective of the cost to hospitals to acquire these products. Furthermore, some therapeutic radiopharmaceutical manufacturers already submit ASP data for separately payable drugs and biologicals and, therefore, are familiar with the submission process, including its timing and other requirements. As we stated previously, we continue to believe that the use of ASP information for OPPS payment would provide an opportunity to improve payment accuracy for nonpass-through, separately payable therapeutic radiopharmaceuticals by applying an established methodology that has already been successfully implemented under the OPPS for other separately payable drugs and biologicals. The OPPS has relied upon ASP information as an accurate method for providing payment for drugs and biologicals for several years.
Comment: One commenter requested that CMS not include payment rates based on mean unit cost for therapeutic radiopharmaceuticals in the Addenda to the CY 2010 OPPS/ASC final rule with comment period when a manufacturer intends to report ASP information for CY 2010. The commenter offered this recommendation in order to avoid having other payers that utilize Medicare payment rates adopt these payment rates that the commenter believes will never be those paid to hospitals in CY 2010.
Response: We believe that payment at mean unit cost would appropriately pay for the average hospital acquisition cost and associated handling costs of nonpass-through, separately payable therapeutic radiopharmaceuticals if ASP data are not available. Therefore, we have included the mean unit cost amounts for nonpass-through, separately payable therapeutic radiopharmaceuticals in Addenda A and B to this CY 2010 OPPS/ASC final rule with comment period that would be used for payment in any CY 2010 calendar quarter for which ASP information is not submitted by the product's manufacturer(s). Inclusion of mean unit cost for all nonpass-through, separately payable therapeutic radiopharmaceuticals in the addenda is unique to CY 2010, because we have no ASP information available for these products based on their payment in CY 2009. For future years, based on our usual final rule addenda publication policy, we note that if a radiopharmaceutical manufacturer has submitted ASP for OPPS payment in October 2010, as long as our CY 2011 payment methodology for nonpass-through, separately payable therapeutic radiopharmaceuticals relies on ASP, we would publish a payment rate in the CY 2011 OPPS/ASC final rule with comment period that reflects the radiopharmaceutical's third quarter CY 2010 ASP information.
We follow this final rule addenda publication policy based on our general expectation that drugs and biologicals with ASP information available for payment in the fourth quarter of CY 2009 will have ASP information available for payment in the first quarter of CY 2010. Therefore, we believe that posting illustrative CY 2010 payment rates for drugs and biologicals based on the October 2009 ASP information, rather than mean unit cost, provides a better illustration of the likely payment rates for these product in January 2010. In the event that we have no ASP information for a therapeutic radiopharmaceutical or any other drug or biological paid based on the ASP methodology for any quarter of CY 2010, the applicable mean unit cost for payment of the product in CY 2010 is available on the CMS web site in the OPPS drug median file that is posted as supporting information for this final rule with comment period at: http://www.cms.hhs.gov/HospitalOutpatientPPS/HORD/list.asp#TopOfPage.
Comment: One commenter requested that CMS establish a temporary Level II HCPCS C-code, effective January 1, 2010, to be used in CY 2010 to report the product currently described by HCPCS code A9605 (Samarium Sm-153 Lexidronam, per 50 millicuries). The commenter recommended that CMS replace the current “per 50 millicuries” in the code descriptor with “per treatment dose” in the HCPCS C-code descriptor. The commenter stated that the current code descriptor for HCPCS code A9605 is problematic for coding and ASP reporting purposes because of the decay in radiopharmaceutical radioactivity. Under the existing code descriptor of “per 50 millicuries,” while the manufacturer would report ASP for the HCPCS code based on the radioactivity level of the product at the time of sale, the hospital would report units of the HCPCS code based on the dose administered to the patient at a later point in time, and there would be a mismatch between the reported price and the dose actually administered to the patient. The commenter concluded that reporting the sales and administration of this product on a “per treatment dose” basis would allow ASP information to be aligned with payment for the product under the OPPS, taking into consideration radioactive decay over time since administration would always occur after the manufacturer's sale of the product.
Response: We understand the concerns raised by the commenter in regards to the current code descriptor for A9605. In response to these concerns, CMS' HCPCS Workgroup has decided to create a new HCPCS code, A9604 Samarium SM-153 lexidronam, therapeutic, per treatment dose, up to 150 millicuries), effective January 1, 2010, and delete existing HCPCS code A9605. This new code should facilitate alignment between ASP reporting by the manufacturer and hospital reporting of administration on a “per treatment dose” basis. We note that the default payment rate for HCPCS code A9604 included in Addendum A and B to this final rule with comment period is based on the per-day mean unit cost of HCPCS code A9605. We believe that the CY 2008 hospital per-day cost of HCPCS code A9605 reflects the cost of a single treatment dose and, therefore, it is the mean per-day cost that we will use for payment of new HCPCS code A9604 in CY 2010 if ASP information is not available.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to pay all nonpass-through, separately payable therapeutic radiopharmaceuticals at ASP+4 percent based on ASP information, if available, for a “patient-ready” dose beginning on January 1, 2010, and updated on a quarterly basis for products for which manufacturers report ASP data. We are defining a “patient-ready” dose for OPPS purposes as including all component materials of the radiopharmaceutical, at a minimum, and any other processing the manufacturer requires to produce the radiopharmaceutical that it sells that are reflected in the sales price, including radiolabeling, as long as any fees paid for such processing done on behalf of the manufacturer meet the definition of “bona fide service fees” under § 414.802. We also are finalizing our CY 2010 proposal, without modification, to base nonpass-through, separately payable therapeutic radiopharmaceutical payment on mean Start Printed Page 60526 unit cost derived from CY 2008 claims data when ASP pricing is not available. The nonpass-through therapeutic radiopharmaceuticals that are separately payable in CY 2010 are identified in Table 42 below. The CY 2010 payment rates for these products included in Addenda A and B to this final rule with comment period are based on mean unit cost. Moreover, we note that, should ASP be submitted timely for January 2010 OPPS payment, according to our usual process for updating the payment rates for separately payable drugs and biologicals on a quarterly basis if updated ASP information is available, these payment rates will be updated through the January 2010 OPPS quarterly release.
| CY 2010 HCPCS code | CY 2010 long descriptor | Final CY 2010 APC | Final CY 2010 SI |
|---|---|---|---|
| A9517 | Iodine I–131 sodium iodide capsule(s), therapeutic, per millicurie | 1064 | K |
| A9530 | Iodine I–131 sodium iodide solution, therapeutic, per millicurie | 1150 | K |
| A9543 | Yttrium Y–90 ibritumomab tiuxetan, therapeutic, per treatment dose, up to 40 millicuries | 1643 | K |
| A9545 | Iodine I–131 tositumomab, therapeutic, per treatment dose | 1645 | K |
| A9563 | Sodium phosphate P–32, therapeutic, per millicurie | 1675 | K |
| A9564 | Chromic phosphate P–32 suspension, therapeutic, per millicurie | 1676 | K |
| A9600 | Strontium Sr-89 chloride, therapeutic, per millicurie | 0701 | K |
| A9604 | Samarium SM–153 lexidronam, therapeutic, per treatment dose, up to 150 millicuries | 1295 | K |
6. Payment for Nonpass-Through Drugs, Biologicals, and Radiopharmaceuticals With HCPCS Codes, but Without OPPS Hospital Claims Data
Public Law 108–173 does not address the OPPS payment in CY 2005 and after for drugs, biologicals, and radiopharmaceuticals that have assigned HCPCS codes, but that do not have a reference AWP or approval for payment as pass-through drugs or biologicals. Because there is no statutory provision that dictated payment for such drugs, biologicals, and radiopharmaceuticals in CY 2005, and because we had no hospital claims data to use in establishing a payment rate for them, we investigated several payment options for CY 2005 and discussed them in detail in the CY 2005 OPPS final rule with comment period (69 FR 65797 through 65799).
For CYs 2005 to 2007, we implemented a policy to provide separate payment for new drugs, biologicals, and radiopharmaceuticals with HCPCS codes (specifically those new drug, biological, and radiopharmaceutical HCPCS codes in each of those calendar years that did not crosswalk to predecessor HCPCS codes) but which did not have pass-through status, at a rate that was equivalent to the payment they received in the physician's office setting, established in accordance with the ASP methodology for drugs and biologicals, and based on charges adjusted to cost for radiopharmaceuticals. For CYs 2008 and 2009, we finalized a policy to provide payment for new drugs (excluding contrast agents) and biologicals (excluding implantable biologicals for CY 2009) with HCPCS codes, but which did not have pass-through status and were without OPPS hospital claims data, at ASP+5 percent and ASP+4 percent, respectively, consistent with the final OPPS payment methodology for other separately payable drugs and biologicals. New therapeutic radiopharmaceuticals were paid at charges adjusted to cost based on the statutory requirement for CY 2008 and CY 2009 and payment for new diagnostic radiopharmaceuticals was packaged in both years. In the CY 2010 OPPS/ASC proposed rule (74 FR 35336 through 35337), for CY 2010, we proposed to continue the CY 2009 payment methodology for new drugs (excluding contrast agents) and nonimplantable biologicals and extend the methodology to payment for new therapeutic radiopharmaceuticals, when their period of payment at charges adjusted to cost no longer would apply. Therefore, for CY 2010, we proposed to provide payment for new drugs (excluding contrast agents), nonimplantable biologicals, and therapeutic radiopharmaceuticals with HCPCS codes (those new CY 2010 drug (excluding contrast agents), nonimplantable biological, and therapeutic radiopharmaceutical HCPCS codes that do not crosswalk to CY 2009 HCPCS codes), but which do not have pass-through status and are without OPPS hospital claims data, at ASP+4 percent, consistent with the proposed CY 2010 payment methodology for other separately payable nonpass-through drugs, nonimplantable biologicals, and therapeutic radiopharmaceuticals. We believed this proposed policy would ensure that new nonpass-through drugs, nonimplantable biologicals, and therapeutic radiopharmaceuticals would be treated like other drugs, nonimplantable biologicals, and therapeutic radiopharmaceuticals under the OPPS, unless they are granted pass-through status. Only if they are pass-through drugs, nonimplantable biologicals, or therapeutic radiopharmaceuticals would they receive a different payment for CY 2010, generally equivalent to the payment these drugs and biologicals would receive in the physician's office setting, consistent with the requirements of the statute. We proposed to continue packaging payment for all new nonpass-through diagnostic radiopharmaceuticals, contrast agents, and implantable biologicals with HCPCS codes (those new CY 2010 diagnostic radiopharmaceutical, contrast agent, and implantable biological HCPCS codes that do not crosswalk to predecessor HCPCS codes), consistent with the proposed packaging of all existing nonpass-through diagnostic radiopharmaceuticals, contrast agents and implantable biologicals, as discussed in more detail in the proposed rule (74 FR 35323 through 35324).
In accordance with the OPPS ASP methodology, in the absence of ASP data, in the CY 2010 OPPS/ASC proposed rule (74 FR 35336), for CY 2010, we proposed to continue the policy we implemented beginning in CY 2005 of using the WAC for the product to establish the initial payment rate for new nonpass-through drugs and biologicals with HCPCS codes, but which are without OPPS claims data. However, we note that if the WAC is Start Printed Page 60527 also unavailable, we would make payment at 95 percent of the product's most recent AWP. We also proposed to assign status indicator “K” to HCPCS codes for new drugs and nonimplantable biologicals without OPPS claims data and for which we have not granted pass-through status. We further noted that, with respect to new items for which we do not have ASP data, once their ASP data become available in later quarter submissions, their payment rates under the OPPS would be adjusted so that the rates would be based on the ASP methodology and set to the finalized ASP-based amount (proposed for CY 2010 at ASP+4 percent) for items that have not been granted pass-through status.
We did not receive any public comments specific to these proposals. While commenters, in general, objected to payment for drugs and biologicals at ASP+4 percent, these comments were not specific to new drugs and biologicals with HCPCS codes but without OPPS claims data. Further, we summarize the general public comments on payment for separately payable drugs and provide our responses in section V.B.3.b. of this final rule with comment period. In addition, commenters on the CY 2010 OPPS/ASC proposed rule objected to packaging payment for diagnostic radiopharmaceuticals and contrast agents in general, but these comments were not directed to new diagnostic radiopharmaceuticals or contrast agents with HCPCS codes but without OPPS claims data. We summarize these comments and provide our responses in section V.A.2.d. of this final rule with comment period.
Therefore, we are finalizing our CY 2010 proposals, without modification, as follows: Payment for new drugs (excluding contrast agents), nonimplantable biologicals, and therapeutic radiopharmaceuticals with HCPCS codes, but which do not have pass-through status and are without OPPS hospital claims data, will be made at ASP+4 percent for CY 2010. In cases where ASP information is not available, payment will be made using WAC, and if WAC is also unavailable payment will be made at 95 percent of the most recent AWP. Further, payment for all new nonpass-through diagnostic radiopharmaceuticals, contrast agents, and implantable biologicals with HCPCS codes but without OPPS claims data will be packaged for CY 2010. Finally, we are assigning status indicator “K” to HCPCS codes for new drugs and nonimplantable biologicals without OPPS claims data and for which we have not granted pass-through status in CY 2010.
For CY 2010, we also proposed to base payment for new therapeutic radiopharmaceuticals with HCPCS codes as of January 1, 2010, but which do not have pass-through status, on the WACs for these products if ASP data for these therapeutic radiopharmaceuticals are not available. If the WACs are also unavailable, we proposed to make payment for new therapeutic radiopharmaceuticals at 95 percent of their most recent AWPs because we would not have mean costs from hospital claims data upon which to base payment. Analogous to new drugs and biologicals, we proposed to assign status indicator “K” to HCPCS codes for new therapeutic radiopharmaceuticals for which we have not granted pass-through status.
We did not receive any public comments specific to our proposal for new therapeutic radiopharmaceuticals with HCPCS codes but without pass-through status. However, commenters on the CY 2010 OPPS/ASC proposed rule were supportive of the ASP methodology, in general, for payment for therapeutic radiopharmaceuticals in the HOPD, and we are finalizing an ASP payment methodology for separately payable therapeutic radio- pharmaceuticals for CY 2010 as discussed in section V.B.5. of this final rule with comment period.
Therefore, we are finalizing our CY 2010 proposals, without modification, to provide payment for new therapeutic radiopharmaceuticals with HCPCS codes but without pass-through status, if ASP information is not available, based on WAC. If WAC information is also unavailable, we will make payment for new therapeutic radiopharmaceuticals at 95 percent of their most recent AWP. In addition, we are assigning status indicator “K” to HCPCS codes for new therapeutic radiopharmaceuticals in CY 2010 that do not have pass-through status.
Consistent with other ASP-based payments, for CY 2010, we proposed to announce any changes to the payment amounts for new drugs and biologicals in the CY 2010 OPPS/ASC final rule with comment period and also on a quarterly basis on the CMS Web site during CY 2010 if later quarter ASP submissions (or more recent WACs or AWPs) indicate that changes to the payment rates for these drugs and biologicals are necessary. The payment rates for new therapeutic radiopharmaceuticals would also be changed accordingly, based on later quarter ASP submissions. We note that the new CY 2010 HCPCS codes for drugs, biologicals, and therapeutic radiopharmaceuticals were not available at the time of development of the proposed rule. However, they are included in Addendum B to this CY 2010 OPPS/ASC final rule with comment period. They are assigned comment indicator “NI” in Addendum B to reflect that their interim final OPPS treatment is open to public comment on the CY 2010 OPPS/ASC final rule with comment period.
We did not receive any public comments on our proposal to announce, via the CMS Web site, any changes to the OPPS payment amounts for new drugs and biologicals on a quarterly basis. Therefore, we are finalizing our proposals and will update payment rates for new drugs, biologicals, and therapeutic radiopharmaceuticals, as necessary, in association with our quarterly update process and provide this information on the CMS Web site.
There are several nonpass-through drugs and biologicals that were payable in CY 2008 and/or CY 2009 for which we did not have any CY 2008 hospital claims data available for the proposed rule and for which there were no other HCPCS codes that describe different doses of the same drug but for which we did have pricing information for the ASP methodology. In the CY 2010 OPPS/ASC proposed rule (74 FR 35337), we noted that there are currently no therapeutic radiopharmaceuticals in this category. In order to determine the packaging status of these products for CY 2010, we calculated an estimate of the per day cost of each of these items by multiplying the payment rate for each product based on ASP+4 percent, similar to other nonpass-through drugs and biologicals paid separately under the OPPS, by an estimated average number of units of each product that would typically be furnished to a patient during one administration in the hospital outpatient setting. We proposed to package items for which we estimated the per administration cost to be less than or equal to $65, which is the general packaging threshold that we proposed for drugs, nonimplantable biologicals, and therapeutic radiopharmaceuticals in CY 2010. We proposed to pay separately for items with an estimated per day cost greater than $65 (with the exception of diagnostic radiopharmaceuticals, contrast agents and implantable biologicals, which we proposed to continue to package regardless of cost, as discussed in more detail in the proposed rule (74 FR 35323 through 35324)) in CY 2010. We proposed that the CY 2010 payment for separately payable items without CY 2008 claims Start Printed Page 60528 data would be ASP+4 percent, similar to payment for other separately payable nonpass-through drugs and biologicals under the OPPS. In accordance with the ASP methodology used in the physician's office setting, in the absence of ASP data, we proposed to use the WAC for the product to establish the initial payment rate. However, we note that if the WAC is also unavailable, we would make payment at 95 percent of the most recent AWP available.
We did not receive any public comments on our proposal to use estimated per day costs for these drugs and biologicals or on the resulting packaging status of these drugs and biologicals. Therefore, we are finalizing our CY 2010 proposal, without, modification to use the estimated number of units per day included in Table 43 below to determine estimated per day costs for the corresponding drugs and biologicals for CY 2010. Further, we are finalizing our proposal to package those drugs with an estimated per day cost less than or equal to $65 and to provide separate payment for those drugs and biologicals with estimated per day costs over $65 for CY 2010. For those drugs and biologicals that we determined to be separately payable in CY 2010, payment will be made at ASP+4 percent. If ASP information is not available, payment will be based on WAC or 95 percent of the most recently published AWP if WAC is not available. The final estimated units per day and status indicators for these items are displayed in Table 43 below.
| CY 2010 HCPCS code | CY 2010 long descriptor | Estimated average number of units per administration | Final CY 2010 SI | Final CY 2010 APC |
|---|---|---|---|---|
| 90681 | Rotavirus vaccine, human, attenuated, 2 dose schedule, live, for oral use | 1 | K | 1239 |
| 90696 | Diphtheria, tetanus toxoids, acellular pertussis vaccine and poliovirus vaccine, inactivated (DTaP–IPV), when administered to children 4 through 6 years of age, for intramuscular use | 1 | N | |
| J0364 | Injection, apomorphine hydrochloride, 1 mg | 12 | N | |
| J2724 | Injection, protein c concentrate, intravenous, human, 10 iu | 2240 | K | 1139 |
| J3355 | Injection, urofollitropin, 75 IU | 2 | K | 1741 |
| J9215 | Injection, interferon, alfa-n3, (human leukocyte derived), 250,000 iu | 5 | K | 0865 |
Finally, there were eight drugs and biologicals, shown in Table 31 of the CY 2010 OPPS/ASC proposed rule (74 FR 35337), that were payable in CY 2008, but for which we lacked CY 2008 claims data and any other pricing information for the ASP methodology for the CY 2010 OPPS/ASC proposed rule. In CY 2009, for similar items without CY 2007 claims data and without pricing information for the ASP methodology, we stated that we were unable to determine their per day cost and we packaged these items for the year, assigning these items status indicator “N.”
For CY 2010, we proposed to change the status indicator for eight drugs and biologicals shown in Table 31 of the CY 2010 OPPS/ASC proposed rule (74 FR 35337) to status indicator “E” (Not paid by Medicare when submitted on outpatient claims (any outpatient bill type)) as we understood that these drugs and biologicals are not currently sold or have been identified as obsolete. In addition, we proposed to provide separate payment for these drugs and biologicals if pricing information reflecting recent sales becomes available mid-year in CY 2010 for the ASP methodology. If pricing information becomes available, we would assign the products status indicator “K” and pay for them separately for the remainder of CY 2010.
We did not receive any public comments on our proposal to change the status indicators for drugs and biologicals without claims data or pricing information for the ASP methodology. Therefore, we are finalizing our CY 2010 proposal, without modification, to assign status indicator “E” to these drugs and biologicals. As we have used updated claims data and ASP pricing information for this final rule with comment period, we have newly identified for this final rule with comment period CPT codes 90393 (Vaccinia immune globulin, human, for intramuscular use); 90477 (Adenovirus vaccine, type 7, live, for oral use); 90644 (Meningococcal conjugate vaccine, serogroups C & Y and Hemophilus influenza b vaccine, tetanus toxoid conjugate (Hib-MenCY–TT), 4-dose schedule, when administered to children 2–15 months of age, for intramuscular use); and 90670 (Pneumococcal conjugate vaccine, 13 valent, for intramuscular use) as lacking CY 2008 claims data and any other pricing information for the ASP methodology. Therefore, in addition to the HCPCS codes we proposed to assign status indicator “E” for CY 2010 on this basis in the proposed rule, we are assigning status indicator “E” to CPT codes 90393, 90477, 90644 and 90670 for CY 2010. All drugs and biologicals without CY 2008 hospital claims data and data based on the ASP methodology that are assigned status indicator “E” on this basis at the time of this final rule with comment period for CY 2010 are displayed in Table 44 below.
| CY 2010 HCPCS code | CY 2010 long descriptor | Final CY 2010 SI |
|---|---|---|
| 90296 | Diphtheria antitoxin, equine, any route | E |
| 90393 | Vaccinia immune globulin, human, for intramuscular use | E |
| Start Printed Page 60529 | ||
| 90477 | Adenovirus vaccine, type 7, live, for oral use | E |
| 90581 | Anthrax vaccine, for subcutaneous use | E |
| 90644 | Meningococcal conjugate vaccine, serogroups C & Y and Hemophilus influenza b vaccine, tetanus toxoid conjugate (Hib-MenCY–TT), 4-dose schedule, when administered to children 2–15 months of age, for intramuscular use | E |
| 90670 | Pneumococcal conjugate vaccine, 13 valent, for intramuscular use | E |
| 90727 | Plague vaccine, for intramuscular use | E |
| J0128 | Injection, abarelix, 10 mg | E |
| J0350 | Injection, anistreplase, per 30 units | E |
| J0395 | Injection, arbutamine hcl, 1 mg | E |
| J1452 | Injection, fomivirsen sodium, intraocular, 1.65 mg | E |
| J2460 | Injection, oxytetracycline HCL, up to 50 mg | E |
VI. Estimate of OPPS Transitional Pass-Through Spending for Drugs, Biologicals, Radiopharmaceuticals, and Devices
A. Background
Section 1833(t)(6)(E) of the Act limits the total projected amount of transitional pass-through payments for drugs, biologicals, radio- pharmaceuticals, and categories of devices for a given year to an “applicable percentage” of total program payments estimated to be made under section 1833(t) of the Act for all covered services furnished for that year under the hospital OPPS. For a year before CY 2004, the applicable percentage was 2.5 percent; for CY 2004 and subsequent years, we specify the applicable percentage up to 2.0 percent.
If we estimate before the beginning of the calendar year that the total amount of pass-through payments in that year would exceed the applicable percentage, section 1833(t)(6)(E)(iii) of the Act requires a uniform reduction in the amount of each of the transitional pass-through payments made in that year to ensure that the limit is not exceeded. We make an estimate of pass-through spending to determine not only whether payments exceed the applicable percentage, but also to determine the appropriate reduction to the conversion factor for the projected level of pass-through spending in the following year in order to ensure that total estimated pass-through spending for the prospective payment year is budget neutral as required by section 1883(t)(6)(E) of the Act.
For devices, developing an estimate of pass-through spending in CY 2010 entails estimating spending for two groups of items. The first group of items consists of device categories that were recently made eligible for pass-through payment and that would continue to be eligible for pass-through payment in CY 2010. The CY 2008 OPPS/ASC final rule with comment period (72 FR 66778) describes the methodology we have used in previous years to develop the pass-through spending estimate for known device categories continuing into the applicable update year. The second group contains items that we know are newly eligible, or project would be newly eligible, for device pass-through payment in the remaining quarters of CY 2009 or beginning in CY 2010. As discussed in section V.A.4. of this final rule with comment period, we proposed that, beginning in CY 2010, the pass-through evaluation process and pass-through payment for implantable biologicals newly approved for pass-through payment beginning on or after January 1, 2010, that are always surgically inserted or implanted (through a surgical incision or a natural orifice) would be the device pass-through process and payment methodology only. Therefore, we proposed that the estimate of pass-through spending for implantable biologicals newly eligible for pass-through payment beginning in CY 2010 would be included in the pass-through spending estimate for this second group of device categories. The sum of the CY 2010 pass-through estimates for these two groups of device categories equals the total CY 2010 pass-through spending estimate for device categories with pass-through status.
For devices eligible for pass-through payment, section 1833(t)(6)(D)(ii) of the Act establishes the pass-through amount as the amount by which the hospital's charges for the device, adjusted to cost, exceeds the portion of the otherwise applicable Medicare OPD fee schedule that the Secretary determines is associated with the device. As discussed in section IV.A.2. of this final rule with comment period, we deduct from the pass-through payment for an identified device category eligible for pass-through payment an amount that reflects the portion of the APC payment amount that we determine is associated with the cost of the device, defined as the device APC offset amount, when we believe that predecessor device costs for the device category newly approved for pass-through payment are already packaged into the existing APC structure. For each device category that becomes newly eligible for device pass-through payment, including an implantable biological under our CY 2010 proposal, we estimate pass-through spending to be the difference between payment for the device category and the device APC offset amount, if applicable, for the procedures that would use the device. If we determine that predecessor device costs for the new device category are not already included in the existing APC structure, the pass-through spending estimate for the device category would be the full payment at charges adjusted to cost.
For drugs and biologicals eligible for pass-through payment, section 1833(t)(6)(D)(i) of the Act establishes the pass-through payment amount as the amount by which the amount authorized under section 1842(o) of the Act (or, if the drug or biological is covered under a competitive acquisition contract under section 1847B of the Act, an amount determined by the Secretary equal to the average price for the drug or biological for all competitive acquisition areas and year established under such section as calculated and adjusted by the Secretary) exceeds the portion of the otherwise applicable fee schedule amount that the Secretary determines is associated with the drug or biological. As we proposed, we are paying for most nonpass-through separately payable drugs and nonimplantable biologicals under the Start Printed Page 60530 CY 2010 OPPS at ASP+4 percent, which represents the otherwise applicable fee schedule amount associated with most pass-through drugs and biologicals, and because we are paying for CY 2010 pass-through drugs and nonimplantable biologicals at ASP+6 percent or the Part B drug CAP rate, if applicable, our estimate of drug and nonimplantable biological pass-through payment for CY 2010 is not zero. Furthermore, payment for certain drugs, specifically diagnostic radiopharmaceuticals, contrast agents, and implantable biologicals without pass-through status, is always packaged into payment for the associated procedures because these products would never be separately paid. However, all pass-through diagnostic radiopharmaceuticals, contrast agents, and those implantable biologicals with pass-through status approved prior to CY 2010 are paid at ASP+6 percent or the Part B drug CAP rate, if applicable, like other pass-through drugs and biologicals. Therefore, our estimate of pass-through payment for all diagnostic radiopharmaceuticals and contrast agents and those implantable biologicals with pass-through status approved prior to CY 2010 is also not zero.
In section V.A.6. of this final rule with comment period, we discuss our policy to determine if the cost of certain “policy-packaged” drugs, including diagnostic radiopharmaceuticals and contrast agents, are already packaged into the existing APC structure. If we determine that a “policy-packaged” drug approved for pass-through payment resembles predecessor diagnostic radiopharmaceuticals or contrast agents already included in the costs of the APCs that would be associated with the drug receiving pass-through payment, as we proposed, we are offsetting the amount of pass-through payment for diagnostic radiopharmaceuticals and contrast agents. For these drugs, the APC offset amount is the portion of the APC payment for the specific procedure performed with the pass-through diagnostic radiopharmaceutical or contrast agent that is attributable to diagnostic radiopharmaceuticals or contrast agents, which we refer to as the “policy-packaged” drug APC offset amount. If we determine that an offset is appropriate for a specific diagnostic radiopharmaceutical or contrast agent receiving pass-through payment, we reduce our estimate of pass-through payment for these drugs by this amount. We have not established a policy to offset pass-through payment for implantable biologicals when approved for pass-through payment as a drug or biological, that is, for CY 2009 and earlier, so we consider full payment at ASP+6 percent for these implantable biologicals receiving biological pass-through payment in our estimate of CY 2010 pass-through spending for drugs and biologicals.
We note that the Part B drug CAP program has been suspended beginning January 1, 2009. We refer readers to the Medicare Learning Network (MLN) Matters Special Edition article SE0833 for more information on this suspension. As of the publication of the CY 2010 OPPS/ASC proposed rule and this final rule with comment period, the Part B drug CAP program has not been reinstituted. Therefore, for this final rule with comment period, we are continuing to not have an effective Part B drug CAP rate for pass-through drugs and biologicals. Similar to estimates for devices, the first group of drugs and biologicals requiring a pass-through payment estimate consists of those products that were recently made eligible for pass-through payment and that continue to be eligible for pass-through payment in CY 2010. The second group contains drugs and nonimplantable biologicals that we know are newly eligible, or project would be newly eligible, in the remaining quarters of CY 2009 or beginning in CY 2010. The sum of the CY 2010 pass-through estimates for these two groups of drugs and biologicals equals the total CY 2010 pass-through spending estimate for drugs and biologicals with pass-through status.
B. Estimate of Pass-Through Spending
For CY 2010, we proposed to set the applicable pass-through payment percentage limit at 2.0 percent of the total projected OPPS payments for CY 2010, consistent with our OPPS policy from CY 2004 through 2009 (74 FR 35339).
For the first group of devices for pass-through payment estimate purposes, there were no device categories receiving pass-through payment in CY 2009 that would continue for payment during CY 2010 (74 FR 35339) and, therefore, we proposed a device pass-through payment estimate for the first group of pass-through device categories of $0.
We also proposed for CY 2010 to use the device pass-through process and payment methodology for implantable biologicals that are always surgically inserted or implanted (through a surgical incision or a natural orifice). We proposed to consider existing implantable biologicals approved for pass-through payment under the drugs and biologicals pass-through provision prior to CY 2010 as drugs and biologicals for pass-through payment estimate purposes. We proposed to continue to consider these implantable biologicals that have been approved for pass-through status prior to CY 2010 drugs and biologicals until they expire from pass-through status. Therefore, the proposed pass-through spending estimate for the first group of pass-through devices did not include implantable biologicals that were granted pass-through status prior to CY 2010. Finally, we proposed to provide payment for implantable biologicals newly eligible for pass-through payment beginning in CY 2010 based on hospital charges adjusted to cost, rather than the ASP methodology that is applicable to pass-through drugs and biologicals. Therefore, we proposed that, beginning in CY 2010, the estimate of pass-through spending for implantable biologicals first paid as pass-through devices in CY 2010 would be based on the payment methodology for pass-through devices and would be included in the device pass-through spending estimate.
In estimating our proposed CY 2010 pass-through spending for device categories in the second group, that is, device categories that we knew at the time of the development of the proposed rule would be newly eligible for pass-through payment in CY 2010 (of which there were none), additional device categories (including categories that describe implantable biologicals) that we estimated could be approved for pass-through status subsequent to the development of the proposed rule and before January 1, 2010, and contingent projections for new categories (including categories that describe implantable biologicals in the second through fourth quarters of CY 2010), we proposed to use the general methodology described in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66778), while also taking into account recent OPPS experience in approving new pass-through device categories. There were no new device categories (including categories that describe implantable biologicals) for CY 2010 of which we were aware at the time of development of the proposed rule. The estimate of CY 2010 pass-through spending for this second group of device categories was $10.0 million for the proposed rule (74 FR 35339).
Employing our established methodology that the estimate of pass-through device spending in CY 2010 incorporates CY 2010 estimates of pass-through spending for known device categories continuing in CY 2010, those known or projected to be first effective Start Printed Page 60531 January 1, 2010, and those device categories projected to be approved during subsequent quarters of CY 2009 or CY 2010, our proposed CY 2010 estimate of total pass-through spending for device categories was $10.0 million (74 FR 35339).
To estimate CY 2010 proposed pass-through spending for drugs and biologicals in the first group, specifically those drugs (including radiopharmaceuticals and contrast agents) and biologicals (including implantable biologicals) recently made eligible for pass-through payment and continuing on pass-through status for CY 2010, we proposed to utilize the most recent Medicare physician's office data regarding their utilization, information provided in the respective pass-through applications, historical hospital claims data, pharmaceutical industry information, and clinical information regarding those drugs or biologicals, in order to project the CY 2010 OPPS utilization of the products.
For the known drugs and biologicals (excluding diagnostic radio- pharmaceuticals, contrast agents, and implantable biologicals) that would be continuing on pass-through status in CY 2010, we estimated the proposed pass-through payment amount as the difference between ASP+6 percent or the Part B drug CAP rate, as applicable, and ASP+4 percent, aggregated across the projected CY 2010 OPPS utilization of these products. Because payment for a diagnostic radiopharmaceutical or contrast agent would be packaged if the product were not paid separately due to its pass-through status, we included in the pass-through estimate the difference between payment for the drug or biological at ASP+6 percent (or WAC+6 percent, or 95 percent of AWP, if ASP information is not available) and the “policy-packaged” drug APC offset amount, if we determined that the diagnostic radiopharmaceutical or contrast agent approved for pass-through payment resembles predecessor diagnostic radiopharmaceuticals or contrast agents already included in the costs of the APCs that would be associated with the drug receiving pass-through payment. Because payment for an implantable biological continuing on pass-through status in CY 2010 would be packaged if the product were not paid separately due to its pass-through status and because we have not established a pass-through payment offset policy for implantable biologicals when approved for pass-through payment as biologicals, that is, for CY 2009 and earlier, we included in the proposed pass-through spending estimate the full payment for these implantable biologicals at ASP+6 percent (or WAC+6 percent or 95 percent of AWP, if ASP information is not available). We note that our spending estimate for this first group of drugs and biologicals was stated in the CY 2010 OPPS/ASC proposed rule as $8.9 million (74 FR 35340), while our estimate for the second group of drugs and biologicals was reported as $19.1 million. We inadvertently mislabeled these two spending estimates in the CY 2010 OPPS/ASC proposed rule. For this first group of drugs and biologicals, the proposed spending estimate should have been reported as $19.1 million and the second group should have been reported as $8.9 million.
To estimate CY 2010 pass-through spending for drugs and nonimplantable biologicals in the second group (that is, drugs and nonimplantable biologicals that we knew at the time of development of the proposed rule would be newly eligible for pass-through payment in CY 2010, additional drugs and nonimplantable biologicals that we estimated could be approved for pass-through status subsequent to the development of the proposed rule and before January 1, 2010, and projections for new drugs and nonimplantable biologicals that could be initially eligible for pass-through payment in the second through fourth quarters of CY 2010), we proposed to use utilization estimates from pass-through applicants, pharmaceutical industry data, clinical information, recent trends in the per unit ASPs of hospital outpatient drugs, and projected annual changes in service volume and intensity as our basis for making the CY 2010 proposed pass-through payment estimate. We also considered the most recent OPPS experience in approving new pass-through drugs and nonimplantable biologicals. As noted earlier, we also proposed to include new implantable biologicals that we expect to be approved for pass-through status as devices beginning in CY 2010 in the second group of items considered for device pass-through estimate purposes. Therefore, we did not include implantable biologicals in the second group of items in the proposed drug and biological pass-through spending estimate.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35314 through 35317), we proposed to revise our pass-through payment policy regarding “new” drugs and biologicals that were not receiving hospital outpatient payment as of December 31, 1996, and that also met the other criteria for receiving pass-through payment. Specifically, we proposed to change the start date of the pass-through payment eligibility period for a “new” drug or biological from the first date on which pass-through payment is made to the date on which payment is first made for a drug or biological as an outpatient hospital service under Part B, using the date of first sale of the drug or biological in the United States after FDA approval as a proxy, to better reflect the statutory provisions for pass-through payment under section 1833(t)(6) of the Act. We expected that a number of the drugs and biologicals currently receiving pass-through payment in CY 2009 would not be eligible for pass-through payment under the proposed revised definition of the pass-through payment eligibility period, Accordingly, for the CY 2010 OPPS/ASC proposed rule, we reduced our estimate of CY 2010 pass-through spending for new drugs and nonimplantable biologicals in the second group that could be initially eligible for pass-through payment beginning in CY 2010 to take into consideration the potential effect of the proposed CY 2010 pass-through payment eligibility period policy on the future number of drugs and biologicals newly approved for pass-through payment, in comparison with our historical OPPS experience over the past several years.
As noted above, we inadvertently mislabeled the spending estimates for the two groups of drugs and biologicals in the CY 2010 OPPS/ASC proposed rule. Therefore, while we reported that the spending estimate for this second group of drugs and biologicals in the CY 2010 OPPS/ASC proposed rule was $19.1 million (74 FR 35340), the estimate that should have been reported for this second group of drugs and biologicals was $8.9 million.
We did not receive any public comments on the proposed methodology to estimate pass-through spending for drugs, biologicals, radiopharmaceuticals, and device categories in CY 2010. However, we did receive public comments on our proposal to use the first date of sale in the United States after FDA approval as a proxy for the first date of payment under Medicare Part B as an outpatient hospital service for determining the pass-through payment eligibility period for pass-through drugs and nonimplantable biologicals under the OPPS for CY 2010, which would have reduced the pass-through payment estimate for drugs and nonimplantable biologicals. These public comments, our responses, and our final policy for CY 2010 are discussed in section V.A.5 of this final rule with comment period. As with our current policy, in CY 2010 the Start Printed Page 60532 pass-through payment eligibility period and the period of pass-through payment period will run concurrently. Thus, for our final CY 2010 pass-through spending estimate for new drugs and nonimplantable biologicals that could be initially eligible for pass-through payment beginning in CY 2010, we have no need to reduce our estimate of pass-through spending to take into consideration a policy change in the pass-through payment eligibility period. Therefore, we are finalizing our proposed methodology to estimate annual pass-through spending for devices and drugs and nonimplantable biologicals, with the modification as described above.
As stated in section V.A.4. of this final rule with comment period, as we proposed, beginning in CY 2010, implantable biologicals that are always surgically inserted or implanted (through a surgical incision or a natural orifice) will be evaluated under the device pass-through process and paid according to the device payment methodology. We are continuing to consider implantable biologicals approved for pass-through payment under the drug and biological pass-through provision prior to CY 2010 as drugs and biologicals for pass-through payment estimate purposes. These implantable biologicals that have been approved for pass-through status prior to CY 2010 continue to be considered drugs and biologicals until they expire from pass-through status. Therefore, the final pass-through spending estimate for the first group of pass-through device categories does not include implantable biologicals that have been granted pass-through status prior to CY 2010.
In section V.A.4. of this final rule with comment period, as we proposed, we are providing that payment for implantable biologicals newly eligible for pass-through payment beginning in CY 2010 is based on hospital charges adjusted to cost, rather than the ASP methodology that is applicable to pass-through drugs and biologicals. Therefore, we are providing that, beginning in CY 2010, the estimate of pass-through spending for implantable biologicals first paid as pass-through devices in CY 2010 is based on the payment methodology for pass-through devices, and is included in the final CY 2010 device pass-through spending estimate for the second group of pass-through device categories.
The final CY 2010 pass-through spending estimate for the first group of pass-through device categories is $0. The estimate of CY 2010 pass-through spending for the second group of pass-through device categories is $10.0 million for this final rule with comment period, as it was for the proposed rule (74 FR 35339). Our final CY 2010 estimate of total pass-through spending for device categories is $10.0 million.
The estimate for pass-through spending for the first group of drugs and biologicals is $28.9 million for CY 2010. The estimate for pass-through spending for the second group of drugs and biologicals is $6.7 million for CY 2010. As stated above, the final estimates differ, in part, from our proposed rule estimates in order to reflect our final policy regarding the pass-through payment eligibility period. As discussed in section V.A. of this final rule with comment period, radiopharmaceuticals are considered drugs for pass-through purposes. Therefore, we have included radiopharmaceuticals in our CY 2010 pass-through spending estimate for drugs and biologicals. Our final CY 2010 estimate of total pass-through spending for drugs and biologicals is $35.6 million.
In summary, in accordance with the methodology described above in this section, we estimate that total pass-through spending for the device categories and the drugs and biologicals that are continuing to receive pass-through payment in CY 2010 and those device categories, drugs, and nonimplantable biologicals that first become eligible for pass-through payment during CY 2010 is approximately $45.5 million, which represents 0.14 percent of total OPPS projected payments for CY 2010. We estimate that pass-through spending in CY 2010 will not amount to 2.0 percent of total projected OPPS CY 2010 program spending.
VII. OPPS Payment for Brachytherapy Sources
A. Background
Section 1833(t)(2)(H) of the Act, as added by section 621(b)(2)(C) of Public Law 108–173 (MMA), mandated the creation of additional groups of covered OPD services that classify devices of brachytherapy consisting of a seed or seeds (or radioactive source) (“brachytherapy sources”) separately from other services or groups of services. The additional groups must reflect the number, isotope, and radioactive intensity of the brachytherapy sources furnished and include separate groups for palladium-103 and iodine-125 sources.
Section 1833(t)(16)(C) of the Act, as added by section 621(b)(1) of Public Law 108–173, established payment for brachytherapy sources furnished from January 1, 2004, through December 31, 2006, based on a hospital's charges for each brachytherapy source furnished adjusted to cost. Under section 1833(t)(16)(C) of the Act, charges for the brachytherapy sources may not be used in determining any outlier payments under the OPPS for that period of payment. Consistent with our practice under the OPPS to exclude items paid at cost from budget neutrality consideration, these items were excluded from budget neutrality for that time period as well.
In our CY 2007 annual OPPS rulemaking, we proposed and finalized a policy of prospective payment based on median costs for the 11 brachytherapy sources for which we had claims data. We based the prospective payment rates on median costs for each source from our CY 2005 claims data (71 FR 68102 through 71 FR 68115).
Subsequent to publication of the CY 2007 OPPS/ASC final rule with comment period, section 107 of Public Law 109–432 (MIEA–TRHCA) amended section 1833 of the Act. Specifically, section 107(a) of Public Law 109–432 amended section 1833(t)(16)(C) of the Act by extending the payment period for brachytherapy sources based on a hospital's charges adjusted to cost for 1 additional year, through December 31, 2007. Therefore, we continued to pay for brachytherapy sources based on charges adjusted to cost for CY 2007.
Section 107(b)(1) of Public Law 109–432 amended section 1833(t)(2)(H) of the Act by adding a requirement for the establishment of separate payment groups for “stranded and non-stranded” brachytherapy sources furnished on or after July 1, 2007, in addition to the existing requirements for separate payment groups based on the number, isotope, and radioactive intensity of brachytherapy sources under section 1833(t)(2)(H) of the Act. Section 107(b)(2) of Pub. L. 109–432 authorized the Secretary to implement this requirement by “program instruction or otherwise.” We note that public commenters who responded to the CY 2007 OPPS/ASC proposed rule asserted that stranded sources, which they described as embedded into the stranded suture material and separated within the strand by material of an absorbable nature at specified intervals, had greater production costs than non-stranded sources (71 FR 68113 through 68114).
As a result of the statutory requirement to create separate groups for stranded and non-stranded sources as of July 1, 2007, we established several coding changes through a transmittal, effective July 1, 2007 (Transmittal 1259, dated June 1, 2007). Based on public Start Printed Page 60533 comments received on the CY 2007 OPPS/ASC proposed rule and industry input, we were aware of three sources available in stranded and non-stranded forms at that time: iodine-125; palladium-103; and cesium-131 (72 FR 42746). We created six new HCPCS codes to differentiate the stranded and non-stranded versions of iodine, palladium, and cesium sources.
In Transmittal 1259, we indicated that if we receive information that any of the other sources now designated as non-stranded are also FDA-approved and marketed as a stranded source, we would create a code for the stranded source. We also established two “Not Otherwise Specified” (NOS) codes for billing stranded and non-stranded sources that are not yet known to us and for which we do not have source-specific codes. We established HCPCS code C2698 (Brachytherapy source, stranded, not otherwise specified, per source) for stranded NOS sources and HCPCS code C2699 (Brachytherapy source, non-stranded, not otherwise specified, per source) for non-stranded NOS sources.
In the CY 2008 OPPS/ASC final rule with comment period (72 FR 66784), we again finalized prospective payment for brachytherapy sources, beginning in CY 2008, with payment rates determined using the CY 2006 claims-based costs per source for each brachytherapy source. Consistent with our policy regarding APC payments made on a prospective basis, we finalized the policy in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66686) to subject the cost of brachytherapy sources to the outlier provision of section 1833(t)(5) of the Act, and to also subject brachytherapy source payment weights to scaling for purposes of budget neutrality. Therefore, brachytherapy sources could receive outlier payments if the costs of furnishing brachytherapy sources met the criteria for outlier payment. In addition, as noted in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66683), implementation of prospective payment for brachytherapy sources would provide opportunities for hospitals to receive additional payments under certain circumstances through the 7.1 percent rural SCH adjustment.
For CY 2008, we also proposed and finalized a policy regarding payment for new brachytherapy sources for which we have no claims data (72 FR 42749 and 72 FR 66786, respectively). We indicated we would assign future new HCPCS codes for new brachytherapy sources to their own APCs, with prospective payment rates set based on our consideration of external data and other relevant information regarding the expected costs of the sources to hospitals. Finally, we proposed and finalized our policy to discontinue using status indicator “H” (Pass-Through Device Categories. Separate cost based pass-through payment; not subject to co-payment) because we would not be paying charges adjusted to costs after December 31, 2007, and instead adopted a policy of using status indicator “K” (which includes, among others, “Brachytherapy Sources. Paid under OPPS; separate APC payment”) for CY 2008 (72 FR 42749 and 72 FR 66785, respectively).
After we finalized these proposals for CY 2008, section 106(a) of Pub. L. 110–173 (MMSEA) extended the charges-adjusted-to-cost payment methodology for brachytherapy sources for an additional 6 months, through June 30, 2008. Because our final CY 2008 policies paid for brachytherapy sources at prospective rates based on median costs, we were unable to implement these policies during this extension.
In the CY 2009 OPPS/ASC proposed rule (73 FR 41502), we again proposed prospective payment rates for brachytherapy sources for CY 2009. We proposed to pay for brachytherapy sources at prospective rates based on their source-specific median costs as calculated from CY 2007 claims data available for CY 2009 ratesetting. Subsequent to issuance of the CY 2009 OPPS/ASC proposed rule, Public Law 110–275 (MIPPA) was enacted on July 15, 2008. Section 142 of Public Law 110–275 amended section 1833(t)(16)(C) of the Act, as amended by section 106(a) of Public Law 110–173 (MMSEA), to further extend the payment period for brachytherapy sources based on a hospital's charges adjusted to cost from July 1, 2008, through December 31, 2009. Therefore, we continued to pay for brachytherapy sources at charges adjusted to cost in CY 2008 from July 1 through December 31, and we maintained the assignment of status indicator “H” to brachytherapy sources for claims processing purposes in CY 2008. For CY 2009, we have continued to pay for all separately payable brachytherapy sources based on a hospital's charges adjusted to cost. Because brachytherapy sources are paid at charges adjusted to cost, we did not subject them to outlier payments under section 1833(t)(5) of the Act, or subject brachytherapy source payment weights to scaling for purposes of budget neutrality. Moreover, during the CY 2009 period of payment at charges adjusted to cost, brachytherapy sources are not eligible for the 7.1 percent rural SCH adjustment (as discussed in detail in section II.E. of this final rule with comment period).
Furthermore, for CY 2009, we did not adopt the policy we established in the CY 2008 OPPS/ASC final rule with comment period of paying stranded and non-stranded NOS codes for brachytherapy sources, HCPCS codes C2698 and C2699, based on a rate equal to the lowest stranded or non-stranded prospective payment for such sources. Also, for CY 2009, we did not adopt the policy we established in the CY 2008 OPPS/ASC final rule with comment period regarding payment for new brachytherapy sources for which we have no claims data. NOS HCPCS codes C2698 and C2699 and newly established specific source codes are paid at charges adjusted to cost through December 31, 2009, consistent with section 142 of Public Law 110–275.
For CY 2009, we finalized our proposal to create new status indicator “U” (Brachytherapy Sources. Paid under OPPS; separate APC payment) for brachytherapy source payment, instead of using status indicator “K” as proposed and finalized for CY 2008 for prospective payment, or status indicator “H,” used during the period of charges adjusted to cost payment. As noted in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68670), assigning a status indicator, such as status indicator “K,” to several types of items and services with potentially differing payment policies added unnecessary complexity to our operations. Status indicator “U” is used only for brachytherapy sources, regardless of their specific payment methodology for any period of time.
At the February 2009 meeting, the APC Panel recommended paying for brachytherapy sources in CY 2010 using a prospective payment methodology based on median costs from claims data. The APC Panel reviewed CY 2007 and CY 2008 brachytherapy source median costs from claims data and noted the stability of the data from year to year.
B. OPPS Payment Policy
Under section 142 of Public Law 110–275, payment for brachytherapy sources is mandated at charges adjusted to cost only through CY 2009. In the CY 2010 OPPS/ASC proposed rule (74 FR 35342), we proposed to adopt for CY 2010 the general OPPS prospective payment methodology for brachytherapy sources, consistent with section 1833(t)(2)(C) of the Act.
As we have previously stated (72 FR 66780 and 73 FR 41502), we believe that adopting the general OPPS prospective payment methodology for brachytherapy sources is appropriate for Start Printed Page 60534 a number of reasons. The general OPPS payment methodology uses median costs based on claims data to set the relative payment weights for hospital outpatient services. This payment methodology results in more consistent, predictable, and equitable payment amounts per source across hospitals by eliminating some of the extremely high and low payment amounts resulting from payment based on hospitals' charges adjusted to cost. We believe the OPPS prospective payment methodology would also provide hospitals with incentives for efficiency in the provision of brachytherapy services to Medicare beneficiaries. Moreover, this approach is consistent with our payment methodology for the vast majority of items and services paid under the OPPS.
We proposed to use CY 2008 claims data for setting the CY 2010 payment rates for brachytherapy sources, as we proposed for most other items and services that will be paid under the CY 2010 OPPS. For CY 2008, we have a full year of claims data for each of the separately payable sources, including iodine, palladium, and cesium sources that have stranded and non-stranded configurations. As indicated earlier, the APC Panel, at the February 2009 meeting, recommended using the median cost data for CY 2010 rates. Our proposal was consistent with the APC Panel's recommendation.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35342), we proposed to adopt the other payment policies for brachytherapy sources we finalized in previous final rules. We proposed to pay for the stranded and non-stranded NOS codes, HCPCS codes C2698 and C2699, at a rate equal to the lowest stranded or non-stranded prospective payment rate for such sources, respectively, on a per source basis (as opposed, for example, to a per mCi), which is based on the policy we established in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66785). The proposed payment methodology for NOS sources would provide payment to a hospital for new sources, while encouraging interested parties to quickly bring new sources to our attention so that specific coding and payment could be established.
We also proposed to implement the policy we established in the CY 2008 OPPS/ASC final rule with comment period (which was superseded by section 142 of Public Law 110–275) regarding payment for new brachytherapy sources for which we have no claims data, based on the same reasons we discussed in that final rule with comment period (72 FR 66786). That policy is intended to enable us to assign future new HCPCS codes for new brachytherapy sources to their own APCs, with prospective payment rates set based on our consideration of external data and other relevant information regarding the expected costs of the sources to hospitals.
Consistent with our policy regarding APC payments made on a prospective basis, we proposed to subject brachytherapy sources to outlier payments under section 1833(t)(5) of the Act, and also to subject brachytherapy source payment weights to scaling for purposes of budget neutrality. Therefore, brachytherapy sources could receive outlier payments if the costs of furnishing brachytherapy sources meet the criteria for outlier payment. In addition, as noted in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66683), implementation of prospective payments for brachytherapy sources would provide opportunities for hospitals to receive additional payments in CY 2010 under certain circumstances through the 7.1 percent rural adjustment as described in section II.E. of the proposed rule (74 FR 35295) and this final rule with comment period.
Therefore, in the CY 2010 OPPS/ASC proposed rule, we proposed to pay for brachytherapy sources at prospective payment rates based on their source-specific median costs for CY 2010. The separately payable brachytherapy source HCPCS codes, long descriptors, APCs, status indicators, and approximate median costs that we proposed for CY 2010 were presented in Table 32 of the proposed rule (74 FR 35342).
Comment: A number of commenters recommended that CMS continue to pay for brachytherapy sources separately based on hospitals' charges adjusted to cost due to the commenters' ongoing concerns regarding Medicare hospital claims data for brachytherapy sources; the commenters provided various examples of issues of concern. Some commenters were concerned that characteristics of high dose rate (HDR) iridium-192, which is a renewable source whose life decays over a 90-day period and is used to treat multiple patients, makes establishment of fair and adequate payment difficult on a fixed prospective basis. The commenters also claimed that the CMS brachytherapy source data continue to show huge variations in per unit costs on claims across hospitals. Several commenters stated that one half of the current brachytherapy sources have proposed payment rates based on 50 or fewer hospitals reporting claims for these sources. Some commenters also indicated that “rank order anomalies” exist in proposed payment rates for brachytherapy sources, citing that HCPCS code C2635 (Brachytherapy source, non-stranded, High Activity, Palladium-103, greater than 2.2 mCi (NIST), per source) always costs more than low activity sources (HCPCS code C2640, Brachytherapy source, stranded, Palladium-103, per source, and HCPCS code C2641, Brachytherapy source, non-stranded, Palladium-103, per source), yet hospital claims data do not reflect this difference. A number of commenters believed that the current charges-adjusted-to-cost methodology is more accurate and has been tested over time. A few commenters argued that the charges-adjusted-to-cost methodology provides overall cost savings to the Medicare program compared to the prospective payment methodology proposed for CY 2010, according to an analysis performed by the brachytherapy source industry. The commenters thus concluded that implementing prospective brachytherapy source payment would increase aggregate Medicare expenditures for brachytherapy sources compared with the charges-adjusted-to-cost payment methodology.
Several commenters supported the CY 2010 proposal to pay for brachytherapy sources prospectively based on median costs from claims data. One commenter asserted that hospital-specific payments based on the charges-adjusted-to-cost payment methodology violate the intent of a prospective payment system, namely to provide incentives to improve efficiency and control costs. The commenter believed that hospital-specific payments could be manipulated because hospitals know the CCR used to determine payments for brachytherapy sources.
Response: As we stated in the CY 2008 final rule with comment period (72 FR 66782), we believe that median costs based on hospital claims data for brachytherapy sources have produced reasonably consistent per-source cost estimates over the past several years, comparable to the patterns we have observed for many other OPPS services whose payments are set based upon relative payment weights from claims data. We believe that our per-source payment methodology specific to each source's radioisotope, radioactive intensity, and stranded or non-stranded configuration, supplemented by payment based on the number of sources used in a specific clinical case, adequately accounts for the major expected sources of variability across treatments.
As we also explained in the CY 2008 OPPS/ASC final rule with comment Start Printed Page 60535 period (72 FR 66782), a prospective payment system such as the OPPS relies on the concept of averaging, where the payment may be more or less than the estimated cost of providing a service for a particular patient, but with the exception of outlier cases, it is adequate to ensure access to appropriate care. In the case of brachytherapy sources for which the law requires separate payment groups, without packaging, the costs of these individual items could be expected to show greater variation than some other APCs under the OPPS because higher variability in costs for some component items and services is not balanced with lower variability for others and because relative weights are typically estimated using a smaller set of claims.
Nevertheless, we believe that prospective payment for brachytherapy sources based on median costs from claims calculated according to the standard OPPS methodology is appropriate at this time and would provide hospitals with the greatest incentives for efficiency in furnishing brachytherapy treatment. Under the budget-neutral OPPS, it is the relativity of costs of services, not their absolute costs, that is important, and we believe that brachytherapy sources can now be appropriately paid according to the standard OPPS payment approach. Moreover, OPPS payments for all services are similarly subjected to the same 2-year lag in costs from claims data available for ratesetting. Therefore, we believe the relative costs of OPPS services should generally be appropriate. It is important that the same measure of central tendency (median cost) from claims be used to establish the payment weights for all OPPS services in order to provide appropriate payment for all of these services. The inflation rate of medical services is taken into consideration through the conversion factor, which is updated annually to account for inflation and used to calculate payment rates from the relative payment weights based on median costs.
It is not uncommon for OPPS prospective payment rates to be based on claims from a relatively small number of hospitals that furnished the service in the year of claims data available for the OPPS update year. We are not concerned that some sources may have median costs and proposed payment rates based on 50 or fewer providers, as are some commenters. Fifty hospitals may report hundreds of brachytherapy source claims for many cases and comprise the universe of providers using particular low volume sources, for which we are required to pay separately by statute. Further, our methodology for estimating median costs for brachytherapy sources utilizes all line-item charges for those sources, which allows us to use all hospital reported charge and estimated cost information to set payment rates for these items. This is in contrast to our limitation of relying on “natural” single and “pseudo” single procedure claims to set APC payment rates for services with packaged costs. We have no reason to believe that prospective payment rates based on claims from those providers furnishing a particular source do not appropriately reflect the cost of that source to hospitals.
As for most other OPPS services, we note that the median costs for brachytherapy sources are based upon the costs of those providers that furnished the sources in CY 2008. Hospitals individually determine their charge for an item or service, and one of Medicare's primary requirements for setting a charge is that it be reasonably and consistently related to the cost of the item or service for that facility (Medicare Provider Reimbursement Manual-I, Section 2203). We then estimate a cost from that charge using the hospital's most recent Medicare hospital cost report data in our standard OPPS ratesetting process. In as much as we paid hospitals at charges adjusted to cost for brachytherapy sources in CY 2008 based on these exact charges, we believe hospital's individual charges to be accurate for their institution.
In the case of high and low activity iodine-125 sources, our claims data showed that the cost of the high activity source is greater than the low activity sources, yet this relationship is reversed for palladium-103 sources, as the commenter pointed out. We have no information about the expected cost differential between high and low activity sources of various isotopes other than what is available in our claims and hospital cost report data. For high activity palladium-103, only 16 hospitals provided this source in CY 2008, compared to 166 and 268 providers for low activity palladium sources described by HCPCS codes C2640 and C2641, respectively. Clearly, fewer providers furnished high activity palladium-103 sources, and we expect that the hospital cost distribution for those hospitals could be different than the cost distribution of the large number of providers reporting the low activity sources. These varied cost distributions clearly contribute to the observed relationship in median costs between the different types of sources, yet we see no reason why our standard ratesetting methodology for brachytherapy sources that relies on all claims from all hospitals furnishing brachytherapy sources would not yield valid median costs for those hospitals furnishing the different brachytherapy sources upon which CY 2010 prospective payments rates are based.
When the statutory requirement for payment of brachytherapy sources at a hospital's charges adjusted to cost ends on December 31, 2009 (section 1833(t)(16)(C) of the Act), prospective payment for brachytherapy sources based on their median costs would make the source payment an integral part of the OPPS, rather than a separate cost-based payment methodology within the OPPS. We believe that consistent and predictable prospectively established payment rates under the OPPS for brachytherapy sources are appropriate because we do not believe that the hospital resource costs associated with specific brachytherapy sources would vary greatly across hospitals or clinical conditions under treatment, other than through differences in the numbers of sources utilized that would be accounted for in the standard OPPS payment methodology we are finalizing.
We agree that sources such as HDR irirdium-192 have a fixed active life and must be replaced every 90 days; as a result, hospitals' per-treatment cost for the source would be dependent on the number of treatments furnished per source. The source cost must be amortized over the life of the source. Therefore, in establishing their charges for HDR iridium, we expect hospitals to project the number of treatments that would be provided over the life of the source and establish their charges for the source accordingly, as we have stated previously (72 FR 66783). For most such OPPS services, our practice is to establish prospective payment rates based on the median costs from hospitals claims data, to provide incentives for efficient and cost-effective delivery of these services.
We do not agree with the commenters that prospective brachytherapy source payment based on median costs would increase aggregate Medicare expenditures compared to the charges-adjusted-to-cost methodology, or that the charges-adjusted-to-cost methodology would provide overall cost savings to the Medicare program compared to the prospective payment methodology. We also do not believe that the beneficiary copayment in the aggregate would increase under the prospective payment methodology. We have traditionally estimated charge inflation for brachytherapy sources as higher than the market basket inflation Start Printed Page 60536 update applicable to prospective payment under the OPPS. We estimated charge inflation for brachytherapy sources between the 2 most recent years of hospital claims data by comparing the per-unit charge in CY 2008 claims to the per-unit charge in CY 2007 claims across all sources, and we used this estimate in our budget neutrality calculations. We are currently estimating a charge inflation factor of 17.1 percent for brachytherapy sources between CY 2007 and CY 2008 and, over the past several years, we have consistently estimated brachytherapy source charge inflation factors higher than 8 percent. Inflating payment at hospitals' charges adjusted to cost in the CY 2008 claims to CY 2010 using this most recent charge inflation factor and comparing it to an estimate of prospective payment for the same sources suggest that aggregate brachytherapy source payment for CY 2010 at charges adjusted to cost would be slightly higher than prospective payment for brachytherapy sources in CY 2010. Although the commenters did not include the details of their analysis in their comments, it is possible that the analysis did not include a charge inflation factor to increase payment estimated at charges adjusted to cost from CY 2008 to CY 2010.
Comment: One commenter indicated that the proposed source-specific payments were consistent with its experience with the cost per unit of the sources, except for the proposed payment for HCPCS code C2634 (Brachytherapy source, non-stranded, High Activity, Iodine-125, greater than 1.01 mCi (NIST), per source). The commenter noted that the proposed payment rate for HCPCS code C2634 is $60, yet its invoices for high activity I–125, that is, HCPCS code C2634, range between $174 and $689. The commenter also stated that high activity I–125 sources are ordered based on a range of activity levels. The commenter suggested that there may have been errors in hospital reporting of HCPCS code C2634 in CY 2008 that resulted in the low proposed payment rate. The commenter requested that CMS reevaluate the proposed payment rate for HCPCS code C2634 for CY 2010 using average cost data from manufacturers.
Response: We are pleased that the proposed CY 2010 payment rates for all but one of the brachytherapy sources are consistent with the commenter's experience. The CY 2008 median cost of HCPCS code C2634 for this final rule with comment period is approximately $59, compared with approximately $31 in CY 2006 and approximately $38 in CY 2007. The CY 2008 median cost is somewhat higher than the previous 2 years, and we acknowledge that the variability in the activity of sources reported with HCPCS code C2634 could explain some of the variability in cost for this source. Furthermore, we note that the CY 2008 median cost for HCPCS code C2634 is based on 18,602 units, over 267 days, from 48 providers. We believe that some variation in relative cost from year to year is to be expected in a prospective payment system, particularly for low volume items.
For all APCs whose payment rates are based upon relative payment weights, we note that the quality and accuracy of reported units and charges significantly influence the final median costs that are the basis for our payments. Beyond our standard OPPS trimming methodology (described in section II.A.2. of this final rule with comment period) that we apply to those claims that have passed various types of claims processing edits, it is not our policy to judge the accuracy of hospital coding and charging for the purpose of ratesetting. Moreover, we do not believe it is necessary to incorporate external cost data from manufacturers of brachytherapy sources or others because, in a relative weight system like the OPPS, it is the relativity of the costs to one another, rather than absolute cost, that is important in setting payment rates. External data lack relativity to the estimated costs derived from the claims and cost report data and generally are not appropriate for determining relative weights that result in payment rates when costs derives from hospital claims and cost report data for services are available.
Comment: One commenter suggested that brachytherapy sources are not reported consistently by all providers using a specific revenue center and recommended that CMS maintain payment at charges adjusted to cost until cost data are improved by refined information resulting from the new cost center for high cost supplies.
Response: In analyzing the reporting of brachytherapy sources in CY 2008 claims, we found that the great majority of brachytherapy sources are reported under revenue code 0278 (Other Implants). Under the policy finalized in the FY 2009 IPPS final rule (73 FR 48462 through 48463), we finalized a definition of a new” Implantable Devices Charged to Patients” cost center to which costs and charges under revenue code 0278 would map in the future. Thus, brachytherapy sources would generally be subject to the “Implantable Devices Charged to Patients” cost center for future cost estimation under the OPPS, potentially leading to greater accuracy in cost estimation for these devices as noted by the commenter. This new cost center was available for use for cost reporting periods beginning on or after May 1, 2009, and was discussed in Transmittal 20 (dated July 2009) that updated Chapter 36, Hospital and Hospital Health Care Complex Cost Report (Form CMS 2552–96) of the Medicare Provider Reimbursement Manual, Part 2, to provide Line 55.30 to report “Implantable Devices Charged to Patients.” The proposed draft cost report Form CMS–2552–10, published in the Federal Register for public comment on July 2, 2009 (74 FR 31738), provides new line 69 to report “Implantable Devices Charged to Patients.” The proposed cost report can be viewed at: http://www.cms.hhs.gov/PaperworkReductionActof1995/PRAL/itemdetail.asp?filterType=none&filterByDID=-99&sortByDID=2&sortOrder=descending&itemID=CMS1224069&intNumPerPage=10.
We have stated previously that we continue to emphasize our preference for long-term cost reporting changes and broad education initiatives to address the accuracy of claims data (73 FR 68524). This recent change to include a new cost center will ultimately influence both the IPPS and OPPS relative weights in the future. Nevertheless, in the meantime, we believe it is fully appropriate to utilize our current cost estimates for brachytherapy sources and all other implantable devices in calculating payment weights under the OPPS because these are our best current estimates of costs as derived from claims and cost report data. When hospital-specific CCRs from the new cost center are available for ratesetting in several years, we will incorporate those into the revenue code-to-cost center crosswalk that we use for OPPS cost estimation. However, at the present time, we believe our current methodology that generally utilizes the available single medical supply CCR leads to appropriate cost estimates for brachytherapy sources, and we see no reason why payment at charges adjusted to cost, which applies an hospital-specific overall ancillary CCR to hospital charges for brachytherapy sources, would lead to a more accurate cost estimate for these items. The hospital-specific overall ancillary CCR is based on costs and charges for a wide range of OPPS services, and we have no reason to believe that hospital markup practices for brachytherapy sources are similar to the relationship between costs Start Printed Page 60537 and charges represented in this very general CCR.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to pay for brachytherapy sources prospectively based on CY 2008 median costs from historical hospital claims data. In addition, we will pay the stranded and non-stranded NOS codes, HCPCS codes C2698 and C2699, at a rate equal to the lowest stranded or non-stranded prospective payment rate for such sources, respectively, on a per source basis. Payment for new brachytherapy sources, which may be established quarterly, will be made through their own APCs, with prospective payment rates set based on our consideration of external data and other relevant information regarding the expected costs of the sources to hospitals because we would have no information from claims data on the costs of these new sources to hospitals. Finally, in CY 2010, brachytherapy sources will be subject to outlier payments, their payment weights subject to scaling for purposes of budget neutrality, and, under some circumstances, their payment subject to the 7.1 percent rural adjustment as discussed in section II.E. of this final rule with comment period.
Table 45 below displays the separately payable brachytherapy source HCPCS codes, long descriptors, APCs, status indicators, and approximate median costs for CY 2010.
| CY 2010 HCPCS Code | CY 2010 long descriptor | Final CY 2010 APC | Final CY 2010 SI | Final CY 2010 approximate APC median cost |
|---|---|---|---|---|
| A9527 | Iodine I–125, sodium iodide solution, therapeutic, per millicurie | 2632 | U | $38 |
| C1716 | Brachytherapy source, non-stranded, Gold-198, per source | 1716 | U | 42 |
| C1717 | Brachytherapy source, non-stranded, High Dose Rate Iridium-192, per source | 1717 | U | 229 |
| C1719 | Brachytherapy source, non-stranded, Non-High Dose Rate Iridium-192, per source | 1719 | U | 63 |
| C2616 | Brachytherapy source, non-stranded, Yttrium-90, per source | 2616 | U | 15,635 |
| C2634 | Brachytherapy source, non-stranded, High Activity, Iodine-125, greater than 1.01 mCi (NIST), per source | 2634 | U | 59 |
| C2635 | Brachytherapy source, non-stranded, High Activity, Palladium-103, greater than 2.2 mCi (NIST), per source | 2635 | U | 28 |
| C2636 | Brachytherapy linear source, non-stranded, Palladium-103, per 1MM | 2636 | U | 19 |
| C2638 | Brachytherapy source, stranded, Iodine-125, per source | 2638 | U | 42 |
| C2639 | Brachytherapy source, non-stranded, Iodine-125, per source | 2639 | U | 36 |
| C2640 | Brachytherapy source, stranded, Palladium-103, per source | 2640 | U | 60 |
| C2641 | Brachytherapy source, non-stranded, Palladium-103, per source | 2641 | U | 57 |
| C2642 | Brachytherapy source, stranded, Cesium-131, per source | 2642 | U | 109 |
| C2643 | Brachytherapy source, non-stranded, Cesium-131, per source | 2643 | U | 65 |
| C2698 | Brachytherapy source, stranded, not otherwise specified, per source | 2698 | U | *42 |
| C2699 | Brachytherapy source, non-stranded, not otherwise specified, per source | 2699 | U | *28 |
| * Median cost is that of the lowest cost stranded or non-stranded source upon which CY 2010 payment for the NOS HCPCS code is based. | ||||
We continue to invite hospitals and other parties to submit recommendations to us for new HCPCS codes to describe new brachytherapy sources consisting of a radioactive isotope, including a detailed rationale to support recommended new sources. Such recommendations should be directed to the Division of Outpatient Care, Mail Stop C4–05–17, Centers for Medicare and Medicaid Services, 7500 Security Boulevard, Baltimore, MD 21244. We will continue to add new brachytherapy source codes and descriptors to our systems for payment on a quarterly basis.
VIII. OPPS Payment for Drug Administration Services
A. Background
In CY 2005, in response to the recommendations made by public commenters and the hospital industry, OPPS transitioned from Level II HCPCS Q-codes to the use of CPT codes for drug administration services. These CPT codes allowed specific reporting of services regarding the number of hours for an infusion and provided consistency in coding between Medicare and other payers. (For a discussion regarding coding and payment for drug administration services prior to CY 2005, we refer readers to the CY 2008 OPPS/ASC final rule with comment period (72 FR 66787).)
While hospitals began adopting CPT codes for outpatient drug administration services in CY 2005, physicians paid under the MPFS were using HCPCS G-codes in CY 2005 to report office-based drug administration services. These HCPCS G-codes were developed in anticipation of substantial revisions to the drug administration CPT codes by the CPT Editorial Panel that were expected for CY 2006.
In CY 2006, as anticipated, the CPT Editorial Panel revised its coding structure for drug administration services and incorporated new concepts, such as initial, sequential, and concurrent services, into a structure that previously distinguished services based on type of administration (chemotherapy/nonchemotherapy), method of administration (injection/infusion/push), and for infusion services, first hour and additional hours. For CY 2006, we implemented the CY 2006 drug administration CPT codes that did not reflect the concepts of initial, sequential, and concurrent services under the OPPS, and we created HCPCS C-codes that generally paralleled the CY 2005 CPT codes for reporting these other services.
For CY 2007, as a result of public comments on the proposed rule and feedback from the hospital community and the APC Panel, we implemented the full set of CPT codes for drug administration services, including codes incorporating the concepts of initial, sequential, and concurrent services. In addition, the CY 2007 update process offered us the first opportunity to consider data gathered from the use of CY 2005 CPT codes for purposes of ratesetting. For CY 2007, we used CY 2005 claims data to implement a six- Start Printed Page 60538 level APC structure for drug administration services. In CY 2008, we continued to use the full set of CPT codes for drug administration services and continued our assignment of drug administration services to this six-level APC structure.
For CY 2009, we continued to allow hospitals to use the full set of CPT codes for drug administration services but moved from a six-level APC structure to a five-level APC structure. We note that, while there were changes in the CPT numerical coding for nonchemotherapy drug administration services in CY 2009, the existing CPT codes were only renumbered, and there were no significant changes to the code descriptors themselves. As we discussed in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68672), the CY 2009 ratesetting process afforded us the first opportunity to examine hospital claims data for the full set of CPT codes that reflected the concepts of initial, sequential, and concurrent services. For CY 2009, we performed our standard annual OPPS review of the clinical and resource characteristics of the drug administration CPT codes assigned to the six-level CY 2008 APC structure based on the CY 2007 claims data available for the CY 2009 OPPS/ASC proposed rule. As a result of our hospital cost analysis and detailed clinical review, we adopted a five-level APC structure for CY 2009 drug administration services to more appropriately reflect their resource utilization in APCs that also group clinically similar services. As we noted in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68671), these APCs generally demonstrated the clinically expected and actually observed comparative relationships between the median costs of different types of drug administration services, including initial and additional services; chemotherapy and other diagnostic, prophylactic, or therapeutic services; injections and infusions; and simple and complex methods of drug administration. In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68673), we indicated our belief that the five-level APC structure was the most appropriate structure based on updated hospital claims data for the full range of CPT codes for drug administration for the CY 2009 OPPS/ASC final rule with comment period because the structure resulted in payment groups with greater clinical and resource homogeneity.
B. Coding and Payment for Drug Administration Services
In the CY 2010 OPPS/ASC proposed rule (74 FR 35343), we proposed for CY 2010 to continue to use the full set of CPT codes for drug administration services. In addition, as a part of our standard annual review, we analyzed the assignments of CPT codes for drug administration into the five-level APC structure and, based on the results of this review, proposed to continue a five-level APC structure for CY 2010. Further, we proposed some minor reconfigurations of the APCs as described below to account for changes in HCPCS code-specific median costs resulting from updated CY 2008 claims data and the most recent cost report data, and the CY 2010 drug payment proposal that is discussed in section V.B.3.b. of the proposed rule (74 FR 35326 through 35333) and this final rule with comment period.
In the CY 2007 OPPS/ASC final rule with comment period (71 FR 68117), we explained that we expected CPT codes for additional hours of infusion to be reported with CPT codes for the initial hour of drug infusion. This would result in a substantial number of claims for drug administration services that were unusable for ratesetting purposes because multiple services would be present on the same bill and result in essentially no correctly coded claims upon which to set the median costs for the CPT codes describing additional hours of infusion. (We refer readers to section II.A.1.b. of the proposed rule (74 FR 35239 through 35241) and this final rule with comment period for a further discussion of multiple bills and our ratesetting methodology.) In order to use these claims for ratesetting purposes for both the initial drug administration codes and the additional hour drug administration codes, we adopted the policy of adding the additional hour drug administration codes to the bypass list in order to create “pseudo” single claims that would be useable for OPPS ratesetting purposes. After the creation of these “pseudo” single claims, we applied the standard OPPS methodology to calculate HCPCS code-specific median costs for these initial and additional hour drug administration codes.
As we explained further in the CY 2007 OPPS/ASC final rule with comment period, bypassing these additional hour drug administration CPT codes and developing additional “per unit” claims provided a methodology for calculating median costs for these previously packaged drug administration services which attributed all of their line-item cost data to their assigned APCs. However, we noted that this methodology allocates all packaged costs on claims for drug administration services to the associated initial hour of infusion code. Because these additional hours of infusion codes were always reported with other drug administration services, we expected that the packaging related to additional hours of infusion would be appropriately assigned to the initial drug administration service also included on the same claim. While we stated our belief that there are some packaged costs that are clinically related to the second and subsequent hours of infusion, especially for infusions of packaged drugs spanning several hours, we were not able to accurately assign representative portions of packaged costs to multiple different services due to the limitations of our claims data.
We indicated that, while this methodology did not assign any packaged costs to the additional hours of drug administration codes, we believed this methodology took into account all of the packaging on claims for drug administration services and provided a reasonable framework for developing median costs for drug administration services that were often provided in combination with one another.
Since this approach was first adopted for CY 2007, we have updated and expanded the bypass methodology to reflect changing drug administration HCPCS codes that are recognized under the OPPS. We placed all of the add-on CPT codes for drug administration services, including the sequential infusion and intravenous push codes, on the bypass list in CY 2009 (73 FR 68513) and proposed to include them in CY 2010 (74 FR 35242 through 35252) in order to continue this framework for transforming these otherwise unusable multiple bills into “pseudo” single claims that can be used for OPPS ratesetting purposes. Table 33 of the proposed rule (74 FR 35345 through 35349) displayed the proposed configurations of the five drug administration APCs for CY 2010. In proposing to reassign several HCPCS codes for CY 2010, we took into consideration the resource characteristics of the services, as reflected in their HCPCS code-specific median costs and their clinical characteristics. We believed the proposed APC configurations group drug administration services that share sufficiently similar clinical and resource characteristics, taking into consideration updated CY 2008 claims data and the most recent cost report data and Start Printed Page 60539 common clinical scenarios that have been described to us.
Comment: Several commenters supported the proposal to include the drug administration add-on codes on the bypass list. The commenters stated that, by including these codes in the bypass methodology, more single bills can be used for ratesetting purposes.
One commenter recommended that CPT code 96368 (Intravenous infusion, for therapy, prophylaxis, or diagnosis (specify substance or drug): concurrent infusion (List separately in addition to code for primary procedure)) be included on the bypass list in order to ensure consistency with the treatment of other drug administration codes.
Response: As stated in the CY 2007 OPPS/ASC final rule with comment period (71 FR 68117), we expect CPT codes for additional hours of infusion to be reported with CPT codes for the initial hour of drug infusion. This would result in a substantial number of claims for drug administration services that would be unusable for ratesetting purposes because multiple services would be present on the same bill and result in essentially no correctly coded claims upon which to set the median costs for the CPT codes describing additional hours of infusion. In order to use these claims for ratesetting purposes for both the initial drug administration codes and the additional hour drug administration codes, we adopted the policy of adding the additional hour drug administration codes on the bypass list in order to create “pseudo” single claims that would be useable for OPPS ratesetting purposes. We continue to believe that bypassing these additional hour drug administration CPT codes and developing additional “per unit” claims provide a methodology for calculating median costs for these previously packaged additional hour drug administration services, which attributes all of their line-item cost data to their assigned APCs. Although we understand that this methodology does not assign any packaged costs to the additional hours of drug administration codes, we continue to believe this methodology takes into account all of the packaging on claims for drug administration services and provides a reasonable framework for developing median costs for drug administration services that are often provided in combination with one another.
As discussed above, since this approach was first adopted for CY 2007, we have updated and expanded the bypass methodology to reflect changing drug administration HCPCS codes that are recognized under the OPPS. We placed all of the add-on CPT codes for drug administration services, including the sequential infusion and intravenous push codes, on the bypass list in CY 2009 (73 FR 68513) and proposed to include them in CY 2010 (74 FR 35242 through 35252) in order to continue this framework for transforming these otherwise unusable multiple bills into “pseudo” single claims that can be used for OPPS ratesetting purposes.
We have not added CPT code 96368 (or its predecessor CPT code 90768) on the bypass list because our CY 2010 policy unconditionally packages payment for this service and, therefore, it is not a candidate for the bypass list. The purpose of the bypass list is to develop “pseudo” single claims so that there are more data available to determine the median costs of separately payable services for ratesetting purposes. Including packaged codes would be contrary to the purpose of the bypass list.
We refer readers to section II.A.1.b. of this final rule with comment period for a full discussion of our final bypass policy and list for CY 2010.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to use the full set of CPT codes for drug administration and include all separately paid drug administration add-on HCPCS codes on the CY 2010 bypass list. We will not add CPT code 96368 on the bypass list because it is not a separately paid service and, therefore, it is not a candidate for the bypass list.
Comment: Several commenters expressed support for the proposed five-level APC structure for drug administration services. Some commenters requested that CMS continue to evaluate the five-level structure annually. In addition, several commenters specifically supported the proposed CY 2010 reconfiguration of the HCPCS code assignments to the drug administration APCs.
One commenter stated that the data used to propose reassignments of drug administration codes to drug administration APCs for the proposed rule were inadequate. The commenter explained that changes to CPT codes require hospitals to train staff and implement guidelines for code use and, therefore, accurate claims hospital data for updated CPT codes are not immediately available from the first year of their use. The commenter added that differences in definitions for drug administration codes by Medicare contractors contribute to incomplete and inconsistent data.
Response: In proposing to reassign several HCPCS codes for CY 2010, we took into consideration the resource characteristics of the services, as reflected in their HCPCS code-specific median costs and their clinical characteristics. We believe the proposed APC configurations group drug administration services that share sufficiently similar clinical and resource characteristics, taking into consideration updated CY 2008 claims data and the most recent cost report data and common clinical scenarios that have been described to us.
We disagree with the commenter who believed that our costs from hospital claims data are inadequate. We believe that the hospital claims data for drug administration HCPCS codes are robust and representative of the costs of the many hospitals performing these services. Multiple drug administration HCPCS codes are reported on several hundred thousand hospital outpatient claims annually and almost all drug administration HCPCS codes are reported on at least several thousand claims. The data that we have reviewed for CY 2010 do not dramatically differ from previous years' data for these high volume services furnished by thousands of hospitals. This is evidenced in the number of hospitals billing for drug administration services, the frequency of specific drug administration services, and the resulting median costs of the drug administration services.
Finally, we note that it is our standard practice to annually review the configuration of all APCs. Therefore, as part of our standard methodology, we expect to continue to review the configuration of drug administration APCs in future years.
Comment: A few commenters requested that HCPCS code C8957 (Intravenous infusion for therapy/diagnosis; initiation of prolonged infusion (more than eight hours), requiring use of portable or implantable pump) not be reassigned to APC 0439 (Level IV Drug Administration) as proposed for CY 2010. Instead, the commenters requested that HCPCS code C8957 continue to be assigned to APC 0440 (Level V Drug Administration) for CY 2010. In addition, one commenter requested that CPT code 96521 (Refilling and maintenance of portable pump) not be reassigned to APC 0439 as proposed for CY 2010. Instead, the commenter requested that CPT code 96521 continue to be assigned to APC 0440. The commenters stated that HCPCS code C8957 and CPT code 96521 represent prolonged infusions that require the use of a pump and a significant amount of time and nursing resources. Start Printed Page 60540
Response: As is our standard process, for the CY 2010 proposed rule, we reviewed each APC for clinical cohesiveness and resource homogeneity. As the commenters noted, we proposed to reassign HCPCS code C8957 to APC 0439 because we believed that the proposed HCPCS-specific median cost more closely matched the proposed median cost of APC 0439. Upon further review, we agree with the commenters that the clinical characteristics of the procedure described by HCPCS code C8957 that describes a prolonged intravenous infusion lasting more than 8 hours more closely resemble those of procedures assigned to APC 0440. Further, the HCPCS-specific median cost of HCPCS code C8957 (approximately $179) is only slightly less than the median cost of APC 0440 (approximately $218), resulting in our belief that APC 0440 would be the most appropriate assignment of HCPCS code C8957 for CY 2010.
In addition, we proposed to reassign CPT code 96521 to APC 0439 because we believed that the proposed HCPCS-specific median cost more closely matched the median cost of APC 0439. We continue to believe that the HCPCS-specific median cost of CPT code 96521 (approximately $133) closely resembles the median cost of APC 0439 (approximately $126). In addition, we note that while HCPCS code C8957 describes the initiation of a prolonged infusion that we would expect to be resource-intensive, CPT code 96521 describes the refilling and maintenance of a portable infusion pump, a drug administration service that we would expect to require less hospital resources. Therefore, while we believe that there is a compelling reason to assign HCPCS code C8957 to the higher level drug administration APC 0440, we do not find a compelling reason to do the same for CPT code 96521.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal for the five-level APC structure for drug administration services, with a modification to not reassign HCPCS code C8957 to APC 0439 as proposed. Instead, we will continue to assign HCPCS code C8957 to APC 0440 for CY 2010, with a final APC median cost of approximately $218. We are finalizing our proposed CY 2010 assignment of CPT code 96521 to APC 0439, with a final APC median cost of approximately $126.
Comment: A few commenters requested that CPT codes 96376 (Therapeutic, prophylactic, or diagnostic injection (specify substance or drug); each additional sequential intravenous push of the same substance/drug provided in a facility) and 96368 (Intravenous infusion, for therapy, prophylaxis, or diagnosis (specify substance or drug); concurrent infusion) be paid separately in CY 2010. Some commenters stated that CPT code 96376 is similar to CPT code 96374 (Therapeutic, prophylactic, or diagnostic injection (specify substance or drug); intravenous push, single or initial substance/drug) and should be assigned to the same APC as CPT code 96374 for CY 2010. In addition, some commenters indicated that CPT code 96368 is similar to CPT code 96375 (Therapeutic, prophylactic, or diagnostic injection (specify substance or drug); each additional sequential intravenous push of a new substance/drug (List separately in additional to code for primary procedure)) and should be assigned to the same APC as CPT code 96375 for CY 2010. Other commenters noted that because CMS now has claims data upon which to set specific payment rate for these services, the OPPS should pay separately for CPT codes 96376 and 96368.
Response: We agree with the commenters that we have cost data for these CPT codes based on historical hospital claims data. However, we also believe that these codes remain appropriate for packaging and, therefore, we include their costs in payment for the independent services with which they are always associated. As we discussed in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66787 through 66788) and in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68674), in deciding whether to package a service or pay for it separately, we consider a variety of factors, including whether the service is normally provided separately or in conjunction with other services; how likely it is for the costs of the packaged code to be appropriately mapped to the separately payable codes with which it was performed; and whether the expected cost of the service is relatively low. CPT codes 96376 and 96368, by definition, are always provided in association with other drug administration services, and we continue to believe that they are most appropriately packaged under the OPPS.
Furthermore, we do not agree with the commenters that the services described by CPT code 96376 are similar to those described by CPT code 96374. CPT code 96374 is an initial intravenous push code, and, per CPT instructions, special billing guidelines apply. Commonly, this service requires the initial establishment of intravenous access in a patient, a resource-intensive task performed by hospital staff using special supplies. In contrast, CPT code 96376 is an add-on code and is reported for each additional sequential intravenous push of the same substance/drug. In the case of this sequential service, the patient already has established intravenous access, so we would expect the service to require fewer hospital resources. In addition, we do not agree with commenters that the services described by CPT code 96368 are similar to those described by CPT code 96375. CPT code 96368 describes a concurrent intravenous infusion while CPT code 96375 describes a sequential intravenous push, and we would expect these services to require different hospital resources.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to unconditionally package payment for CPT codes 96368 and 96376. These CPT codes are, therefore, assigned status indicator “N” in Addendum B to this final rule with comment period.
Comment: Several commenters submitted questions related to coding for drug administration services. Some commenters requested information on how to code for specific clinical scenarios, while other commenters were concerned about documentation requirements for a stop time for an infusion.
Response: Each of these comments and questions is outside of the scope of the proposals in the CY 2010 OPPS/ASC proposed rule. However, we will consider the possibility of addressing these concerns through other available mechanisms, as appropriate.
In summary, after review of the public comments we received, we are finalizing our proposed coding and payment structure for drug administration as follows. We are finalizing, without modification, our proposal to include all separately payable drug administration add-on codes on the bypass list for CY 2010. In addition, we are finalizing our proposed five-level APC structure for payment of drug administration services in the HOPD for CY 2010, with the exception of a modification to continue to assign HCPCS code C8957 to APC 0440 for CY 2010, rather than APC 0439 as we proposed. Finally, we are finalizing our CY 2010 proposal, without modification, to continue to package payment for CPT codes 96376 and 96368 for CY 2010.
Table 46 below displays the final configurations of the five drug administration APCs for CY 2010.
Start Printed Page 60541 Start Printed Page 60542 Start Printed Page 60543 Start Printed Page 60544 Start Printed Page 60545IX. OPPS Payment for Hospital Outpatient Visits
A. Background
Currently, hospitals report visit HCPCS codes to describe three types of OPPS services: Clinic visits, emergency department visits, and critical care services. For OPPS purposes, we recognize clinic visit codes as those codes defined in the CPT codebook to report evaluation and management (E/M) services provided in the physician's office or in an outpatient or other ambulatory facility. We recognize emergency department visit codes as those codes used to report E/M services provided in the emergency department. Emergency department visit codes consist of five CPT codes that apply to Type A emergency departments, and five Level II HCPCS codes that apply to Type B emergency departments. For OPPS purposes, we recognize critical care codes as those CPT codes used by hospitals to report critical care services that involve the “direct delivery by a physician(s) of medical care for a critically ill or critically injured patient,” as defined by the CPT codebook. In Transmittal 1139, Change Request 5438, dated December 22, 2006, we stated that, under the OPPS, the time that can be reported as critical care is the time spent by a physician and/or hospital staff engaged in active face-to-face critical care of a critically ill or critically injured patient. Under the OPPS, we also recognize HCPCS code G0390 (Trauma response team associated with hospital critical care service) for the reporting of a trauma response in association with critical care services.
As we proposed in the CY 2010 OPPS/ASC proposed rule (74 FR 35349 through 35350), we are continuing to recognize these CPT and HCPCS codes describing clinic visits, Type A and B emergency department visits, critical care services, and trauma team activation provided in association with critical care services for CY 2010. These codes are listed below in Table 47.
| CY 2010 HCPCS Code | CY 2010 Descriptor |
|---|---|
| Clinic Visit HCPCS Codes | |
| 99201 | Office or other outpatient visit for the evaluation and management of a new patient (Level 1). |
| Start Printed Page 60546 | |
| 99202 | Office or other outpatient visit for the evaluation and management of a new patient (Level 2). |
| 99203 | Office or other outpatient visit for the evaluation and management of a new patient (Level 3). |
| 99204 | Office or other outpatient visit for the evaluation and management of a new patient (Level 4). |
| 99205 | Office or other outpatient visit for the evaluation and management of a new patient (Level 5). |
| 99211 | Office or other outpatient visit for the evaluation and management of an established patient (Level 1). |
| 99212 | Office or other outpatient visit for the evaluation and management of an established patient (Level 2). |
| 99213 | Office or other outpatient visit for the evaluation and management of an established patient (Level 3). |
| 99214 | Office or other outpatient visit for the evaluation and management of an established patient (Level 4). |
| 99215 | Office or other outpatient visit for the evaluation and management of an established patient (Level 5). |
| Emergency Department Visit HCPCS Codes | |
| 99281 | Emergency department visit for the evaluation and management of a patient (Level 1). |
| 99282 | Emergency department visit for the evaluation and management of a patient (Level 2). |
| 99283 | Emergency department visit for the evaluation and management of a patient (Level 3). |
| 99284 | Emergency department visit for the evaluation and management of a patient (Level 4). |
| 99285 | Emergency department visit for the evaluation and management of a patient (Level 5). |
| G0380 | Type B emergency department visit (Level 1). |
| G0381 | Type B emergency department visit (Level 2). |
| G0382 | Type B emergency department visit (Level 3). |
| G0383 | Type B emergency department visit (Level 4). |
| G0384 | Type B emergency department visit (Level 5). |
| Critical Care Services HCPCS Codes | |
| 99291 | Critical care, evaluation and management of the critically ill or critically injured patient; first 30–74 minutes. |
| 99292 | Critical care, evaluation and management of the critically ill or critically injured patient; each additional 30 minutes. |
| G0390 | Trauma response associated with hospital critical care service. |
During the February 2009 APC Panel meeting, the APC Panel recommended that CMS present at the next APC Panel meeting an analysis of the most common diagnoses and services associated with Type A and Type B emergency department visits, including an analysis by hospital-specific characteristics, as well as an analysis of CY 2008 claims data for clinic and emergency department (Types A and B) visits. The APC Panel also recommended that the work of the Visits and Observation Subcommittee continue. We adopted these recommendations in the CY 2010 OPPS/ASC proposed rule (74 FR 35350) and provided frequency and cost data from CY 2008 claims for clinic and emergency department visits at the August 2009 meeting of the APC Panel. We plan to provide the requested analysis of the most common diagnoses and services associated with Type A and Type B emergency department visits to the APC Panel at the winter 2010 meeting of the APC Panel.
At its August 2009 meeting, the APC Panel recommended that CMS present an analysis of CY 2009 claims data for clinic and emergency department (Type A and B) visits at the next meeting of the APC Panel. The APC Panel recommended again that CMS provide analyses of the most common diagnoses and services associated with Type A and Type B emergency department visits at the next meeting of the APC Panel, including analysis by hospital-specific characteristics. We are accepting all of these recommendations and will present the available requested data at the winter 2010 meeting of the APC Panel.
B. Policies for Hospital Outpatient Visits
1. Clinic Visits: New and Established Patient Visits
As reflected in Table 47, hospitals use different CPT codes for clinic visits based on whether the patient being treated is a new or an established patient. Beginning in CY 2009, we refined the definitions of new and established patients to reflect whether or not the patient has been registered as an inpatient or outpatient of the hospital within the past 3 years. A patient who has been registered as an inpatient or outpatient of the hospital within the 3 years prior to a visit would be considered to be an established patient for that visit, while a patient who has not been registered as an inpatient or outpatient of the hospital within the 3 years prior to a visit would be considered to be a new patient for that visit. We refer readers to the CY 2009 OPPS/ASC final rule with comment period (73 FR 68677 through 68680) for a full discussion of the refined definitions.
We stated in the CY 2010 OPPS/ASC proposed rule (74 FR 35350) that we continue to believe that defining new or established patient status based on whether the patient has been registered as an inpatient or outpatient of the hospital within the 3 years prior to a visit will reduce hospitals' administrative burden associated with reporting appropriate clinic visit CPT codes. For CY 2010, we proposed to continue recognizing the refined definitions of new and established patients, and our policy of calculating median costs for clinic visits under the OPPS using historical hospital claims data.
Comment: Several commenters recommended that CMS remove the distinction between new and established patient clinic visits, arguing that facilities must expend the same level of resources regardless of whether the patient was registered as an inpatient or an outpatient in the hospital within the past 3 years. According to some commenters, CMS' use of the CPT codes for visits, which differentiate between new and established patients, is contrary to CMS' Start Printed Page 60547 past statements that the CPT guidelines and definitions for E/M visit codes are not applicable in the hospital outpatient setting because they fail to reflect hospital services and resource consumption. In addition, some commenters stated there are significant operational issues involved with implementing the 3-year criterion for hospital clinic visit billing purposes, and expressed concerns that hospitals' incorrect compliance with this requirement could be targeted by Recovery Audit Contractor (RAC) audits and other types of audits. Some commenters argued that any differential in costs that is evident in claims data for new versus established patient visits would be the result of hospitals' erroneous reporting of these codes, rather than any real difference in the level of resources expended treating a new versus an established patient. One commenter characterized the median cost differences between new and established patient visit codes as random and suggested that some providers report CPT codes 99201 and 99211 as “default codes” when reporting clinic visits, which, according to the commenter, raises the costs reflected in the claims data for these codes and artificially impacts the overall APC ratesetting process.
Some commenters asserted that CMS' proposal in the CY 2010 MPFS proposed rule to eliminate the use of consultation codes for physician payment purposes provides a precedent for discontinuing the use of the CPT E/M codes by hospitals. According to the commenters, CMS cited findings in the March 2006 OIG report entitled “Consultations in Medicare: Coding and Reimbursement” that physicians frequently misuse CPT codes for consultation services as a basis for no longer recognizing those codes under the MPFS. The commenters stated that hospitals similarly misuse the clinic visit codes, and that CMS should cease to recognize the clinic visit CPT codes under the OPPS for this reason.
Many commenters suggested that, as an alternative to the clinic visit CPT codes for new and established patients, hospitals bill for visits based on the resources expended in the visit at a level determined by the hospitals' internal reporting guidelines, regardless of whether the patient is new or established. Some commenters supported the use of Level II HCPCS G-codes for hospital clinic visits to represent hospital resources expended, without the distinction between new and established patients. According to the commenters, creation of these HCPCS G-codes would streamline hospital reporting of visits, enable hospitals to correctly code for visits based on established definitions, and facilitate elimination of the new versus established patient visit concept for hospital reporting. The commenters noted that, in the past, providers have resisted implementing hospital-specific HCPCS codes for reporting visits before the implementation of national visit reporting guidelines for hospitals, but suggested that providers may now favor HCPCS G-codes over the existing CPT codes for visits that are tied to CMS' definition of new and established patients for purposes of reporting those codes. Some commenters suggested that CMS discuss the development of clinic visit HCPCS G-codes at the winter 2010 APC Panel meeting and include a proposal for clinic visit HCPCS G-codes in the CY 2011 OPPS/ASC proposed rule.
Response: Because hospital claims data continue to show significant cost differences between new and established patient visits, we continue to believe it is necessary and appropriate to recognize the CPT codes for both new and established patient visits and, in some cases, provide differential payment for new and established patient visits of the same level. For example, the final CY 2010 median cost for the level 3 new patient clinic visit, described by CPT code 99203 and calculated using over 200,000 single claims from CY 2008, is approximately $96, while the final CY 2010 median cost for the level 3 established patient clinic visit, described by CPT code 99213 and calculated using over 4.5 million single claims from CY 2008, is approximately $70. We believe this difference in median costs warrants continued assignment of these CPT codes to different APCs for CY 2010.
Given that we have a substantial volume of single claims from a significant number of hospitals upon which to calculate the median costs for all levels of clinic visits, we do not agree with the commenters that the differences in costs for new versus established patient visits are random or the result of erroneous billing. We expect hospitals to report all HCPCS codes in accordance with correct coding principles, CPT code descriptions, and relevant CMS guidance, which, in this case, specifies that the meanings of “new” and “established” patients as included in the clinic visit CPT code descriptors pertain to whether or not the patient has been registered as an inpatient or an outpatient of the hospital within the past 3 years (73 FR 68679). We have no reason to believe that hospitals are systematically disregarding these principles to the extent that our median costs for clinic visits, which are based on data from millions of single claims, would be artificially skewed.
We also do not agree with the commenters that CMS' proposal in the CY 2010 MPFS proposed rule to eliminate the use of consultation codes for physician payment purposes (74 FR 33553 through 33554) has any direct relevance to the distinction between new and established patient visits under the OPPS. As we stated previously, we have no reason to believe that hospitals are not correctly reporting these services. Furthermore, unlike the MPFS proposal that would require physicians to report the existing CPT codes for new and established patient visits instead of the consultation CPT codes, we could not easily implement a policy to eliminate the use of the clinic visit CPT codes under the OPPS, because there are no other existing codes that hospitals could use to report these services.
We recognize that some commenters believe hospitals would now support the creation of Level II HCPCS G-codes for hospital clinic visits, whereas in the past they generally opposed hospital-specific codes for visits in the absence of national visit reporting guidelines. We welcome any comments hospitals have on alternative coding schemes for reporting hospital clinic visits that would not require hospitals to distinguish between new and established patients, such as the creation of hospital-specific clinic visit HCPCS G-codes or the exclusive use of established patient clinic visit codes. We are particularly interested in commenters' thoughts on how we would develop payment rates for clinic visits under another coding scheme, considering the claims data that we have now for these services demonstrate significant differences in costs between new and established patient clinic visits and could not be easily crosswalked to a structure that does not distinguish between new and established patients. We will consider any ideas that we receive as we prepare for the CY 2011 OPPS/ASC proposed rule.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to continue to define new or established patient status for the purpose of reporting the clinic visit CPT codes, on the basis of whether or not the patient has been registered as an inpatient or outpatient of the hospital within the past 3 years. We also are finalizing our CY 2010 proposal, without modification, to continue our policy of calculating median costs for Start Printed Page 60548 clinic visits under the OPPS using historical hospital claims data. As discussed in detail in section II.A.2.e.(1) of this final rule with comment period and consistent with our CY 2009 policy, when calculating the median costs for the clinic visit APCs (0604 through 0608), we utilized our methodology that excludes those claims for visits that are eligible for payment through the extended assessment and management composite APC 8002 (Level I Extended Assessment and Management Composite). We continue to believe that this approach results in the most accurate cost estimates for APCs 0604 through 0608 for CY 2010.
2. Emergency Department Visits
Since CY 2007, we have recognized two different types of emergency departments for payment purposes under the OPPS—Type A emergency departments and Type B emergency departments. As described in greater detail below, by providing payment for two types of emergency departments, we recognize for OPPS payment purposes both the CPT definition of an emergency department, which requires the facility to be available 24 hours, and the requirements for emergency departments specified in the provisions of the Emergency Medical Treatment and Labor Act (EMTALA) (Pub. L. 99–272), which do not stipulate 24-hour availability but do specify other obligations for hospitals that offer emergency services. For more detailed information on the EMTALA provisions, we refer readers to the CY 2009 OPPS/ASC final rule with comment period (73 FR 68680).
In the CY 2007 OPPS/ASC final rule with comment period (71 FR 68132), we finalized the definition of Type A emergency departments to distinguish them from Type B emergency departments. A Type A emergency department must be available to provide services 24 hours a day, 7 days a week, and meet one or both of the following requirements related to the EMTALA definition of a dedicated emergency department specified at § 489.24(b), specifically: (1) It is licensed by the State in which it is located under the applicable State law as an emergency room or emergency department; or (2) it is held out to the public (by name, posted signs, advertising, or other means) as a place that provides care for emergency medical conditions on an urgent basis without requiring a previously scheduled appointment. For CY 2007 (71 FR 68140), we assigned the five CPT E/M emergency department visit codes for services provided in Type A emergency departments to five created Emergency Visit APCs, specifically APC 0609 (Level 1 Emergency Visits), APC 0613 (Level 2 Emergency Visits), APC 0614 (Level 3 Emergency Visits), APC 0615 (Level 4 Emergency Visits), and APC 0616 (Level 5 Emergency Visits). We defined a Type B emergency department as any dedicated emergency department that incurred EMTALA obligations, but did not meet the CPT definition of an emergency department. For example, a hospital department that may be characterized as a Type B emergency department would meet the definition of a dedicated emergency department, but may not be available 24 hours a day, 7 days a week. Hospitals with such dedicated emergency departments incur EMTALA obligations with respect to an individual who presents to the department and requests, or has a request made on his or her behalf, examination or treatment for a medical condition.
To determine whether visits to Type B emergency departments have different resource costs than visits to either clinics or Type A emergency departments, in the CY 2007 OPPS/ASC final rule with comment period (71 FR 68132), we finalized a set of five HCPCS G-codes for use by hospitals to report visits to all entities that meet the definition of a dedicated emergency department under the EMTALA regulations but that are not Type A emergency departments. These codes are called “Type B emergency department visit codes.” In the CY 2007 OPPS/ASC final rule with comment period (71 FR 68132), we explained that these new HCPCS G-codes would serve as a vehicle to capture median cost and resource differences among visits provided by Type A emergency departments, Type B emergency departments, and clinics. We stated that the reporting of specific HCPCS G-codes for emergency department visits provided in Type B emergency departments would permit us to specifically collect and analyze the hospital resource costs of visits to these facilities in order to determine if, in the future, a proposal for an alternative payment policy might be warranted. We expected hospitals to adjust their charges appropriately to reflect differences in Type A and Type B emergency department visit costs.
As we noted in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68681), the CY 2007 claims data used for that rulemaking were from the first year of claims data available for analysis that included hospital's cost data for these new Type B emergency department HCPCS visit codes. Based on our analysis of the CY 2007 claims data, we confirmed that the median costs of Type B emergency department visits were less than the median costs of Type A emergency department visits for all but the level 5 visit. In other words, the median costs from the CY 2007 hospital claims represented real differences in the hospital resource costs for the same level of visits in a Type A or Type B emergency department. Therefore, for CY 2009, we adopted the August 2008 APC Panel recommendation to assign levels 1 through 4 Type B emergency department visits to their own APCs and to assign the level 5 Type B emergency department visit to the same APC as the level 5 Type A emergency department visit.
We now have CY 2008 cost data for CY 2010 ratesetting for the Type B emergency department HCPCS G-codes, representing a second year of claims data for these Type B emergency department visit HCPCS codes. In the CY 2010 OPPS/ASC proposed rule (74 FR 35351 through 35353), we presented our observation of frequency and patterns of billing based on the CY 2008 claims available at that time. We also repeated some of our analyses of Type B emergency department visits using the available CY 2008 claims and cost report data to confirm that Type B emergency department visit costs are generally lower than Type A emergency department visit costs and to assess whether there are systematic differences in the costs of Type A and Type B emergency department visits by Medicare contractor. The pattern of relative cost differences between Type A and Type B emergency department visits was largely consistent with the distributions we observed in the CY 2007 data, with the exception that, in the CY 2008 claims data available for the proposed rule, we observed a relatively lower HCPCS code-specific median cost associated with level 5 Type B emergency department visits compared to the HCPCS-code specific median cost of level 5 Type A emergency department visits. In contrast, in our CY 2007 claims data, we observed similar resource costs for level 5 Type A and Type B emergency department visits. We also determined that there are no significant differences in how Medicare contractors have interpreted our Type A and Type B emergency department visit reporting policies.
We shared cost and frequency data with the Visits and Observation Subcommittee of the APC Panel during the February 2009 meeting, and in the CY 2010 OPPS/ASC proposed rule (74 Start Printed Page 60549 FR 35353), we proposed to pay for Type B emergency department visits in CY 2010 consistent with their median costs. Specifically, we proposed to pay for levels 1 through 4 Type B emergency department visits through four levels of APCs: APC 0626 (Level 1 Type B Emergency Visits), APC 0627 (Level 2 Type B Emergency Visits), APC 0628 (Level 3 Type B Emergency Visits), and APC 0629 (Level 4 Type B Emergency Visits). In addition, we proposed to adopt new APC 0630 (Level 5 Type B Emergency Visits) and to pay for level 5 Type B emergency department visits through this new APC. We proposed to assign HCPCS codes G0380, G0381, G0382, G0383, and G0384 (the levels 1, 2, 3, 4, and 5 Type B emergency department visit Level II HCPCS codes) to APCs 0626, 0627, 0628, 0629, and 0630, respectively, for CY 2010. These HCPCS codes would be the only HCPCS codes assigned to these APCs. Furthermore, to distinguish new APC 0630 from the APC for the level 5 Type A emergency visits, we proposed to modify the title of the current level 5 Type A emergency visit APC to incorporate Type A in the title. Therefore, the revised title of APC 0616 would be “Level 5 Type A Emergency Visits.”
We noted in the CY 2010 OPPS/ASC proposed rule (74 FR 35353) that the proposed policy to pay for Type B emergency department visits based on their median costs is consistent with the APC Panel's March 2008 recommendation for payment of Type B emergency department visits. As part of its recommended configuration of APCs for Type B emergency department visits in CY 2009, the APC Panel also stated that, given the limited CY 2007 claims data available for Type B emergency department visits, CMS should reconsider payment adjustments as more claims data become available. In general, the APC Panel's recommended CY 2009 configuration paid appropriately for each level of the Type B emergency department visits, based on the resource costs of the Type B emergency department visits that are reflected in claims data. We stated in the proposed rule that we believe our proposed CY 2010 configuration also would pay appropriately for each level of Type B emergency department visits based on estimated resource costs from more recent claims data.
For this final rule with comment period, based on updated CY 2008 claims data, we note that 344 hospitals billed at least one Type B emergency department visit code in CY 2008, with a total frequency of visits provided in Type B emergency departments of approximately 220,000. All except 5 of the 344 hospitals reporting Type B emergency department visits in CY 2008 also reported Type A emergency department visits. Overall, many more hospitals (approximately 3,238 total hospitals) reported Type A emergency department visits than Type B emergency department visits. For comparison purposes, the total frequency of visits provided in hospital outpatient clinics and Type A emergency departments is approximately 17.5 million and 11.6 million, respectively. The median costs for the Type B emergency department visit APCs, as compared to the Type A emergency department visit APCs and the clinic visit APCs, are shown in Table 48 below.
| Visit level | Final CY 2010 clinic visit approximate APC median cost | Final CY 2010 type B emergency department approximate APC median cost | Final CY 2010 type A emergency visit approximate APC median cost |
|---|---|---|---|
| Level 1 | $57 | $45 | $53 |
| Level 2 | 69 | 62 | 87 |
| Level 3 | 88 | 97 | 139 |
| Level 4 | 112 | 141 | 221 |
| Level 5 | 166 | 230 | 327 |
As demonstrated in Table 48, the median costs of the lowest level visits, based on the CY 2008 claims and cost report data available for this final rule with comment period, continue to be similar across all settings, including clinic and Type A and B emergency departments. Visit levels 2 and 3 share similar resource costs in the clinic and Type B emergency department settings, while visits provided in Type A emergency departments have higher estimated resource costs at these levels. The level 4 clinic visit APC is less resource-intensive than the level 4 Type B emergency department visit APC, which is similarly less resource-intensive than the level 4 Type A emergency department visit APC. Similarly, the level 5 clinic visit APC is less resource-intensive than the level 5 Type B emergency department visit APC, which is less resource-intensive than the level 5 Type A emergency department visit APC.
This pattern of relative cost differences between Type A and Type B emergency department visits is largely consistent with the distributions we observed in the CY 2007 data, with the exception that, in the updated CY 2008 claims data, we observe a relatively lower HCPCS code-specific median cost associated with level 5 Type B emergency department visits compared to the HCPCS-code specific median cost of level 5 Type A emergency department visits. In contrast, in our CY 2007 claims data, we observed similar resource costs for level 5 Type A and Type B emergency department visits. In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68683), we hypothesized that, for the highest level of emergency department visits, the resources required would be the same in both emergency department settings. Now that more data on Type B emergency department visits are available and hospitals have more experience billing for Type B services, we observe differences in the resources for the highest level emergency department visits to Type A and Type B emergency departments.
As noted in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68683), we performed data analyses regarding the costs of Type A and Type B emergency department visits in addition to our standard median cost calculations. These analyses included studying the emergency department Start Printed Page 60550 visit costs of hospitals that billed Type B emergency department visits only, analyzing the cost data for hospitals that billed both Type A and Type B emergency department visits, and evaluating whether there were differences in the costs of Type A and Type B emergency department visits by Medicare contractor to ascertain whether there were differences in how Medicare contractors have interpreted our Type A and Type B emergency department visit policies. In the CY 2007 data, we observed that hospitals that billed both Type A and Type B emergency department visits had lower costs for Type B emergency department visits than Type A emergency department visits at all levels except for the level 5 Type B emergency department visit. Our analyses of the differences in Type A and Type B emergency department visit median costs by Medicare contractors did not identify concerning differences. Overall, we observed a distribution of visit costs as expected, including generally lower Type B emergency department visit costs in comparison with Type A emergency department visits, and increasing costs for Type B emergency department visits from levels 1 through 5, similar to the cost increases we observed from levels 1 through 5 for Type A emergency department visits. We also observed a few contractors with more unusual cost distributions for Type B emergency department visits, including relatively similar or higher costs across levels 1 through 5 for Type B emergency department visits. For CY 2009, we concluded that we had no reason to believe that the cost differences between Type A and Type B emergency department visits evident in our aggregate OPPS claims data resulted from varying Medicare contractor criteria as to what defines Type A and Type B emergency departments. We also committed to monitoring these distributions in future years.
As we did for the CY 2010 OPPS/ASC proposed rule, for this final rule with comment period, we repeated some of our analyses of Type B emergency department visits using updated CY 2008 claims data to confirm that Type B emergency department visit costs are generally lower than Type A emergency department visit costs and to again assess whether there are systematic differences in the costs of Type A and Type B emergency department visits by Medicare contractor. As noted above, we observed that hospitals that billed both Type A and Type B emergency department visits had lower costs for Type B emergency department visits than Type A emergency department visits, including level 5 Type B emergency department visits, which is a change from the CY 2007 data. We further evaluated differences in the costs of Type A and Type B emergency department visits by Medicare contractor. Based on our updated analysis of CY 2008 claims, we continue to observe similar patterns in HCPCS code-specific median cost differences between Type A and Type B emergency department visits as observed in the CY 2007 claims. Hospitals in the jurisdictions of most Medicare contractors have generally lower Type B emergency department visit costs in comparison with Type A emergency department visits, as well as increasing costs for Type B emergency department visits from levels 1 through 5, similar to the cost increases we observed from levels 1 through 5 for Type A emergency department visits.
Like last year, we also continue to observe a few Medicare contractors with more unusual cost distributions for Type B emergency department visits, including those with Type B emergency department visit costs that are relatively similar or higher than Type A emergency department visit costs across levels 1 through 5. Some of these Medicare contractors are the same contractors that we noted had more unusual cost distributions for Type B emergency department visits relative to Type A emergency department visit costs in the CY 2007 claims data. In order to confirm that these Medicare contractors are applying our policies consistently, we examined the HCPCS code-specific median costs for Type A and Type B emergency department visits for the hospitals in each Medicare contractor's area. For almost all of these Medicare contractors, we see one or two hospitals with relatively high Type B emergency department visit costs relative to Type B emergency department visit costs nationwide or with Type B emergency department visit costs that are relatively similar to or higher than Type A emergency department visit costs. These one or two hospitals have sufficient visit volumes to influence the calculation of the HCPCS code-specific median costs for their respective Medicare contractors.
Comment: Several commenters supported CMS' proposal to create a new APC for level 5 Type B emergency department visits. One commenter encouraged CMS to adopt the recommendation made by the APC Panel at the August 2009 meeting to provide an analysis of the most common diagnoses and services associated with Type A and Type B emergency department visits at the next meeting of the APC Panel, including analysis by hospital-specific characteristics, as well as an analysis of CY 2009 claims data for Type A and B emergency department visit APCs.
Response: We appreciate commenters' support of our proposal to create a new APC for level 5 Type B emergency department visits. Our updated analyses of Type B emergency department visits costs for this CY 2010 OPPS/ASC final rule with comment period confirm that the median costs of Type B emergency department visits are less than the median costs of Type A emergency department visits across all levels. Our updated analyses also confirm that there are no significant differences in how Medicare contractors have interpreted our Type A and Type B emergency department visit reporting policies. The median costs from CY 2008 hospital claims represent real differences in the hospital resource costs for the same level of visit in a Type A or Type B emergency department.
Therefore, as we proposed, for the CY 2010 OPPS, we are continuing to pay for Type B emergency department visits in CY 2010 consistent with their median costs. Specifically, we are continuing to pay levels 1 through 4 Type B emergency department visits through four levels of APCs: APC 0626 (Level 1 Type B Emergency Visits), APC 0627 (Level 2 Type B Emergency Visits), APC 0628 (Level 3 Type B Emergency Visits), and APC 0629 (Level 4 Type B Emergency Visits). In addition, we are adopting new APC 0630 (Level 5 Type B Emergency Visits) and will pay for level 5 Type B emergency department visits through this new APC. We are assigning HCPCS codes G0380, G0381, G0382, G0383, and G0384 (the levels 1, 2, 3, 4, and 5 Type B emergency department visit Level II HCPCS codes) to APCs 0626, 0627, 0628, 0629, and 0630, respectively, for CY 2010. These HCPCS codes are the only HCPCS codes assigned to these APCs. Furthermore, to distinguish new APC 0630 from the APC for the level 5 Type A emergency visits, as we proposed, we are modifying the title of the current level 5 Type A emergency visit APC to incorporate Type A in the title. Therefore, the revised title of APC 0616 is “Level 5 Type A Emergency Visits.” We believe our CY 2010 configuration pays appropriately for each level of Type B emergency department visits based on estimated resource costs from more recent claims data.
As stated previously, we plan to provide the requested analysis of the most common diagnoses and services associated with Type A and Type B Start Printed Page 60551 emergency department visits to the APC Panel at the winter 2010 meeting of the APC Panel, as well as an analysis of CY 2009 claims data for Type A and B emergency department visit APCs available at that time.
Comment: One commenter expressed concerns regarding the 30 minute minimum to bill critical care services, described by CPT code 99291. The commenter argued that the resources expended in less than 30 minutes warrant payment at the highest level of E/M payment, and recommended that CMS change the criteria for payment for critical care services to include instances of 15 minutes of critical care and instances in which the patient expires in less than 30 minutes, despite the critical care services furnished. According to the commenter, the significant resources utilized during these critical care episodes are not appropriately recognized for payment purposes because they cannot be reported with CPT code 99291 under existing guidelines.
Another commenter requested that CMS consider extending payment for trauma team activations, described by HCPCS code G0390, to level 5 emergency department visits, in addition to critical care services when all other trauma activation criteria are met. According to the commenter, an emergency department that is extremely efficient can send a patient in need of a trauma team to surgery before the 30 minute time threshold for reporting critical care services is met. The commenter stated that, because the hospital would bill a level 5 emergency department visit code, rather than a critical care code, the encounter would not qualify for trauma response payment even though a trauma response team was utilized. The commenter argued that hospitals should receive an APC payment for HCPCS code G0390 under these circumstances because equivalent trauma team resources are expended even though the encounter lasted fewer than 30 minutes and cannot be reported with CPT code 99291.
Response: As we have stated in the past (72 FR 66806), the CPT instructions for reporting of critical care services with CPT code 99291 and the CPT code descriptor specify that the code can only be billed if 30 minutes or more of critical care services are provided. Hospitals must continue to provide a minimum of 30 minutes of critical care services in order to bill CPT code 99291, according to the CPT code descriptor and CPT instructions. We note that hospitals can report the appropriate clinic or emergency department visit code consistent with their internal guidelines if fewer than 30 minutes of critical care is provided. These CPT instructions and our payment policies for covered hospital outpatient services do not apply any differently if the patient dies while undergoing treatment. We do allow hospitals to use the HCPCS–CA modifier to address situations where a procedure on the OPPS inpatient list must be performed to resuscitate or stabilize a patient (whose status is that of an outpatient) with an emergent, life-threatening condition, and the patient dies before being admitted as an inpatient. We refer readers to section II.A.2.d.(7) of this final rule with comment period for more information on how these services are paid under the OPPS.
We do not agree with the commenter that we should modify our policy to recognize HCPCS code G0390 for the reporting of a trauma response in association with critical care services when the hospital provides fewer than 30 minutes of critical care and cannot report CPT code 99291. We believe that it would be extremely unusual for a patient to require trauma team services, be rushed to surgery within 30 minutes of arrival in the emergency department, and not be subsequently admitted to the hospital as an inpatient. Furthermore, hospitals that provide less than 30 minutes of critical care when trauma activation occurs under the circumstances described by the NUBC guidelines that would permit reporting a charge under revenue code series 68x may report a charge under 68x, but they may not report HCPCS code G0390. In these cases, payment for the trauma team activation is packaged into payment for the other services provided to the patient in the encounter, including the associated emergency department visit that is reported.
Comment: One commenter requested clarification regarding “triage only” visits in which a patient is seen by a nurse and triaged in the hospital emergency department but leaves prior to a physician's examination and treatment. The commenter asked if hospitals can bill visit codes for such cases if the patient is not seen by a physician.
Response: As we have stated in the past (73 FR 68686), under the OPPS, unless indicated otherwise, we do not specify the type of hospital staff (for example, nurses or pharmacists) who may provide services in hospitals because the OPPS only makes payment for services provided incident to physicians' services. Hospitals providing services incident to physicians' services may choose a variety of staffing configurations to provide those services, taking into account other relevant factors, including State and local laws, hospital policies, and other Federal requirements such as EMTALA and the Medicare conditions of participation related to hospital staffing. Billing a visit code in addition to another service merely because the patient interacted with hospital staff or spent time in a room for that service is inappropriate. A hospital may bill a visit code based on the hospital's own coding guidelines which must reasonably relate the intensity of hospital resources to different levels of HCPCS codes. Services furnished must be medically necessary and documented.
As described previously in this section, we are adopting our proposal, without modification, to continue paying for Type B emergency department visits in CY 2010 consistent with their median costs through 5 levels of Type emergency department visit APCs.
Table 49 below displays the APC median costs for each level of Type B emergency department visit under our CY 2010 configuration. As more cost data become available and hospitals gain additional experience with reporting visits to Type B emergency departments, we will continue to regularly reevaluate patterns of Type A and Type B emergency department visit reporting to ensure that hospitals continue to bill appropriately and differentially for these services. In addition, according to our usual practice, we will examine trends in cost data over time and consider proposing alternative emergency department visit APC configurations in the future if updated data indicate that changes to the payment structure should be considered.
We also note that, as discussed in section II.A.2.e.(1) of this final rule with comment period and consistent with our CY 2009 policy, when calculating the median costs for the emergency department visit and critical care APCs (0609 through 0617 and 0626 through 0630), we utilized our methodology that excludes those claims for visits that are eligible for payment through the extended assessment and management composite APC 8002 (Level I Extended Assessment and Management Composite). We continue to believe that this approach will result in the most accurate cost estimates for APCs 0604 through 0608 for CY 2010. Start Printed Page 60552
| Type B emergency department level | Final CY 2010 APC assignment | Final CY 2010 approximate APC median cost |
|---|---|---|
| Level 1 | 0626 | $45 |
| Level 2 | 0627 | 62 |
| Level 3 | 0628 | 97 |
| Level 4 | 0629 | 141 |
| Level 5 | 0630 | 230 |
3. Visit Reporting Guidelines
Since April 7, 2000, we have instructed hospitals to report facility resources for clinic and emergency department hospital outpatient visits using the CPT E/M codes and to develop internal hospital guidelines for reporting the appropriate visit level. Because a national set of hospital-specific codes and guidelines do not currently exist, we have advised hospitals that each hospital's internal guidelines that determine the levels of clinic and emergency department visits to be reported should follow the intent of the CPT code descriptors, in that the guidelines should be designed to reasonably relate the intensity of hospital resources to the different levels of effort represented by the codes.
As noted in detail in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66802 through 66805), we observed a normal and stable distribution of clinic and emergency department visit levels in hospital claims over the past several years. The data indicated that hospitals, on average, were billing all five levels of visit codes with varying frequency, in a consistent pattern over time. Overall, both the clinic and emergency department visit distributions indicated that hospitals were billing consistently over time and in a manner that distinguished between visit levels, resulting in relatively normal distributions nationally for the OPPS, as well as for specific classes of hospitals. The results of these analyses were generally consistent with our understanding of the clinical and resource characteristics of different levels of hospital outpatient clinic and emergency department visits. In the CY 2008 OPPS/ASC proposed rule (72 FR 42764 through 42765), we specifically invited public comment as to whether a pressing need for national guidelines continued at this point in the maturation of the OPPS, or if the current system where hospitals create and apply their own internal guidelines to report visits was currently more practical and appropriately flexible for hospitals. We explained that although we have reiterated our goal since CY 2000 of creating national guidelines, this complex undertaking for these important and common hospital services was proving more challenging than we initially thought as we received new and expanded information from the public on current hospital reporting practices that led to appropriate payment for the hospital resources associated with clinic and emergency department visits. We stated our belief that many hospitals had worked diligently and carefully to develop and implement their own internal guidelines that reflected the scope and types of services they provided throughout the hospital outpatient system. Based on public comments, as well as our own knowledge of how clinics operate, it seemed unlikely that one set of straightforward national guidelines could apply to the reporting of visits in all hospitals and specialty clinics. In addition, the stable distribution of clinic and emergency department visits reported under the OPPS over the past several years indicated that hospitals, both nationally in the aggregate and grouped by specific hospital classes, were generally billing in an appropriate and consistent manner as we would expect in a system that accurately distinguished among different levels of service based on the associated hospital resources.
Therefore, we did not propose to implement national visit guidelines for clinic or emergency department visits for CY 2008. Since publication of the CY 2008 OPPS/ASC final rule with comment period, we have again examined the distribution of clinic and Type A emergency department visit levels based upon updated CY 2008 claims data available for the CY 2010 proposed rule and for this final rule with comment period and confirmed that we continue to observe a normal and stable distribution of clinic and emergency department visit levels in hospital claims. We continue to believe that, based on the use of their own internal guidelines, hospitals are generally billing in an appropriate and consistent manner that distinguishes among different levels of visits based on their required hospital resources. As a result of our updated analyses, we are encouraging hospitals to continue to report visits during CY 2010 according to their own internal hospital guidelines. In the absence of national guidelines, we will continue to regularly reevaluate patterns of hospital outpatient visit reporting at varying levels of disaggregation below the national level to ensure that hospitals continue to bill appropriately and differentially for these services. As originally noted in detail in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66648), we continue to expect that hospitals will not purposely change their visit guidelines or otherwise upcode clinic and emergency department visits for purposes of extended assessment and management composite APC payment.
In addition, we note our continued expectation that hospitals' internal guidelines will comport with the principles listed in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66805). We encourage hospitals with more specific questions related to the creation of internal guidelines to contact their local fiscal intermediary or MAC.
Comment: Several commenters supported CMS' policy of requiring hospitals to use their own internal guidelines to distinguish among different levels of visits based on their required hospital resources and did not favor the implementation of national guidelines at any point in the future. Other commenters expressed appreciation for CMS' approach of studying the challenges associated with national guidelines prior to their implementation. However, many commenters urged CMS to move forward with the implementation of national guidelines for hospitals to report clinic visits because of several problems that they believe continue to exist due to the lack of such guidelines, such as variations in hospitals' internal guidelines that may result in Start Printed Page 60553 inconsistent cost data upon which payment rates for visits are based. Some commenters noted that some Medicare contractors, including RACs, use their own auditing methods rather than reviewing each hospital's internal guidelines while conducting medical review.
The commenters urged CMS to adopt national guidelines no later than CY 2011 due to the burden hospitals would face if they had to implement national visit coding guidelines concurrently with the ICD–10–CM and ICD–10–PCS changes required by FY 2013. According to the commenters, the national guidelines should be clear, concise, and specific with little or no room for varying interpretations, and hospitals should have at least 1 year to prepare for the transition. Many commenters indicated that the American Hospital Association (AHA) will reconvene an expert panel to submit a request to the AMA CPT Editorial Panel to create CPT codes for hospital visits and encouraged CMS to be engaged in and supportive of the recommendations of the expert panel.
Several commenters also recommended that, in the absence of national guidelines for hospital visit reporting, CMS provide additional guidance relating to the specific services that should be included or bundled into the visit levels reported by hospitals. One commenter requested that CMS ask the AMA to supplement its CPT Codebook to include a compilation of instructions from CMS regarding appropriate reporting of hospital visits, such as the 11 principles specified in the CY 2008 OPPS/ASC final rule with comment period that hospitals should follow in developing internal guidelines for reporting visits.
Another commenter performed extensive review of a large sample of hospital emergency department visits to determine whether the distributions observed in this sample resembled the distribution described by CMS and printed in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66804). The commenter explained that the results are similar to those of CMS at the national level, but that emergency departments have increased the proportion of level 4 and 5 emergency department visits in recent years, and that several outlier providers are billing significantly more high level visits than expected based on their geographic location and hospital type. Therefore, the commenter concluded that national guidelines could help slow rapidly increasing health care costs.
Response: We acknowledge that it would be desirable to many hospitals to have national guidelines. However, we also understand that it would be disruptive and administratively burdensome to other hospitals that have successfully adopted internal guidelines to implement any new set of national guidelines while we address the problems that would be inevitable in the case of any new set of guidelines that would be applied by thousands of hospitals. As noted in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66806), we encourage fiscal intermediaries and MACs to review a hospital's internal guidelines when an audit occurs. While we also would encourage RACs to review a hospital's internal guidelines when an audit occurs, we note that currently there are no RAC activities involving visit services. RAC audits may involve CMS-approved issues only and must be posted to each RAC's Web site.
We agree with the commenters that national guidelines should be clear, concise, and specific with little or no room for varying interpretations, and that hospitals should have at least 1 year to prepare for the transition. We look forward to reviewing any recommendations that result from the AHA-convened expert panel referenced by the commenters. If the AMA were to create facility-specific CPT codes for reporting visits provided in HOPDs, we would certainly consider such codes for OPPS use. We also appreciate the visit level distribution analysis provided to us by one commenter and note that, in the absence of national guidelines, we will continue to regularly reevaluate patterns of hospital outpatient visit reporting at varying levels of disaggregation below the national level to ensure that hospitals continue to bill appropriately and differentially for these services. We reiterate our expectation that hospitals' internal guidelines fully comply with the principles listed in the CY 2008 OPPS/ASC final rule with comment period (72 FR 68805).
Regarding the public comments requesting clarification of services that should be included or bundled into visit codes, as we have stated in the past (73 FR 68685), hospitals should separately report all HCPCS codes in accordance with correct coding principles, CPT code descriptions, and any additional CMS guidance, when applicable. We refer readers to the July 2008 OPPS quarterly update (Transmittal 1536, Change Request 6094, issued on June 19, 2008) for further clarification about the reporting of CPT codes for hospital outpatient services paid under the OPPS. In that transmittal, we note that, while CPT codes generally are created to describe and report physician services, they also are used by other providers/suppliers to describe and report services that they provide. Therefore, the CPT code descriptors do not necessarily reflect the facility component of a service furnished by the hospital. Some CPT code descriptors include reference to a physician performing a service. For OPPS purposes, unless indicated otherwise, the usage of the term “physician” does not restrict the reporting of the code or application of related policies to physicians only, but applies to all practitioners, hospitals, providers, or suppliers eligible to bill the relevant CPT codes in accordance with applicable portions of the Act, the Medicare regulations, and other Medicare guidance. In cases where there are separate codes for the technical component, professional component, and/or complete procedure, hospitals should report the code that represents the technical component for their facility services. If there is no separate technical component code for the service, hospitals should report the code that represents the complete procedure. Consistent with past input we have received from many hospitals, hospital associations, the APC Panel, and others, we will continue to utilize CPT codes for reporting services under the OPPS whenever possible to minimize hospitals' reporting burden.
We do not agree with the commenter that we should ask the AMA to supplement its CPT Codebook to include a compilation of instructions from CMS regarding appropriate reporting of hospital visits. Under the OPPS, we develop policies specifically and exclusively for purposes of the Medicare program, while the CPT Codebook provides instructions that are applicable to hospital coding for all payers, unless those payers choose to implement different individual policies. If hospitals believe the inclusion of such information in the CPT Codebook is necessary and appropriate, they may directly request the AMA to do so.
Comment: One commenter requested that CMS recognize CPT codes 99363 (Anticoagulation management for an outpatient taking warfarin, physician review and interpretation of International Normalized Ratio (INR) testing, patient instructions, dosage adjustment (as needed), and ordering of additional tests; initial 90 days of therapy (must include a minimum of 8 INR measurements)) and 99364 (Anticoagulation management for an outpatient taking warfarin, physician review and interpretation of International Normalized Ratio (INR) testing, patient instructions, dosage Start Printed Page 60554 adjustment (as needed), and ordering of additional tests; each subsequent 90 days of therapy (must include a minimum of 3 INR measurements)), which are currently assigned status indicator “B” (Codes that are not recognized by OPPS when submitted on an outpatient hospital Part B bill type (12x and 13x)), as payable under the OPPS. The commenter stated that making these CPT codes payable under the OPPS is appropriate because they accurately describe anticoagulation management services. The commenter argued that recognition of these CPT codes would reduce patient liability because they are billed only once every 90 days.
Response: While we appreciate the commenter's concern about patient liability, we cannot assess whether recognition of CPT codes 99363 and 99364 as payable under the OPPS would actually reduce the cumulative amount of copayment a patient may have to pay for all of the different services that may be involved in anticoagulation management, which may be provided at varying time intervals and with very different levels of intensity for individual patients. We expect that a patient undergoing anticoagulation management by hospital staff for a significant medical condition would periodically have hospital visits, and we would package payment for the non-face-to-face management of the patient's therapy between visits into payment for the visits themselves. Our usual policy is to package payment for the hospital resources associated with managing patients' medical conditions between hospital encounters for patients who undergo surgery or receive hospital visits for any medical condition, including diabetes, hypertension, or cardiac arrhythmias, and we do not believe that payment for anticoagulation management services should be made differently than payment for other medical or surgical management services. Therefore, we see no reason to recognize CPT codes 99363 and 99364 for payment under the OPPS.
After consideration of the public comment we received, we are finalizing our CY 2010 proposal, without modification, to continue to assign status indicator “B” to CPT codes 99363 and 99364 to indicate that these codes are not recognized for payment under the OPPS. We expect that hospitals will continue to consider the hospital resources required to manage patients, including patients requiring anticoagulation management, between hospital encounters when setting their charges for the services furnished in those encounters.
As we have stated in the past (73 FR 68686), we note that billing a visit code in addition to another service merely because the patient interacted with hospital staff or spent time in a room for that service is inappropriate. A hospital may bill a visit code based on the hospital's own coding guidelines, which must reasonably relate the intensity of hospital resources to the different levels of HCPCS codes. Services furnished must be medically necessary and documented. For example, CPT code 85610 (Prothrombin time) is a code that describes performance of the prothrombin time test. If the only service provided is a venipuncture and laboratory test to determine the prothrombin time, this service is the only service that should be billed. If a hospital provides a distinct, separately identifiable service in addition to the test, the hospital is responsible for billing the code that most closely describes the service provided.
We appreciate all of the comments we have received in the past from the public on visit guidelines, and we encourage continued submission of comments throughout the year that would assist us and other stakeholders interested in the development of national guidelines. Until national guidelines are established, hospitals should continue using their own internal guidelines to determine the appropriate reporting of different levels of clinic and emergency department visits. While we understand the interest of some hospitals in having us move quickly to promulgate national guidelines that would ensure standardized reporting of hospital outpatient visit levels, we believe that the issues and concerns identified both by us and others are important and require serious consideration prior to the implementation of national guidelines. Because of our commitment to provide hospitals with 6 to 12 months notice prior to implementation of national guidelines, we would not implement national guidelines prior to CY 2011. Our goal is to ensure that OPPS national or hospital-specific visit guidelines continue to facilitate consistent and accurate reporting of hospital outpatient visits in a manner that is resource-based and supportive of appropriate OPPS payments for the efficient and effective provision of visits in hospital outpatient settings.
X. Payment for Partial Hospitalization Services
A. Background
Partial hospitalization is an intensive outpatient program of psychiatric services provided to patients as an alternative to inpatient psychiatric care for individuals who have an acute mental illness. Section 1833(t)(1)(B)(i) of the Act provides the Secretary with the authority to designate the HOPD services to be covered under the OPPS. The Medicare regulations at § 419.21 that implement this provision specify that payments under the OPPS will be made for partial hospitalization services furnished by community mental health centers (CMHCs) as well as those services furnished by hospitals to their outpatients. Section 1833(t)(2)(C) of the Act requires the Secretary to establish relative payment weights for covered HOPD services (and any APCs) based on median (or mean, at the election of the Secretary) hospital costs using data on claims from 1996 and data from the most recent available cost reports. Because a day of care is the unit that defines the structure and scheduling of partial hospitalization services, we established a per diem payment methodology for the partial hospitalization program (PHP) APC, effective for services furnished on or after August 1, 2000 (65 FR 18452).
Historically, the median per diem cost for CMHCs greatly exceeded the median per diem cost for hospital-based PHPs and fluctuated significantly from year to year, while the median per diem cost for hospital-based PHPs remained relatively constant ($200–$225). We believe that CMHCs may have increased and decreased their charges in response to Medicare payment policies. In developing the CY 2008 update, we began an effort to strengthen the PHP benefit through extensive data analysis and policy and payment changes. We began this effort as a result of the significant fluctuations and declines in the CMHC PHP median per diem costs (we refer readers to the CY 2008 OPPS/ASC final rule with comment period (72 FR 66670 through 66676) for a detailed discussion). The analysis included an examination of revenue-to-cost center mapping, refinements to the per diem methodology, and an in-depth analysis of the number of units of service furnished per day.
For CY 2008, we proposed and finalized two refinements to the methodology for computing the PHP median that we believe resulted in more accurate per diem medians. First, we remapped 10 revenue codes that are common among hospital-based PHP claims to the most appropriate cost centers (72 FR 66671 through 66672). Typically, we map the revenue code to the most specific cost center with a provider-specific CCR. However, if the hospital does not have a CCR for any of Start Printed Page 60555 the listed cost centers, we consider the overall hospital CCR as the default. For partial hospitalization services, the revenue center codes billed by hospital-based PHPs are mapped to Primary Cost Center 3550 (Psychiatric/Psychological Services). If that cost center is not available, they are mapped to the Secondary Cost Center 6000 (Clinic). We use the overall facility CCR for CMHCs because PHPs are CMHCs' only Medicare cost, and CMHCs do not have the same cost structure as hospitals. Therefore, for CMHCs, we use the CCR from the outpatient provider-specific file. A closer examination of the revenue-code-to-cost-center crosswalk revealed that 10 of the revenue center codes used by hospital-based PHPs did not map to a Primary Cost Center of 3550 or a Secondary Cost Center of 6000. We believe this occurred because these codes may also be used for services that are not furnished in a PHP or services that are not psychiatric related (for example, occupational therapy). As discussed in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66671 through 66672), we updated this analysis using more recent PHP claims and CCR data. After remapping codes, we computed an alternate cost for each line item of the hospital-based PHP claims. Remapping those 10 revenue center codes reduced the number of lines that defaulted to the hospitals' overall CCR and thus created a more accurate estimate of PHP per diem costs for a significant number of claims.
Secondly, we refined our methodology for calculating median PHP per diem costs by computing a separate per diem cost for each day rather than for each claim. When there were multiple days of care entered on a claim, a unique cost was computed for each day of care. We only assigned costs for line items on days when a payment was made. All of these costs were then arrayed from lowest to highest, and the middle value of the array was considered the median per diem cost. A complete discussion of the refined method of computing the PHP median per diem cost can be found in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66672).
After completing extensive data analysis, we continued to observe a clear downward trend in the median per diem cost based on the CY 2006 data used to develop the median per diem cost under the CY 2008 OPPS/ASC final rule with comment period. The analysis revealed that fewer PHP services were being provided in a given day. We believed, and continue to believe, that the data reflect the level of cost for the type of services that were being provided and continue to be provided.
Because partial hospitalization is provided in lieu of inpatient care, it should be a highly structured and clinically intensive program, usually lasting most of the day. In order to improve the level of services furnished in a PHP day, in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66673), we reiterated our expectation that hospitals and CMHCs must provide a comprehensive program consistent with the statutory intent. We also indicated our intent to explore changes to our regulations and claims processing systems in order to deny payment for low intensity days.
For CY 2009, we implemented several regulatory, policy, and payment changes, including a two-tiered payment approach for PHP services under which we pay one amount for days with 3 units of service (APC 0172 (Level I Partial Hospitalization)) and a higher amount for days with 4 or more units of service (APC 0173 (Level II Partial Hospitalization)). We implemented this payment approach to reflect the lower costs of a less intensive day while still paying programs that provide 4 or more units of service an amount that recognizes that they have provided a more intensive day of care. In this way, we pay more appropriately for the level of care provided while still allowing PHPs necessary scheduling flexibility (73 FR 68689). As we stated in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68688), it was never our intention that days with only 3 units of service become the number of services provided in a typical day. Our intention was to provide payment to cover days that consisted of 3 units of service only in certain limited circumstances. For example, we believe 3 units of service a day may be appropriate when a patient is transitioning towards discharge or when a patient is required to leave the PHP early for the day due to an unexpected medical appointment. We refer readers to section X.C.2. of the CY 2009 OPPS/ASC final rule with comment period (73 FR 68688 through 68695) for a full discussion of this requirement.
For CY 2009, we proposed to calculate the payment rates for PHP APCs 0172 and 0173 using both hospital-based and CMHC PHP data (73 FR 41513). After consideration of the public comments received on our proposal, we decided to base payment rates for the two-tiered approach on hospital-based PHP data only. As we explained in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68689), using the CMHC data for CY 2009 would have significantly reduced the CY 2009 PHP rates and negatively impacted hospital-based PHPs. Because hospital-based PHPs are geographically diverse, whereas CMHCs are located in only a few States, we were concerned that a significant drop in the rate could result in hospital-based PHPs closing and lead to possible beneficiary access to care problems. To calculate the CY 2009 PHP payment rate for APC 0172, we used the median per diem cost for hospital-based PHP days with 3 units of service to derive a PHP payment rate of $157. For APC 0173, we used the median per diem cost for hospital-based PHP days with 4 or more units of service to derive a CY 2009 PHP payment rate of $200.
In addition, for CY 2009, we finalized our policy to deny payment for any PHP claims for days when fewer than 3 units of therapeutic services are provided. As noted in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68694), we believe that 3 units of service should be the minimum number of services allowed in a PHP day because a day with 1 or 2 units of service does not meet the statutory intent of a PHP program. Three units of service are a minimum threshold that permits unforeseen circumstances, such as medical appointments, while allowing payment, but maintains the integrity of the PHP benefit.
Further, for CY 2009, we revised the regulations at § 410.43 to codify existing basic PHP patient eligibility criteria and added a reference to current physician certification requirements at § 424.24. We believed these changes would help strengthen the PHP benefit by conforming our regulations to our longstanding policy (73 FR 68694 through 68695). Specifically, we revised § 410.43 to add a reference to existing regulations at § 424.24(e) that require that PHP services be furnished pursuant to a physician certification and plan of care. While the requirements at § 424.24(e) are not new, we included the reference in § 410.43 to provide a more complete description of our expectations for PHP programs in one regulatory section. We also revised § 410.43 to add the following patient eligibility criteria and clarify that PHPs are intended for patients who—(1) require a minimum of 20 hours per week of therapeutic services as evidenced in their plan of care; (2) are likely to benefit from a coordinated program of services and require more than isolated sessions of outpatient treatment; (3) do not require 24-hour care; (4) have an adequate support system while not actively engaged in the program; (5) have a mental health Start Printed Page 60556 diagnosis; (6) are not judged to be dangerous to self or others; and (7) have the cognitive and emotional ability to participate in the active treatment process and can tolerate the intensity of the PHP. We refer readers to section X.C.2. of the CY 2009 OPPS/ASC final rule with comment period (73 FR 68694 through 68695) for a full discussion of this requirement.
Lastly, in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68695 through 68697), we revised the partial hospitalization benefit to include several coding updates. We removed three PHP billable codes (CPT codes 90899 (Unlisted psychiatric service or procedure), 90853 (Group psychotherapy other than of a multiple-family group), and 90857 (Interactive group psychotherapy)), and created two new timed HCPCS codes (GO410 (Group psychotherapy other than of a multiple-family group, in a partial hospitalization setting, approximately 45 to 50 minutes) and G0411 (Interactive group psychotherapy in a partial hospitalization setting, approximately 45 to 50 minutes)). The elimination of CPT code 90899 was a result of our concerns about the type of services that may be billed using an unlisted CPT code when a more appropriate code may be available that better describes the services for which PHP payment may be made. The decision to eliminate the two group therapy CPT codes (90853 and 90857) and replace them with two new parallel timed HCPCS G-codes (G–0410 and G–0411) was based on the need for consistency. As most of the current PHP codes already include time estimates, we wanted to maintain consistency with the existing HCPCS codes used in the PHP by applying a time descriptor to the group therapy codes. In addition to these coding updates, we also decided to eliminate CPT code 90849 (multi-family group psychotherapy) as a billable PHP code because we believed that CPT code 90849 focuses the service on the needs of the family and not specifically on the needs of the patient, which is not consistent with the intent of the statute that treatment in a PHP be focused on the patient's condition (73 FR 68696).
B. PHP APC Update for CY 2010
For the CY 2010 OPPS/ASC proposed rule (74 FR 35356), we used CY 2008 claims data and computed median per diem costs in the following three categories: (1) All days; (2) days with 3 units of service; and (3) days with 4 or more units of service. These updated median per diem costs were computed separately for CMHCs and hospital-based PHPs and are shown in Table 50 below.
| CMHCs | Hospital-based PHPs | Combined | |
|---|---|---|---|
| All Days | $140 | $200 | $144 |
| Days with 3 units of service | 129 | 149 | 131 |
| Days with 4 units or more units of service | 173 | 213 | 175 |
Using CY 2008 data and the refined methodology for computing PHP per diem costs that we adopted in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66672), we computed the median per diem cost from all claims of $144. The data indicate that CMHCs continue to provide far fewer days with 4 or more units of service (33 percent compared to 70 percent for hospital-based PHPs) and that the CMHC median per diem cost for 4 or more units of service ($173) is substantially lower than the comparable data from hospital-based PHPs ($213). The median per diem cost for claims containing 4 or more units of service for all PHP claims, regardless of site of service, is $175. Medians for claims containing 3 units of service are $129 for CMHCs, $149 for hospital-based PHPs, and $131 for all PHP claims, regardless of site of service.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35356), for CY 2010, we proposed to continue to use the two-tiered payment approach for PHP services established in CY 2009. In addition, for CY 2010, we proposed to use only hospital-based PHP data to develop the two PHP APC per diem payment rates for the following reasons. If we used combined CMHC and hospital-based PHP data to develop the rates, the two per diem payment rates would be reduced by approximately $26 for APC 0172 and $25 for APC 0173. We are concerned about further reducing both PHP APC per diem payment rates without knowing the impact of the policy and payment changes we made in CY 2009. Because there is a 2-year delay between data collection and rulemaking, the changes we made in CY 2009 will not be reflected in the claims data until next year when we are developing the update for CY 2011. The two proposed APCs median per diem rates for PHP services were as follows:
| Proposed APC | Group title | Proposed median per diem rate |
|---|---|---|
| 0172 | Level I Partial Hospitalization (3 services) | $149 |
| 0173 | Level II Partial Hospitalization (4 or more services) | 213 |
In general, public commenters supported the two-tiered PHP APC per diem payment approach in the proposed rule, the proposed CY 2010 payment rates, and the use of hospital-based PHP data only for generating the PHP APC payment rates.
Comment: A majority of the commenters supported the proposed PHP rates for CY 2010, as well as the two-tiered PHP APC payment structure (with high and low intensity rates). Many of these commenters strongly recommended that CMS use only hospital-based PHP data to determine the final rates. The commenters believed Start Printed Page 60557 that hospital-based data are more reliable, predictable, national in scope, consistent, and stable, and that hospital-based data are meeting the intent of the PHP statute and CMS rules.
Several commenters urged CMS not to combine the CMHC and hospital-based PHP data to set the final CY 2010 PHP payment rates. The commenters pointed out that the volatility and significant fluctuation of the CMHC costs and changes in data since 2000 has continued to place the reliability of the CMHC data in doubt. One commenter urged CMS to use PHP hospital-based data only to set the rates because CMS does not know the impact of the comprehensive policy and payment changes made to PHP services during 2009.
The commenters also recommended that, if CMS were to change the methodology to establish the per diem payment rate in CY 2010 and beyond, CMS adopt two additional APCs for separate CMHCs PHP payment rates. The commenter recommended establishing site specific APCs for PHP services where the hospital-based PHP APCs for Level I (3 units of service) and Level II (4 or more units of service) would be established using hospital cost data and CMHC-based PHP APCs for Level I (3 units of service) and Level II (4 or more units of service) would be established using CMHC data. One commenter pointed out that while the aggregate number of PHP service providers has remained relatively stable over time, the number of hospital-based PHPs has dropped by 16 percent, while the number of CMHC PHPs has increased by 53 percent (with the majority of CMHCs located in Florida, Louisiana, and Texas). The commenters reported that 80 percent of the States have two or more hospital-based programs, and only 30 percent of States have more than one CMHC.
Response: We appreciate the commenters' support for our proposals on the two-tiered payment approach and use of hospital-based PHP data only to develop the CY 2010 payment rates. As we continue to evaluate ways to reflect CMHC costs in establishing PHP future rates, we will take the recommendation to establish site-specific PHP APCs into consideration. After consideration of the public comments we received, we have decided to retain the two-tiered payment approach for CY 2010, using hospital-based PHP data only.
Comment: Several commenters urged CMS to propose an APC code or payment rate for PHP claims for days with fewer than 3 units of service or, at a minimum, the commenters want CMS to suspend for medical review claims with fewer than units of services per day.
Response: We continue to believe that days with 1 or 2 units of service are inconsistent with a benefit designed as a full-day program to substitute for inpatient care. PHP is furnished in lieu of an inpatient psychiatric hospitalization and is intended to be a highly structured and clinically intense program, usually lasting most of the day. We believe that 3 units of service should be the minimum number of services allowed in a PHP day because a day with 1 or 2 units of service does not meet the statutory intent of a PHP program. Our intention was to provide payment to cover days that consisted of 3 units of service only in certain limited circumstances. Therefore, we believe that 3 units of service are a minimum threshold that permits unforeseen circumstances, such as medical appointments, while allowing payment, but still maintains the integrity of a comprehensive program. If there are legitimate instances when 1 or 2 units of service days are justified, the provider has the option of appealing the denial of payment for that day, as specified in the Medicare Claims Processing Manual (Pub. 100–04), Chapter 29 and Chapter 30, Section 30.2.2.
Comment: One commenter stated that the number of rural hospital-based PHPs has declined during the CY 2003–2006 period and indicated that a study conducted last year found that rural areas are being hit with the loss of PHP programs. A few commenters were greatly relieved by the projection of an increase in the reimbursement rate for Level II PHP services. They believed another cut in rates would jeopardize the existence of the PHP benefit, reduce the financial viability of PHPs, and probably lead to closure of many PHPs, thus affecting access to care for this vulnerable population. In addition, because hospital outpatient mental health services paid under the OPPS are capped at the PHP per diem payment rate, many commenters were concerned about overall access to outpatient mental health treatment. The commenters urged CMS to keep mental health services available to all.
Response: We have established the final CY 2010 payment rate based on hospital-based PHP data, yielding an increase in the median for days with 4 or more units of service compared to CY 2009 payment rates. This increase will benefit all PHP programs, including those in rural areas. The CY 2010 payment rates for Level I (3 units of service) shows a decrease in CY 2010 compared to CY 2009. We believe using the CMHC data would significantly reduce the current rate and negatively impact hospital-based PHPs and CMHCs, resulting possibly in reduced access to care. Because hospital-based PHPs are geographically diverse, whereas CMHCs are located in only a few states, we are concerned that a significant drop in the rate could result in hospital-based PHPs closing and leading to possible access problems. For this reason, we are using hospital-based PHP data only to calculate the CY 2010 median per diem payment rates.
Comment: One commenter expressed concern that CMS' data and analysis regarding the median per diem costs for PHP services have been and remain fundamentally flawed. The commenter recommended that CMS conduct further research and, in particular, conduct detailed provider-level research to better understand the costs necessary to deliver PHP services in hospital and CMHC settings. The commenter recommended the payment rate for PHP services (APC 0173) be set at approximately $325 per patient day and no less than the CY 2007 payment rate of $234.73.
Response: We base the PHP APC per diem payment rates on providers' charges reported on claims adjusted by the providers' CCRs. This approach is consistent with the method used to compute payment rates for other APCs under the OPPS, except that, for PHPs for which the unit of service is a day, we sum the charges for a given day and then determine the median cost of all days. We expect that a provider's charges will reflect the level of services provided, which has a relationship to the cost of providing those services. In Medicare cost reporting, the total charges are to be reported along with the provider's cost. To the extent that a provider is submitting bills that have charges that do not directly relate to the delivery or provision of services, its CCRs will be unpredictable and would distort the costs of the services provided.
In developing the CY 2010 PHP APC per diem payment rates, we excluded days that have only 1 or 2 units of service. In addition, we did not include days where no payment was made to avoid diluting the cost. To calculate the Level I PHP APC payment rate, we used days with 3 units of service, and to calculate the Level II PHP APC payment rate, we used days with 4 or more units of service. We believe our methodology accurately reflects the median cost of providing these two levels of PHP services. As discussed previously, we made several refinements to our Start Printed Page 60558 methodology for computing the per diem costs that more accurately reflect the per diem cost of providing PHP services.
Comment: Many commenters stated that cost report information for CMHCs is not currently included in the Healthcare Cost Report Information System (HCRIS) and recommended that CMS base its calculations only on the cost report information that the agency can verify directly and not on data provided by the fiscal intermediaries or MACs. The commenters believed that CMS should calculate payment rates using only cost data from those cost reports currently in and accessible through the HCRIS.
Response: We understand the commenters concern about making CMHC data available through the HCRIS, and we are taking steps to make the data available in the future. For CY 2010, we will use PHP hospital-based data only to set the PHP APC payment rates. The hospital-based PHP data are based on cost report data currently in and accessible through the HCRIS.
Comment: Several commenters expressed concern that there are additional services furnished by CMHCs that are currently provided to PHP patients for which the providers are not reimbursed. The commenters pointed out that the Substance Abuse and Mental Health Services Administration recognizes these additional services by including payment for treatment of mental illness, not just for substance abuse treatment, and for costs for other services, including locating housing. The commenter included a list of HCPCS H-codes as an example of additional services as specified in Table 52 below.
| HCPCS H-code | Description |
|---|---|
| H0001 | Alcohol and/or drug assessment. |
| H0004 | Behavioral health counseling and therapy, per 15 minutes. |
| H0028 | Alcohol and/or drug prevention problem identification and referral service (e.g., student assistance and employee assistance programs), does not include assessment. |
| H0029 | Alcohol and/or drug prevention alternatives service (services for populations that exclude alcohol and other drug use, e.g. alcohol free social events). |
| H0030 | Behavioral health hotline service. |
| H0031 | Mental health assessment, by non-physician. |
| H0032 | Mental health service plan development by non-physician. |
| H0033 | Oral medication administration, direct observation. |
| H0034 | Medication training and support, per 15 minutes. |
| H0047 | Alcohol and/or other drug abuse services, not otherwise specified. |
| H0049 | Alcohol and/or drug screening. |
| H0050 | Alcohol and/or drug services, brief intervention, per 15 minutes. |
| H1011 | Family assessment by licensed behavioral health professional for state defined purposes. |
| H2000 | Comprehensive multidisciplinary evaluation. |
| H2010 | Comprehensive medication services, per 15 minutes. |
| H2011 | Crisis intervention service, per 15 minutes. |
| H2014 | Skills training and development, per 15 minutes. |
| H2027 | Psycho educational service, per 15 minutes. |
Response: Partial hospitalization services are specifically defined in section 1861(ff) of the Act and are a Medicare benefit category. Because there is no benefit category for substance abuse programs, any such program would have to meet requirements established for PHPs, including the requirements that a physician certify that the patient would otherwise require inpatient psychiatric care in the absence of the partial hospitalization services and that the program provides active treatment (section 1835(a)(2)(F) of the Act and 42 CFR 424.27(e)). PHP services involving direct patient care costs are payable under Medicare. The HCPCS H-codes listed above are not payable by Medicare. However, certain services for substance abuse are payable under a PHP because a PHP provides for patient education, mental health assessment, occupational therapy, and behavioral health treatment/services among other services. For a complete list of services covered under a PHP under Medicare, we refer readers to the Medicare Claims Processing Manual (Pub. 100–04), Chapter 4, Section 260. Medicare does not pay for services such as 12-step programs. Many of the HCPCS H-codes listed duplicate allowable PHP service codes, for example, patient education and training. However, the PHP service codes generally are not defined in 15-minute increments.
Comment: One commenter suggested that CMS consider alternative arrangements for partial hospitalization and hospital outpatient services for the future. The commenter suggested removing PHP from the APC codes and instead establish a separate payment system (similar to home health) by establishing a reasonable base rate for PHP for Level II PHP services at a slightly higher level (such as $220–$225 per day), annually adjusting the base rate by a conservative inflation factor such as the CPI, establishing quality criteria to judge performance, and dropping the payment level for Level I PHP services so that only 4 or more services are recognized for payment.
Response: Currently, the statute does not provide for a separate payment system for partial hospitalization services. Therefore, a statutory change would be required to establish an independent payment system for PHPs. Regarding the commenter's recommendation to establish quality criteria, we agree that establishing benchmarks and indicators would be useful, and we encourage providers to share that information with us. We believe that creating a rate specific to days with three services is consistent with our policy to require CMHCs and hospital-based PHPs to provide a minimum of 3 units of service per day in order to receive payment. Although we do not expect Level I PHP service days to be frequent, we do recognize that there are times when a patient may need a less intensive day of service. Therefore, we continue to recognize the need for a two-tiered payment system: one payment for those less intensive days with three services; and another payment for those more intensive days with four or more services.
Comment: A few commenters urged CMS to find a way to strengthen the integrity of the program by developing and implementing standards of participation. The commenters recommended the implementation of standards of care with emphasis on quality of services and urged CMS to develop conditions of participation that would be a useful regulatory tool for Start Printed Page 60559 PHP providers. The commenters suggested that the development process must include all providers and other stakeholders. Several commenters offered to work with CMS to reform and improve the PHP by establishing standards for quality of PHP services provided; adopting CMHC facility-level quality measures and a reporting regime; and assisting with accreditation and cost reporting reforms.
Response: We agree with the commenters that standards of participation is an area that should be addressed, and we are exploring proposing conditions for coverage for CMHCs to establish minimum standards for patient rights, physical environment, staffing, and documentation requirements. We believe that adding conditions for coverage would contribute to more consistency between CMHCs and hospital-based PHPs.
Comment: One commenter suggested that CMS use fiscal intermediaries and MACs to work with hospitals and CMHC providers to establish separate PHP lines on their appropriate Medicare cost reports to arrive at a CCR for PHPs rather than the default psychiatric, clinics, or overall outpatient CCR lines. The commenter believed that, nationally, the CCRs for the PHPs are being understated by applying overall CCRs and/or clinic CCRs and, thus, penalizing the structured intensive partial programs.
Response: We note that most hospitals do not have a cost center for partial hospitalization; therefore, we have used the CCR as specific to PHP as possible. As described earlier in section X.A. of this final rule with comment period, for CY 2008, we proposed and finalized two refinements to the methodology for computing the PHP median per diem cost that we believe resulted in a more accurate median per diem cost. The first of the two CY 2008 refinements was a remapping of 10 revenue codes-to-cost centers for hospital-based PHP claims. We believe that the CY 2008 refinement to the mapping approach continues to be the best method for assigning the most appropriate cost center for hospital-based PHP claims. For a detailed explanation of the remapping of revenue codes for hospital-based PHP claims, we refer readers to the CY 2008 OPPS/ASC proposed rule (72 FR 42691 through 42692) and the CY 2008 OPPS/ASC final rule with comment period (72 FR 66671 through 66672).
In addition, we note that this remapping refinement applies only to hospital-based PHP claims and not to CMHC claims. In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68690), in response to commenters' request that CMS apply the remapping of revenue codes to cost centers to CMHC claims, we stated that we cannot apply the same mapping method to CMHCs because PHP is the CMHCs' only Medicare cost and CMHCs do not have the same cost structure as hospitals.
Comment: One commenter expressed concern that the PHP benefit lacked flexibility. The commenter believed that the rigid guidelines for attendance of 5 plus days a week (20 hours) could create excessive overutilization at times. The commenter stated that it would be more beneficial to restructure PHP to be a more flexible, less costly, outcome-based system of care.
Response: Partial hospitalization is an intensive outpatient program of psychiatric services provided to patients as an alternative to inpatient psychiatric hospitalization or as a step down to shorten an inpatient stay and transition a patient to a less intensive level of care. We understand the commenters' concerns about the 20 hours per week requirement with regard to scheduling flexibility, but we were concerned that if we reduce the minimum number of hours lower than the current guideline, the low end of the range will become the new minimum. Therefore, instead of reducing the number of hours a patient needs in order to be eligible to receive the benefit, we clarified that the patient eligibility requirement that patients require 20 hours of therapeutic services is evidenced in a patient's plan of care rather than in the actual hours of therapeutic services a patient receives. The intent of this eligibility requirement is that, for most weeks, we expect attendance conforming to the patient's plan of care. We recognize that there may be times at the beginning (or end) of a patient's transition into (or out of) a PHP where the patient may not receive 20 hours of therapeutic services.
Comment: One commenter expressed concern about the proposed cuts for Partial Hospitalization (APC 0173) and the impact the cuts will have on its hospital and community. The commenter reviewed the history of APC payment rates for these services and noted the trend of the payment rates decreasing over the past 7 years. The commenter pointed out that it experienced a significant increase in the staff salary and benefit costs. The commenter expressed concern that the decreasing payment rate and increasing expenses will make it difficult for the hospital to sustain these services and access to care for Medicare beneficiaries could worsen.
Response: Hospital costs per day for PHP services for APC 0173 have remained in the range of $200—$225 for CY 2000 through CY 2010. This is the reason we have decided to use hospital-based PHP data only for computing the CY 2010 PHP payment rates, as this approach will lead to payment stability for CY 2010.
In summary, after consideration of the public comments we received, we are adopting as final our CY 2010 proposal to retain the two-tiered payment approach for PHP services and to use only hospital-based PHP data in computing the payment. The two updated PHP APC per diem median costs that we are finalizing for CY 2010 are shown in Table 53 below.
| APC | Group title | Median per diem costs |
|---|---|---|
| 0172 | Level I Partial Hospitalization (3 services) | $148 |
| 0173 | Level II Partial Hospitalization (4 or more services) | 209 |
C. Separate Threshold for Outlier Payments to CMHCs
In the November 7, 2003 final rule with comment period (68 FR 63469), we indicated that, given the difference in PHP charges between hospitals and CMHCs, we did not believe it was appropriate to make outlier payments to CMHCs using the outlier percentage target amount and threshold established for hospitals. Prior to that time, there was a significant difference in the amount of outlier payments made to hospitals and CMHCs for PHP services. In addition, further analysis indicated that using the same OPPS outlier threshold for both hospitals and CMHCs did not limit outlier payments to high cost cases and resulted in excessive outlier payments to CMHCs. Therefore, Start Printed Page 60560 beginning in CY 2004, we established a separate outlier threshold for CMHCs. The separate outlier threshold for CMHCs has resulted in more commensurate outlier payments.
In CY 2004, the separate outlier threshold for CMHCs resulted in $1.8 million in outlier payments to CMHCs. In CY 2005, the separate outlier threshold for CMHCs resulted in $0.5 million in outlier payments to CMHCs. In contrast, in CY 2003, more than $30 million was paid to CMHCs in outlier payments. We believe this difference in outlier payments indicates that the separate outlier threshold for CMHCs has been successful in keeping outlier payments to CMHCs in line with the percentage of OPPS payments made to CMHCs. Table 54 below includes a listing of the outlier target amounts and the portion of the target amount allocated to CMHCs for PHP outliers for CYs 2004 through 2009.
| Year | Outlier target amount percentage | Portion of target amount allocated to CMHCs for PHP outliers (in percent) |
|---|---|---|
| CY 2004 | 2.0 | 0.5 |
| CY 2005 | 2.0 | 0.6 |
| CY 2006 | 1.0 | 0.6 |
| CY 2007 | 1.0 | 0.15 |
| CY 2008 | 1.0 | 0.02 |
| CY 2009 | 1.0 | 0.12 |
As noted in section II.F. of the CY 2010 OPPS/ASC proposed rule (74 FR 35296), for CY 2010, we proposed to continue our policy of identifying 1.0 percent of the aggregate total payments under the OPPS for outlier payments. We proposed that a portion of that 1.0 percent, an amount equal to 0.02 percent of outlier payments (or 0.0002 percent of total OPPS payments), would be allocated to CMHCs for PHP outliers. As discussed in section II.F. of the CY 2010 OPPS/ASC proposed rule (74 FR 35296), we proposed to set a dollar threshold in addition to an APC multiplier threshold for OPPS outlier payments. However, because the PHP APC is the only APC for which CMHCs may receive payment under the OPPS, we would not expect to redirect outlier payments by imposing a dollar threshold. Therefore, we did not propose to set a dollar threshold for CMHC outliers. As noted in section II.F. of the CY 2010 OPPS/ASC proposed rule (74 FR 35296), we proposed to set the outlier threshold for CMHCs for CY 2010 at 3.40 times the APC payment amount and the CY 2010 outlier payment percentage applicable to costs in excess of the threshold at 50 percent. Specifically, we proposed that if a CMHC's cost for partial hospitalization services, paid under either APC 0172 or APC 0173, exceeds 3.40 times the payment for APC 0173, the outlier payment would be calculated as 50 percent of the amount by which the cost exceeds 3.40 times the APC 0173 payment rate.
Comment: Several commenters suggested that instead of creating separate threshold for outlier payments to CMHCs, it would be beneficial to eliminate the outlier payment and use those funds allocated to outlier payments to bolster payments for services provided by CMHCs.
Response: We note that the Secretary shall provide an outlier payment for each covered OPD service (or group of services) in accordance with the requirements set forth in section1833(t)(5) of the Act and the applicable regulations. Because CMHCs are a provider of PHP services, outlier payments must be provided for them in accordance with the statute. We note that eliminating outlier payments for CMHCs would not result in an increase in the PHP rate, but rather would provide additional funding for hospital outlier payments for all HOPD services.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal to set a separate outlier threshold for CMHCs. As discussed in section II.F. of this final rule with comment period, using more recent data for this final rule with comment period, we set the target for hospital outpatient outlier payments at 1.0 percent of total estimated OPPS payments. We allocated a portion of that 1.0 percent, an amount equal to 0.03 percent of outlier payments and 0.0003 percent of total estimated OPPS payments to CMHCs for PHP outliers. For CY 2010, as proposed, we are setting the outlier threshold at 3.40 multiplied by the APC amount and CY 2010 outlier percentage applicable to costs in excess of the threshold at 50 percent.
XI. Procedures That Will Be Paid Only as Inpatient Procedures
A. Background
Section 1833(t)(1)(B)(i) of the Act gives the Secretary broad authority to determine the services to be covered and paid for under the OPPS. Before implementation of the OPPS in August 2000, Medicare paid reasonable costs for services provided in the HOPD. The claims submitted were subject to medical review by the fiscal intermediaries to determine the appropriateness of providing certain services in the outpatient setting. We did not specify in our regulations those services that were appropriate to provide only in the inpatient setting and that, therefore, should be payable only when provided in that setting.
In the April 7, 2000 final rule with comment period (65 FR 18455), we identified procedures that are typically provided only in an inpatient setting and, therefore, would not be paid by Medicare under the OPPS. These procedures comprise what is referred to as the “inpatient list.” The inpatient list specifies those services for which the hospital will be paid only when provided in the inpatient setting because of the nature of the procedure, the underlying physical condition of the patient, or the need for at least 24 hours of postoperative recovery time or monitoring before the patient can be safely discharged. As we discussed in that rule and in the November 30, 2001 final rule with comment period (66 FR 59856), we may use any of a number of criteria we have specified when reviewing procedures to determine whether or not they should be removed from the inpatient list and assigned to an APC group for payment under the OPPS when provided in the hospital outpatient setting. Those criteria include the following:
- Most outpatient departments are equipped to provide the services to the Medicare population.
- The simplest procedure described by the code may be performed in most outpatient departments.
- The procedure is related to codes that we have already removed from the inpatient list.
In the November 1, 2002 final rule with comment period (67 FR 66741), we added the following criteria for use in reviewing procedures to determine whether they should be removed from the inpatient list and assigned to an APC group for payment under the OPPS:
- A determination is made that the procedure is being performed in numerous hospitals on an outpatient basis; or
- A determination is made that the procedure can be appropriately and safely performed in an ASC, and is on the list of approved ASC procedures or has been proposed by us for addition to the ASC list.
The list of codes that we proposed to be paid by Medicare in CY 2010 only as inpatient procedures was included as Start Printed Page 60561 Addendum E to the CY 2010 OPPS/ASC proposed rule.
B. Changes to the Inpatient List
In the CY 2010 OPPS/ASC proposed rule (74 FR 35358), we proposed to use for the CY 2010 OPPS the same methodology as described in the November 15, 2004 final rule with comment period (69 FR 65835) to identify a subset of procedures currently on the inpatient list that are being performed a significant amount of the time on an outpatient basis. Using this methodology, we identified three procedures that met the criteria for potential removal from the inpatient list. We then clinically reviewed these three potential procedures for possible removal from the inpatient list and found them to be appropriate candidates for removal from the inpatient list. During the February 2009 meeting of the APC Panel, we solicited the APC Panel's input on the appropriateness of removing the following three procedures from the CY 2010 inpatient list: CPT codes 21256 (Reconstruction of orbit with osteotomies (extracranial) and with bone grafts (includes obtaining autografts) (eg, micro-ophthalmia)); 27179 (Open treatment of slipped femoral epiphysis; osteoplasty of femoral neck (Heyman type procedure)); and 51060 (Transvesical ureterolithotomy).
In addition to presenting to the APC Panel the three procedures above, we also presented utilization data for the first 9 months of CY 2008 for two other specific procedures, in response to a request by the APC Panel from the March 2008 meeting: CPT code 20660 (Application of cranial tongs, caliper or stereotactic frame, including removal (separate procedure)), a procedure that we removed from the inpatient list for CY 2009; and CPT code 64818 (Sympathectomy, lumbar), a procedure that we maintained on the inpatient list for CY 2009.
Following the discussion at the February 2009 meeting, the APC Panel recommended that CMS remove from the CY 2010 inpatient list CPT codes 21256, 27179, and 51060. The APC Panel also recommended that CPT code 64818 remain on the inpatient list for CY 2010. The APC Panel made no recommendation regarding CPT code 20660.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35358), we proposed to accept the APC Panel's recommendations to remove the procedures described by CPT codes 21256, 27179, and 51060 from the inpatient list because we agree with the APC Panel that the procedures may be appropriately provided as hospital outpatient procedures for some Medicare beneficiaries. We also proposed to retain CPT code 64818 on the inpatient list because we agree with the APC Panel that this procedure should be provided to Medicare beneficiaries only in the hospital inpatient setting. The three procedures we proposed to remove from the inpatient list for CY 2010 and their CPT codes, long descriptors, and proposed APC assignments were displayed in Table 37 of the CY 2010 OPPS/ASC proposed rule (74 FR 35358).
Comment: Several commenters supported the proposal to remove the procedures reported by CPT codes 21256, 27179, and 51060 from the inpatient list, one commenter opposed removing the procedure coded by CPT code 51060, and one commenter expressed concern about removing any of the three procedures proposed for removal from the inpatient list. The commenter that requested that CMS retain CPT code 51060 on the inpatient list reported that the procedure is an open surgical procedure that is much more extensive than a hernia repair. In that commenter's experience, patients who undergo this surgery are not able to go home the same day as surgery because most require parenteral pain medication and ongoing monitoring of the pain, and of possible ileus or hematuria. Another commenter provided no rationale for objecting to the removal of the three procedures as proposed by CMS beyond stating that if CMS does not eliminate the entire inpatient list, then the commenter would have serious concerns about removing the three procedures.
Response: We appreciate the commenters' support of our proposal. In response to the commenters who expressed concern about removing one or more of the three procedures from the inpatient list, we reevaluated the three procedures using more recent utilization data and further clinical review by CMS medical advisors. As a result of that reevaluation, we remain convinced that all three procedures may be safely performed in the HOPD for some Medicare beneficiaries. As we have indicated previously, the removal of a procedure from the inpatient list does not signify a determination by CMS that the procedure should be performed in the HOPD for all beneficiaries. The removal only indicated that CMS is relying on the individual beneficiary's surgeon to advise the most appropriate setting for the procedure based on the beneficiary's medical condition. In fact, as evidenced by the utilization data over the years, most of the newly-removed procedures from the inpatient list continue to be commonly provided in the inpatient setting after they are removed from the list. The removal of a procedure from the inpatient list simply is recognition that there is evidence that the procedure may be safely performed for some beneficiaries who are outpatients and represents no directive about whether the inpatient setting or the outpatient setting is more appropriate in any particular circumstance.
Comment: Several commenters requested the removal of additional procedures from the inpatient list. Although the commenters requested that CMS remove a total of 20 additional procedures from the inpatient list, 4 of the requested codes were not on the proposed CY 2010 inpatient list. All of the codes requested for removal are displayed in Table 55 below.
As identified by asterisks, 11 of the procedures displayed in the chart below were submitted by one commenter representing a group of hospitals. This commenter stated that each of the procedures requested for removal from the inpatient list was carefully reviewed and could be safely provided to Medicare beneficiaries in the HOPD. The commenter reported that research and investigation indicated that clinical criteria sets such as the Milliman Care Guidelines support the safe provision of the 11 procedures in outpatient settings. In addition, the commenter stated that the group's hospitals have physicians providing the procedures safely in the outpatient setting for non-Medicare patients who are in the same age group as the Medicare population.
| CY 2009 HCPCS code | CY 2009 short descriptor | Proposed CY 2010 SI |
|---|---|---|
| 01402 | Anesth, knee arthroplasty | C |
| 22548 | Neck spine fusion | C |
| *22554 | Neck spine fusion | C |
| *22585 | Additional spinal fusion | C |
| *22851 | Apply spine prosth device | T |
| 27447 | Total knee arthroplasty | C |
| 28805 | Amputation thru metatarsal | C |
| *32662 | Thoracoscopy, surgical | C |
| *37182 | Insert hepatic shunt (tips) | C |
| *37215 | Transcath stent, cca w/eps | C |
| Start Printed Page 60562 | ||
| *44950 | Appendectomy | C |
| 44955 | Appendectomy, add-on | C |
| 44960 | Appendectomy | C |
| 55866 | Laparo radical prostatectomy | C |
| *60505 | Explore parathyroid glands | C |
| *63047 | Removal spinal lamina | T |
| 63075 | Neck spine disk surgery | T |
| *63076 | Neck spine disk surgery | C |
| *63267 | Excise intraspinal lesion | C |
| 64999 | Nervous system surgery | T |
| * Submitted by commenter representing a group of hospitals. | ||
Response: In response to the commenters' requests, we reviewed utilization and clinical information for each of the procedures suggested for removal from the inpatient list. Of the 16 procedures reviewed (those with status indicator “C” in the chart above) our medical advisors agreed with the commenters that it would be appropriate to remove 5 of them from the inpatient list. Thus, for CY 2010, we are removing from the inpatient list the procedures reported by CPT codes 28805 (Amputation, foot; transmetatarsal); 37215 (Transcatheter placement of intravascular stent(s), cervical carotid artery, percutaneous; with distal embolic protection); 44950 (Appendectomy); 44955 (Appendectomy; when done for indicated purpose at time of other major procedure (not as separate procedure) (List separately in addition to code for primary procedure)); and 63076 (Discectomy, anterior, with decompression of spinal cord and/or nerve root(s), including osteophytectomy; cervical, each additional interspace (List separately in addition to code for primary procedure)). These procedures and the APCs to which they are assigned for CY 2010 are displayed in Table 56 below.
The clinical and utilization data for the other 11 procedures did not support the appropriateness of providing the procedures to Medicare beneficiaries in the HOPD. We believe that Medicare beneficiaries who undergo any of these 11 procedures should do so only as inpatients.
Comment: Many commenters suggested that CMS eliminate the inpatient list. They believed that each patient's status should be determined by the physician who can establish the most appropriate care plan for the individual. The commenters pointed out the many safety provisions that are met by hospitals participating in the Medicare program as evidence that hospitals would provide care safely and appropriately.
A few of the commenters stated that the inconsistency between the Medicare payment policies for hospitals and physicians of allowing physicians to receive full payment for inpatient procedures that are performed on Medicare beneficiaries in the HOPD who are not inpatients, but denying hospitals payment for those same procedures, gives physicians little incentive to avoid providing inpatient procedures to Medicare beneficiaries who are outpatients. Several commenters suggested that if CMS maintains the inpatient list, the associated payment restrictions be applied consistently to both hospitals and physicians in order to promote a collaborative effort in documentation and clinical care plans.
One commenter stated that the inpatient list, which requires that Medicare beneficiaries be handled differently than the rest of the patient population in some circumstances, creates chaos for the physicians and hospitals who are trying to apply consistent clinical criteria to determine appropriate levels of care for all of their patients.
Many of the commenters argued that there are a variety of circumstances that result in procedures on the inpatient list being performed without an inpatient admission and that hospitals should not be held accountable for those situations. For example, they explained that sometimes during the intraoperative period, due to clinical circumstances, the surgeon performs a procedure that is on the inpatient list in addition to, or rather than, the procedure that was planned.
Finally, the commenters believed that the inpatient list penalizes hospitals unfairly and suggested that if CMS is unwilling to eliminate the inpatient list, it consider developing an appeals process to address circumstances in which payment for a service provided on an outpatient basis is denied due to its presence on the inpatient list. The commenters believed that the appeal would give the hospital the opportunity to submit documentation on the physician's intent, the patient's clinical condition, and the circumstances that enabled the patient to be sent home safely without an inpatient stay.
Response: While we understand the commenters' reasons for advocating the elimination of the inpatient list, we continue to believe that the inpatient list serves an important purpose in identifying procedures that cannot be safely and effectively provided to Medicare beneficiaries in the HOPD. We are concerned that the elimination of the inpatient list could result in unsafe or prolonged care in hospital outpatient departments for Medicare beneficiaries. Therefore, we are not discontinuing our use of the inpatient list at this time.
Although the commenters suggested that we apply the same payment restrictions to physicians and hospitals when inpatient procedures are performed inappropriately, payment for physicians' services are outside of the scope of OPPS payment policy and of this OPPS/ASC final rule with comment period. We continue to believe that it is very important for hospitals to educate physicians on Medicare services covered under the OPPS to avoid inadvertently providing services in a hospital outpatient setting that only are covered during an inpatient stay.
We also are concerned about the potential results of eliminating the inpatient list on Medicare beneficiary liability. For instance, we are concerned that, without the inpatient list, Medicare beneficiaries could experience longer stays in HOPDs after some procedures. The APC Panel has discussed its concern about these long stays that frequently exceed 24 hours. Moreover, the financial liability for OPPS copayments for complex surgical procedures and long periods of care in the HOPD and coverage of items such as usually self-administered drugs differs significantly from a beneficiary's inpatient cost-sharing responsibilities and coverage, and the beneficiary may incur higher out-of-pocket costs for prolonged outpatient encounters than for an inpatient stay for the same surgical intervention.
We continue to encourage physicians' awareness of the implications for Medicare beneficiaries and hospitals of performing inpatient list procedures in the HOPD on beneficiaries who are not inpatients. We do not plan to adopt a specific appeals process for claims related to inpatient list procedures performed in the HOPD at this time. The existing processes established for a beneficiary or a provider to appeal a specific claim remain in effect.
Comment: One commenter suggested that CMS implement a method to identify scheduled outpatient procedures that become, through Start Printed Page 60563 intraoperative circumstances, inpatient procedures. The commenter asserted that, due to hospital billing practices, hospital coding staff do not know until well after the surgery is completed that an unscheduled inpatient procedure was performed on an outpatient who was not admitted as an inpatient. The commenter suggested that CMS implement a HCPCS modifier that the hospital could append to the inpatient procedure on the claim and that payment for the claim could be made at the same rate as those coded with the –CA modifier (APC 0375 (Ancillary Outpatient Services When Patient Expires)).
Response: While we appreciate the commenter's suggestion for addressing circumstances when unplanned inpatient list procedures are performed during operative sessions where outpatient surgical procedures were planned, we do not believe there is a need for a modifier to identify those situations. We continue to believe that the inpatient list procedures are not appropriate for performance on Medicare beneficiaries in the HOPD and, therefore, we expect that when such a procedure is performed, the beneficiary would be admitted as an inpatient.
We established payment for ancillary services reported in association with an inpatient procedure to which the –CA modifier is appended in order to provide payment to hospitals for services furnished in those rare cases in which procedures on the inpatient list are performed to resuscitate or stabilize a patient (whose status is that of an outpatient) with an emergent, life-threatening condition, and the patient dies before being admitted as an inpatient. In these situations, hospitals are unable to admit the patients as inpatients. In the circumstances described by the commenter, we see no insurmountable hospital barriers to admitting the patients as inpatients of the hospital and do not believe it would be appropriate to provide payment (through APC 0375) for a mix of surgical procedures provided to patients who survive at the rate developed specifically to pay for the ancillary services furnished in association with procedures reported with the –CA modifier when a patient dies prior to admission as an inpatient. The calculation of the payment rate for APC 0375 is discussed in detail in section II.A.2.d.(7) of this final rule with comment period.
We understand hospitals' dilemma when the decision is made intraoperatively to perform an unscheduled procedure. However, we continue to believe that it is important for hospitals to educate physicians on Medicare services paid under the OPPS to avoid inadvertently providing services in a hospital outpatient setting that would be paid only during an inpatient stay because we believe that the HOPD is not an appropriate site of service for inpatient list procedures.
Comment: Another commenter recommended that CMS expand the use of the –CA modifier to allow payments to the hospitals for performance of procedures on the inpatient list that must be performed to resuscitate or stabilize an outpatient with an emergency, life-threatening condition, but the patient is stabilized medically and transferred to another acute care hospital for admission. In other words, the commenter added, the patient is never admitted to the hospital where the inpatient list procedure was performed.
Response: We established the –CA modifier policy to provide payment to hospitals for services provided in the specific and rare situations in which procedures on the inpatient list are performed in the HOPD to resuscitate or stabilize a patient (whose status is that of an outpatient) with an emergent, life-threatening condition, and the patient dies before being admitted as an inpatient, not as a method for hospitals to recoup costs incurred when inpatient procedures are performed inappropriately on a Medicare beneficiary in the HOPD.
We see no rationale for allowing hospitals to report the –CA modifier for any circumstances other than those for which it was created. In the scenario described by the commenter, there is no evidence of the hospital's insurmountable barriers to admitting the patient, a criterion for use of the –CA modifier. In addition, we are not convinced that there is a need for a modifier to describe these rare events. We also do not believe it would be appropriate to provide payment at the rate developed specifically to pay for the ancillary services furnished in association with procedures reported with the –CA modifier when a patient dies prior to admission as an inpatient (APC 0375), for a mix of surgical procedures provided to patients who survive. The calculation of the payment rate for APC 0375 is discussed in detail in section II.A.2.d.(7) of this final rule with comment period.
Comment: One commenter suggested that CMS may lack information on the types of inpatient procedures that are performed for beneficiaries who are outpatients because those claims are returned to the provider by the I/OCE, thereby preventing CMS from capturing the unpaid services in its claims data. To address this situation, the commenter recommended that CMS modify the I/OCE so that claims for inpatient procedures provided on an outpatient basis could be processed as not payable rather than returned to the provider. The commenter believed that this modification would enable CMS to gather claims data on these procedures and to see how many and what types of procedures physicians believe are appropriate for performance in the HOPD. The claims data also would provide CMS information about the hospital resources expended to care for these Medicare beneficiaries. In addition, the commenter suggested that CMS could share the data with the APC Panel for discussion and review in support of their evaluations of which procedures may appropriately be removed from the inpatient list.
Response: We believe that our outpatient claims data include the claims for inpatient procedures that are performed on Medicare beneficiaries who are outpatients. As would be expected, the volume for these nonpayable procedures is low compared to the number of payable outpatient claims, but we believe that the claims hospitals submit are available to us for examination in our OPPS claims data each year.
The I/OCE logic does not result in claims for inpatient procedures being returned to the provider. Rather, once the inpatient procedure is identified, it is line-item denied and then, with very few exceptions (for example, claims with the –CA modifier and an indication that the beneficiary expired), it assigns line-item edits to result in payment denial for each of the other services on the claim because these services were furnished on the same day as the inpatient procedure. A full description of the I/OCE logic and edits is available on the CMS Web site at: http://www.cms.hhs.gov/OutpatientCodeEdit/.
After consideration of the public comments we received, we are finalizing our proposal to remove the procedures reported by CPT codes 21256, 27179, and 51060 from the inpatient list. We also are removing five additional procedures that public commenters requested be removed from the inpatient list. These procedures are reported by CPT codes 28805 (Amputation, foot; transmetatarsal); 37215 (Transcatheter placement of intravascular stent(s), cervical carotid artery, percutaneous; with distal embolic protection); 44950 (Appendectomy); 44955 (Appendectomy; when done for Start Printed Page 60564 indicated purpose at time of other major procedure (not as separate procedure) (List separately in addition to code for primary procedure)); and 63076 (Discectomy, anterior, with decompression of spinal cord and/or nerve root(s), including osteophytectomy; cervical, each additional interspace (List separately in addition to code for primary procedure)). The final eight procedures we are removing from the inpatient list for CY 2010 and their CPT codes, long descriptors, and final APC assignments are displayed in Table 56 below.
| CY 2010 HCPCS Code | CY 2010 long descriptor | Final CY 2010 APC assignment | Final CY 2010 status indicator |
|---|---|---|---|
| 21256 | Reconstruction of orbit with osteotomies (extracranial) and with bone grafts (includes obtaining autografts) (eg, micro-ophthalmia) | 0256 | T |
| 27179 | Open treatment of slipped femoral epiphysis; osteoplasty of femoral neck (Heyman type procedure) | 0052 | T |
| 28805 | Amputation, foot; transmetatarsal | 0055 | T |
| 37215 | Transcatheter placement of intravascular stent(s), cervical carotid artery, percutaneous; with distal embolic protection | 0229 | T |
| 44950 | Appendectomy | 0153 | T |
| 44955 | Appendectomy; when done for indicated purpose at time of other major procedure (not as separate procedure) (List separately in addition to code for primary procedure) | 0153 | T |
| 51060 | Transvesical ureterolithotomy | 0163 | T |
| 63076 | Discectomy, anterior, with decompression of spinal cord and/or nerve root(s), including osteophytectomy; cervical, each additional interspace (List separately in addition to code for primary procedure) | 0208 | T |
XII. OPPS Nonrecurring Technical and Policy Changes and Clarifications
A. Kidney Disease Education Services
1. Background
Section 152(b) of Public Law 110–275 (MIPPA) amended section 1861(s)(2) of the Act by adding a new subsection (EE) to provide for coverage of kidney disease education (KDE) services as a Medicare Part B benefit for Medicare beneficiaries diagnosed with stage IV chronic kidney disease (CKD) who, according to accepted clinical guidelines identified by the Secretary, will require dialysis or a kidney transplant, effective for services furnished on or after January 1, 2010. Section 152(b) also added a new subsection (ggg) to section 1861 of the Act to define “kidney disease education services” and to specify who may furnish these services as a “qualified person.” Section 1861(ggg)(2)(A)(i) of the Act, as added by section 152(b) of Public Law 110–275, defines a qualified person as a physician (as defined in section 1861(r)(1) of the Act); or a physician assistant, nurse practitioner, or clinical nurse specialist (as defined in section 1861(aa)(5) of the Act) who furnishes services for which payment may be made under the fee schedule established under section 1848 of the Act. Section 1861(ggg)(2)(A)(ii) of the Act also defines a qualified person as a “provider of services located in a rural area (as defined in section 1886(d)(2)(D) [of the Act]).” The definition of a “qualified person” for this benefit includes certain rural providers of services, such as hospitals, critical access hospitals (CAHs), skilled nursing facilities (SNFs), home health agencies (HHAs), comprehensive outpatient rehabilitation facilities (CORFs), and hospices. Section 1861(ggg)(2)(B) of the Act provides that a qualified person does not include a provider of services (other than a provider of services described in section 1861(ggg)(2)(A)(ii)) or a renal dialysis facility.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35358), we proposed to implement the provisions of section 1861(s)(2)(EE) and 1861(ggg) of the Act, as added by section 152(b) of Pub. L. 110–275, mainly through the June 2009 CY 2010 MPFS proposed rule (CMS–1413–P; Medicare Program; Payment Policies under the Physician Fee Schedule and Other Revisions to Part B for CY 2010), hereinafter referred to as the CY 2010 MPFS proposed rule. Specifically, in section II.G.10. of the CY 2010 MPFS proposed rule (74 FR 33617), we proposed to define the Medicare coverage criteria that would be applicable to KDE services and who may provide these services (that is, a “qualified person”), consistent with the provisions of sections 1861(s)(2)(EE) and 1861(ggg) of the Act. In that proposed rule, we also proposed to define a provider of services in a rural area as defined in section 1886(d)(2)(D) of the Act as a hospital, CAH, SNF, CORF, HHA, or hospice that is physically located in a rural area as defined in § 412.64(b)(ii)(C) of the regulations or a hospital or CAH that is reclassified from urban to rural status pursuant to section 1886(d)(8)(E) of the Act, as defined in § 412.103 of the regulations. According to the proposal included in the CY 2010 MPFS proposed rule, a hospital, CAH, SNF, CORF, HHA, or hospice would not be considered to be a qualified person if the facility providing KDE services is located outside of a rural area unless the service is furnished in a hospital or CAH that has reclassified from urban to rural status under § 412.103.
In addition, in the CY 2010 MPFS proposed rule (74 FR 33614), consistent with the provisions of section 1861(ggg) of the Act, we proposed a payment amount for KDE services furnished by a “qualified person.” Specifically, we proposed to establish two new Level II HCPCS G-codes to describe KDE services and to specify the associated relative value units (RVUs) under the MPFS for payment for these codes.
We instructed individuals who wished to comment on the proposed coverage criteria for KDE services under section 1861(ggg) of the Act, including the definition of a “qualified person,” the proposed HCPCS G-codes, and the proposed RVUs for KDE services to submit their comments to CMS in response to the CY 2010 MPFS proposed rule that we describe above. Below we discuss our proposed payment for KDE services furnished by providers of services located in a rural area. We instructed individuals who wished to submit public comments relating to payment for KDE services furnished by providers of services located in a rural area to submit those Start Printed Page 60565 comments in response to the CY 2010 OPPS/ASC proposed rule.
2. Payment for Services Furnished by Providers of Services Located in a Rural Area
In the CY 2010 OPPS/ASC proposed rule (74 FR 35358), we proposed to pay under the MPFS for KDE services under section 1861(ggg) of the Act when the services are furnished by a qualified person that is a hospital, CAH, SNF, CORF, HHA, or hospice that is located in a rural area as defined in section 1886(d)(2)(D) of the Act or a hospital or CAH that is reclassified from urban to rural status pursuant to section 1886(d)(8)(E) of the Act, as defined in § 412.103 of the regulations. Section 152(b) of Public Law 110–275 amended section 1848(j)(3) of the Act to add section 1861(s)(2)(EE) (kidney disease education services) to the list of subsections of section 1861(s)(2) of the Act, which are included in the definition of physician services in section 1848(j)(3) of the Act. However, the statute does not specify the payment methodology for KDE services furnished by providers of these services located in rural areas.
Given that the statute provides the Secretary with the flexibility to pay all qualified persons under the MPFS and there is precedent for paying both diabetes self-management training and medical nutrition therapy services (which we believe KDE is similar to in terms of resource use, specifically staffing and infrastructure) under the MPFS, we proposed to pay all qualified persons for KDE services under the MPFS. This single payment methodology would apply to all qualified persons, including providers of services in a rural area as we proposed to define such providers in the CY 2010 MPFS proposed rule.
The language in section 1861(ggg) of the Act that defines KDE services is similar to the language in section 1861(qq) of the Act that defines “diabetes self-management training services,” which is a medical or other health service under section 1861(s)(2)(S) of the Act. In addition, the language in section 1861(ggg) of the Act is similar to the language in section 1861(vv) of the Act that defines “medical nutrition therapy services,” which is also a medical or other health service under section 1861(s)(2)(V) of the Act. Finally, both diabetes self-management training and medical nutrition therapy are included in the definition of “physicians' services” for purposes of the MPFS at section 1848(j)(3) of the Act, and our standard policy is to pay for both services under the MPFS when they are furnished in an HOPD. Given that the statute permits us to pay all qualified persons under the MPFS and the precedent for paying both diabetes self-management training and medical nutrition therapy under the MPFS when these services are provided in the hospital outpatient setting, we believe that payment under the MPFS is the most appropriate methodology for payment to qualified persons who are providers of services located in a rural area or who are hospitals or CAHs that have been reclassified from urban to rural status pursuant to § 412.103 of the regulations for the KDE services they furnish.
The proposed CY 2010 MPFS payments for HCPCS codes GXX26 (Educational services related to the care of chronic kidney disease; individual, per session; face-to-face), now finalized in the CY 2010 MPFS final rule with comment period as G0420 (Educational services related to the care of chronic kidney disease; individual per session, per hour, face-to-face), and GXX27 (Educational services related to the care of chronic kidney disease; group, per session; face-to-face), now finalized in the CY 2010 MPFS final rule with comment period as G0421(Educational services related to the care of chronic kidney disease; group, per session, per hour, face-to-face), are discussed in the CY 2010 MPFS proposed rule (74 FR 33619). When the qualified person is a rural provider, we proposed to pay the provider the applicable amount under the MPFS and a single payment would be made for each KDE session, limited to no more than six sessions as discussed in the CY 2010 MPFS proposed rule. Subsequently, we would not provide separate payment for both a physician's professional services and the associated facility services if a single session of KDE services was furnished in a rural hospital or CAH. Therefore, because of operational constraints, we proposed that payment would be made to only one qualified person for KDE services on the same day for the same beneficiary. We also note that the MPFS' geographic practice cost index would apply to the calculation of the payment in a particular fee schedule locality because this locality adjustment methodology is applicable to payment for all services paid under the MPFS. We proposed to assign status indicator “A” to HCPCS codes GXX26 and GXX27 in Addendum B to the CY 2010 OPPS/ASC proposed rule to signify that these services, when covered, would be paid under a payment system other than the OPPS, specifically the MPFS in the case of both HCPCS codes.
We instructed individuals who wished to submit public comments on this proposal to pay under the MPFS for covered KDE services furnished by qualified persons who are hospitals, CAHs, SNFs, CORFs, HHAs, or hospices that are located in a rural area or are treated as being rural under § 412.103 to submit those comments in accordance with the instructions for commenting on the CY 2010 OPPS/ASC proposed rule. We instructed individuals who wished to submit public comments on all other aspects of the proposed implementation of sections 1861(s)(2)(EE) and 1861(ggg) of the Act, including, but not limited to, the proposed criteria for coverage of the services, the proposed definition of “session,” the proposed HCPCS G-codes, and the proposed content of the program, to submit those comments in response to the CY 2010 MPFS proposed rule.
Comment: A few commenters objected to the proposed payment to rural providers for KDE services. The commenters believed that the proposed rates were too low for appropriate payment for KDE services and recommended that CMS revise its KDE payment rates to reflect the greater resources required for rural provider to furnish KDE services.
Response: As a result of our review of the public comments and further analysis, we are adjusting the final CY 2010 MPFS RVUs for HCPCS codes G0420 and G0421. Specifically, we reviewed the medical nutrition therapy CPT codes, 97802 (Medical nutrition therapy; initial assessment and intervention, individual, face-to-face with the patient, each 15 minutes) and 97804 (Medical nutrition therapy; group (2 or more individual(s)), each 30 minutes), that we are crosswalking to the KDE codes for payment under the MPFS. We have adjusted the final CY 2010 values for HCPCS codes G0420 and G0421 to reflect not only the final specification of the time of one hour for an individual or group KDE session but also to reflect the appropriate supplies and equipment without duplication. We multiplied the physician work RVUs for HCPCS code G0420 by four and the work RVUs for HCPCS code G0421 by two to account for the fact that we are crosswalking a 15 minute MNT code to a 60 minute KDE code for the individual service and a 30 minute MNT code to a 60 minute KDE code for the group service case. We refer readers to the CY 2010 MPFS final rule with comment period for the CY 2010 RVUs for KDE services that determine payment to rural providers for HCPCS codes G0420 and G0421. Start Printed Page 60566
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to pay under the MPFS for covered KDE services furnished by qualified persons that are hospitals, CAHs, SNFs, CORFs, HHAs, or hospices that are located in a rural area or are treated as being rural under § 412.103. Public comments concerning the definition of a “qualified person,” the proposed HCPCS G-codes, the proposed RVUs for KDE services, the proposed criteria for coverage of the services, the proposed definition of “session,” and the proposed content of the program are discussed in the CY 2010 MPFS final rule with comment period.
B. Pulmonary Rehabilitation, Cardiac Rehabilitation, and Intensive Cardiac Rehabilitation Services
1. Legislative Changes
Section 144(a) of Public Law 110–275 (MIPPA) made a number of changes to the Act to provide Medicare Part B coverage and payment for pulmonary and cardiac rehabilitation services furnished to beneficiaries with chronic obstructive pulmonary disease and certain other conditions, respectively, effective January 1, 2010. Specifically, section 144(a)(1) of the Act amended section 1861(s)(2) of the Act by adding new subparagraphs (CC) and (DD) to specify Medicare Part B coverage of items and services furnished under (1) a cardiac rehabilitation (CR) program (as defined in an added new section 1861(eee)(1) of the Act) or under a pulmonary rehabilitation (PR) program (as defined under an added new section 1861(fff)(1) of the Act; and (2) an intensive cardiac rehabilitation (ICR) program (as defined in an added new section 1861(eee)(4) of the Act). The amendments made by section 144(a) of Public Law 110–275 provide for coverage of CR, PR, and ICR services provided in a physician's office, in a hospital on an outpatient basis, or in other settings as the Secretary determines appropriate. Section 144(a)(2) of Public Law 110–275 amended section 1848(j)(3) to provide for payment for services furnished in an ICR program under the MPFS and also added a new section 1848(b)(5) to provide specific language governing payment for ICR services. Under that specific section, the Secretary shall substitute the Medicare OPD fee schedule amount established under the prospective payment system for hospital outpatient department services under section 1833(t)(3)(D) of the Act for CR (under HCPCS codes 93797 (Physician services for outpatient cardiac rehabilitation; without continuous ECG monitoring (per session)) and 93798 (Physician services for outpatient cardiac rehabilitation; with continuous ECG monitoring (per session)) for CY 2007, or any succeeding HCPCS codes established for cardiac rehabilitation). Section 144(a)(2) also defines under the new section 1848(b)(5) a “session” for each of the component CR items and services defined in subparagraphs (A) through (E) of section 1861(eee)(3) of the Act, when furnished for one hour, as a separate session of ICR, and specified that payment may be made for up to 6 sessions per day of the series of 72 one-hour sessions of ICR services. Section 144(a)(1)(B) also requires that a physician must be immediately available and accessible for medical consultations and medical emergencies at all times items and services are being furnished under CR, ICR, and PR programs, except that in the case of such items and services furnished under such a program in a hospital, such availability shall be presumed.
As we discuss in detail in section II.G.8. of the CY 2010 MPFS proposed rule (74 FR 33606), we proposed to use the MPFS and the OPPS rulemaking processes, and may use the national coverage determination (NCD) process as well, to implement the amendments made by section 144(a) of Public Law 110–275. In the CY 2010 MPFS proposed rule, we specified our policy proposals for implementing Medicare Part B coverage and payment for services furnished in a CR, ICR, and PR program under the MPFS. In the CY 2010 OPPS/ASC proposed rule (74 FR 35360), we proposed the CY 2010 OPPS payment for services in a CR, ICR, or PR program furnished to hospital outpatients.
Comment: A number of commenters asked that CMS confirm that the services of physical therapists are not part of the PR, CR, or ICR benefits authorized by section 144(a)(1) of Public Law 100–275 and are always paid under the physical therapy benefit and that, therefore, the therapy services do not require physician supervision when furnished as part of a PR, CR, or ICR program, including in the HOPD. With regard to PR, they stated that CMS has a longstanding history of recognizing the services of a physical therapist as an integral part of a PR program and requiring that these services be reported and paid as physical therapy services. Specifically, the commenters indicated that in the CY 2002 MPFS regulation (66 FR 55246) and in the current Medicare Claims Processing Manual (Pub. 100–04, Chapter 5, Section 20.A), CMS specifies that when physical therapists treat respiratory conditions, they should report CPT codes for physical therapy in the 97000 series and should not report HCPCS codes G0237 (Therapeutic procedures to increase strength or endurance of respiratory muscles, one on one, face to face, per 15 minutes (includes monitoring)); G0238 (Therapeutic procedures to improve respiratory function or increase strength or endurance of respiratory muscles, one on one, face to face, per 15 minutes (includes monitoring)); or G0239 (Therapeutic procedures to improve respiratory function or increase strength or endurance of respiratory muscles, two or more individuals (includes monitoring)). The commenters added that in the September 25, 2007 Decision Memo for Pulmonary Rehabilitation (CAG–00356N), CMS recognized the importance of physical therapy to patients with pulmonary conditions and stated that these services should be billed and paid under the physical therapy benefit. The commenters argued that a plan of care developed by a physical therapist to improve pulmonary function for a patient with chronic obstructive pulmonary disease (COPD), which meets the medical necessity criteria for physical therapy services, is covered and paid under the physical therapy benefit. They explained that, although it is a covered PR service, the therapy plan of care is separate from the PR benefit authorized by section 144(a)(1) of Public Law 100–275, should continue to be reported under the CPT codes for physical therapy services, and should be paid under the physical therapy benefit. In addition, the commenters requested that CMS confirm that skilled physical therapy services that are rendered in the CR setting by a qualified physical therapist should be conducted, reported, and paid as physical therapy services, and that physician supervision is not necessary in the CR setting when the physical therapist is delivering treatment that clearly meets the criteria for a physical therapy service. The commenters explained that CMS has recognized and codified that physical therapy is a separate benefit and that physical therapists are qualified to perform certain services independent of direct physician supervision.
Response: We expect that most patients participating in PR, CR, or ICR programs authorized by section 144(a)(1) of Public Law 100–275 and covered by Medicare Part B will be debilitated based on their underlying medical condition, age, or other factors. In order to develop a PR, CR, or ICR Start Printed Page 60567 treatment plan, some debilitated patients may require evaluations by therapists on the multidisciplinary team, in addition to assessments by other team members. In order to participate successfully in the prescribed exercise component of the PR, CR, or ICR program, we also expect that these patients may receive individualized treatments by therapists on the multidisciplinary team and others to promote the increased functionality that is a principle goal of PR, CR, and ICR programs. As we stated in the CY 2010 MPFS proposed rule, the items and services furnished by a CR or PR program are individualized and set forth in written treatment plans for each beneficiary (74 FR 33607 and 33611). We believe these evaluations and individualized treatments are a part of the PR, CR, or ICR program. As such, we believe they should be conducted by one or more members of the multidisciplinary team of the PR, CR, or ICR program with the appropriate expertise.
While we have not defined PR, CR, or ICR services as always including therapists' services as part of the comprehensive benefit (74 FR 33608 and 33614), we acknowledged that written treatment plans are highly individualized and that there should be flexibility in the type, amount, frequency, and duration of services provided in each session (74 FR 33607). We expect that physical therapists could conduct assessments and individualized treatments as part of the PR, CR, or ICR program because physical therapists have the knowledge and skills to assist in addressing common problems that lead to physicians ordering PR, CR, or ICR services for their patients, including poor aerobic capacity, poor endurance, and shortness of breath in the context of chronic pulmonary or cardiovascular disease. In the context of PR, while we also stated that individuals requiring PR services have a chronic respiratory disease and are in need of supervised aerobic exercise, we acknowledged that patients require assessments to address individualized needs and the provision of a mix of services necessary to address those needs (74 FR 33613).
Patients in PR, CR, or ICR programs must receive the full complement of care as defined under these benefits, in accordance with their individualized treatment plan, including assessments and prescribed exercise. Additionally, the standard HCPCS coding guidance instructs practitioners and providers to report the code for the procedure or service that most accurately describes the service performed. As stated in Section 20.12.1.b. of Chapter 5 of the Medicare Contractor Beneficiary and Provider Communications Manual, in instances where several component services, which have different CPT/HCPCS codes, may be described in one more comprehensive code, only the single code most accurately describing the procedure performed or service rendered should be reported. Therefore, we would expect that when therapists provide these evaluations and individualized treatment services under a comprehensive PR, CR, or ICR treatment plan, these services would be billed by the hospital as PR, CR, or ICR services under the comprehensive PR, CR, or ICR CPT codes or Level II HCPCS G-codes that apply, and not as physical therapy services. Furthermore, as discussed in section XII.B.4. of this final rule with comment period, for purposes of PR, CR, and ICR services, direct supervision must be provided by a doctor of medicine or osteopathy as defined in section 1861(r)(1) of the Act. This direct supervision rule would also apply to services furnished by therapists on the multidisciplinary team, and these services would be paid to the hospital as PR, CR, or ICR services.
We expect that most patients who meet the diagnosis requirements for coverage of PR, CR, or ICR would receive component services covered under the PR, CR, or ICR benefit as part of a comprehensive PR, CR, or ICR program, subject to the coverage and payment policies that we are finalizing in this CY 2010 OPPS/ASC final rule with comment period and the CY 2010 MPFS final rule with comment period. We understand that some component services of PR, CR, or ICR have previously been furnished to beneficiaries and paid by Medicare under other benefits, such as the outpatient physical therapy benefit. Because section 144(a)(1) of Public Law 100–275 authorized a new comprehensive PR benefit and codified specific benefits for CR and ICR, we believe that hospitals should furnish the full scope of the PR, CR, or ICR benefit, where services will be paid as hospital outpatient services under the OPPS, as comprehensive programs to those patients who qualify for coverage. We would not expect the component services of PR, CR, and ICR programs to be unbundled and billed separately by different providers or practitioners under other benefit categories, such as the physical therapy benefit where services would be paid under the MPFS. Therefore, we expect that it would be uncommon for a patient receiving care under a PR, CR, or ICR treatment plan to also be receiving physical therapy services under a separate physical therapy plan of care. There may be patients with therapy needs that are outside the treatment plan appropriate for PR, CR, or ICR, and such patients should receive medically necessary physical therapy services specific to those other needs. However, we would not expect this to be the norm. Clearly, a single period of care can only be billed as one type of treatment service, so hospitals could never bill both physical therapy and PR, CR, or ICR services for the same time period for the same patient (for example, an hour session from 10 a.m. to 11 a.m. on a single date of service).
We plan to monitor claims data for PR, CR, and ICR services, as well as any additional claims for therapy services. If we detect patterns of care that are inconsistent with our stated expectations for PR, CR, or ICR services and therapy services, we may encourage Medicare contractors to review cases in which a hospital reports both types of services for the same patient during the same span of time (for example, over a several month period) or we may propose changes to our payment methodologies for these services.
After considering the public comments we received, we are clarifying that we would expect component services that are furnished under a PR, CR, or ICR treatment plan to beneficiaries who qualify for PR, CR, or ICR services to be furnished as PR, CR, or ICR services, regardless of whether they are furnished by a physical therapist or other healthcare practitioner, and that all of the coverage and payment requirements for hospital outpatient services, including, but not limited to, the physician supervision requirements for hospital outpatient therapeutic services, apply to those PR, CR, or ICR services. We refer readers to section XII.D.3. of this final rule with comment period for a discussion of the final CY 2010 policies for the direct supervision of hospital outpatient therapeutic services. We expect that hospitals will furnish the comprehensive set of services that is contemplated in the criteria for PR, CR, or ICR programs to beneficiaries who qualify for the benefit.
2. Payments for Services Furnished to Hospital Outpatients in a Pulmonary Rehabilitation Program
In the CY 2010 OPPS/ASC proposed rule (74 FR 35360), we proposed to create for CY 2010 one new Level II HCPCS code for hospitals to report and bill for the services furnished under a PR program as specified in section 1861(fff) of the Act. Specifically, we Start Printed Page 60568 proposed to use HCPCS code GXX30 (Pulmonary rehabilitation, including aerobic exercise (includes monitoring), per session, per day). This proposed new HCPCS G-code would be used by hospitals to report PR services furnished to patients performing physician-prescribed exercises that are targeted to improving the patient's physical functioning and may also include the provision of other aspects of PR, such as education and training. Consistent with our proposal in the CY 2010 MPFS proposed rule, we proposed that hospitals would use HCPCS code GXX30 to report sessions lasting a minimum of 60 minutes each, generally for two to three sessions of PR per week, under the OPPS. We also proposed to allow no more than one session per day because individuals who are furnished services in a PR program have significant respiratory compromise and would not typically be capable of performing more than one session of exercise per day.
We proposed that PR described by HCPCS code GXX30 would be a new comprehensive service. We did not believe there was an existing clinical APC to which this service could be appropriately assigned under the OPPS based on the information currently available to us. We did not believe that any services currently paid under the OPPS were sufficiently similar to PR, based on both clinical and resource characteristics, to justify the initial assignment of HCPCS code GXX30 to the same clinical APC as an existing service. Historically, individual component services that comprise comprehensive PR have been reported separately with existing HCPCS codes that are paid under the OPPS through the individual APC that is most appropriate for each service described by the specific HCPCS code reported.
For payment under the MPFS, we proposed relative value units for new HCPCS code GXX30 for CY 2010 based on the estimated resources and work intensity associated with existing CR and respiratory therapy services. The nonfacility practice expense amount is the component of the MPFS payment that is most comparable to what Medicare pays under the OPPS. Both the MPFS nonfacility practice expense payment and the OPPS payment include payment for the service costs other than the physician professional services that are billed and paid under the MPFS in all service settings. The CY 2010 proposed nonfacility practice expense payment amount under the MPFS was between $10 and $20.
For the CY 2010 OPPS, we proposed to assign HCPCS code GXX30 to New Technology APC 1492 (New Technology—Level IB ($10-$20)), the New Technology APC that provides payment for new services with estimated facility costs between $10 and $20, because we believed that we lacked appropriate hospital cost data from claims to guide the initial assignment of the new HCPCS code that would describe services furnished under the new PR benefit. The New Technology APC payment of $15, at the midpoint of the cost band, would be approximately the same as the proposed CY 2010 MPFS nonfacility practice expense amount for PR services described by HCPCS code GXX30. As discussed above, this is the portion of the proposed MPFS payment that is most comparable to what Medicare would pay under the OPPS. We believed that this proposed temporary assignment to a New Technology APC would allow us to pay appropriately for the service under the OPPS at a rate that is similar to the corresponding physician's office payment amount, while we gathered hospital claims data and experience with the new service on which to base a clinically relevant APC assignment in the future.
Comment: Many commenters claimed that 90 percent of PR services are furnished in HOPDs and that CMS is currently paying considerably more for these services than the proposed payment. The commenters indicated the PR services are commonly furnished in the group setting in the HOPD, although they added that patients commonly require significant one-on-one assistance from hospital staff to encourage and facilitate their full participation in supervised exercise. The commenters objected to the proposal to pay $15 per session under New Technology APC 1492 because they believed that it would reduce payment for PR services to such an extent that hospitals would no longer be able to afford to furnish the services and that access to care would be diminished, rather than expanded as the law intended. Specifically, the commenters were concerned about the proposed payment rate because PR services would be reported under a new HCPCS G-code that would include all component services and assessments furnished in a single session per day with a minimum session duration of 60 minutes and a maximum of 1 session per day. The commenters estimated that the proposed payment for PR services would result in less than 25 percent of the current payment to hospitals for the same component services that are reported under existing HCPCS codes.
The commenters opposed the proposed payment of PR services under a New Technology APC because they believe that CMS has considerable experience with payment for these services under HCPCS codes G0237, G0238, or G0239, which are currently assigned to APC 0077 (Level I Pulmonary Treatment) under the OPPS. They argued that these three existing HCPCS G-codes are now being reported for PR services in HOPDs and that OPPS payment should be made for comprehensive PR services as authorized by section 144(a)(1) of Public Law 100–275 based on the historical payments made for services reported using these codes. They stated that the OPPS currently pays approximately $27 per unit for HCPCS codes G0237, G0238 and G0239 and that, therefore, the proposed payment for PR services would be much less than hospitals are currently being paid for the same services and would seriously erode the quality and quantity of care currently being furnished. The commenters indicated that PR services require considerable hospital staff and overhead costs that include, but are not limited to, the costs of respiratory therapists and the purchase and maintenance of a wide range of expensive exercise equipment, such as oximeters with printers, one channel ECG monitors, dedicated emergency cart/resuscitation equipment, and portable oxygen equipment. They reasoned that payment at $15 per session of one hour's duration would be insufficient to permit hospital PR programs to continue to function because the current sessions are at least 1 hour (and typically 2 hours) long and, therefore, OPPS payment for 1 hour of service reported using HCPCS codes G0237 and G0238 is currently about $100 per hour. Further, they noted that the OPPS pays separately for all assessments and tests under the current policy. They identified the assessment services that are currently paid separately but for which payment would be included in the per session payment for PR services according to the proposal as including, but not limited to, CPT codes 94620 (Pulmonary stress testing; simple (e.g., 6-minute walk test, prolonged exercise test for bronchospasm with pre- and post-spirometry and oximetry)); 94664 (Demonstration and/or evaluation of patient utilization of an aerosol generator, nebulizer, metered dose inhaler or IPPB device); and 94667 (Manipulation chest wall, such as cupping, percussion and vibration to facilitate lung function; initial demonstration and/or evaluation). One Start Printed Page 60569 commenter requested that CMS create two new Level II HCPCS codes for PR sessions, one including the assessment services and one excluding the assessment services.
Some commenters claimed that it is not appropriate to compare the costs of CR to PR services because the overhead costs for PR services are higher than for CR services. They also indicated that it is contradictory for CMS to require that lung volume reduction surgery patients receive no less than 2 hours of PR services per day but to limit coverage of PR for moderate to severe COPD patients to 1 session per day.
The commenters suggested three alternatives to the proposed payment for a single PR HCPCS code through a New Technology APC. One alternative would be to continue to permit hospitals to bill HCPCS codes G0237, G0238, and G0239 for PR services and also to bill separately for the assessment services proposed to be included in the single comprehensive PR HCPCS code (with a separate visit payment for the initial physician or hospital staff assessment of the patient that commenters claimed requires 60 to 90 minutes of professional time). Payment for HCPCS codes G0237, G0238, and G0239 would continue to be made through APC 0077 and the assessment services would be paid through their existing clinical APCs as well. Some commenters suggested a variation of this alternative that would establish an APC with a payment rate of $50 to which HCPCS codes G0237, G0238, and G0239, as currently defined, would be assigned. As a second alternative, the commenters suggested that CMS assign the new comprehensive PR HCPCS code to APC 0078 (Level II Pulmonary Treatment), which had a proposed payment rate of approximately $96, and not allow separate reporting and payment for CPT codes 94620, 94664, and 94667 and the 60 to 90 minute intake visit. Finally, the commenters stated that CMS could establish a payment rate for the new comprehensive PR HCPCS code by multiplying the OPPS payment rate for APC 0077 by 4 because the cost of the new per hour PR code would be at least 4 times the cost of HCPCS codes G0237 and G0238 that describe 15 minutes of care and are assigned to APC 0077. The commenters believed that any of these alternatives would result in a PR payment to hospitals that is appropriate for the cost of the services being furnished.
Response: We examined our hospital and physician claims data for HCPCS codes G0237, G0238, and G0239, and we agree with the commenters that most services for restoration of pulmonary function have historically been furnished in hospitals and that there is considerable evidence of hospital outpatient utilization and cost in our claims data. We found that, in CY 2008, Medicare paid approximately $20 million in aggregate to about 900 hospitals for services reported under HCPCS codes G0237, G0238, and G0239, which describe pulmonary therapy services that commenters believe reflect the costs of the same types of hospital staff, supplies, and overhead that would be used to furnish PR services.
Therefore, for the CY 2010 OPPS, we will pay for PR services in HOPDs under the OPPS through a new clinical APC with a median “per session” cost simulated from historical hospital claims data for similar pulmonary therapy services, rather than assigning the new PR HCPCS G-code to a New Technology APC as we proposed. We have previously used a simulation approach to develop a median cost estimate for a single new CPT or Level II HCPCS code that would previously have been reported under several existing HCPCS codes when furnished in the HOPD, most recently for echocardiography services as discussed in detail in section II.A.2.d.(4) of this final rule with comment period. Specifically, we are assigning the comprehensive Level II HCPCS code G0424 (Pulmonary rehabilitation, including exercise (includes monitoring), per hour, per session) to new clinical APC 0102 (Level II Pulmonary Treatment), with payment based on the aggregate per day simulated “per session” hospital median cost of approximately $50 as calculated from claims data for the existing pulmonary therapy HCPCSG-codes and associated assessments and tests. No other HCPCS codes are assigned to APC 0102 for CY 2010. (APC 0078 has been renamed Level III Pulmonary Treatment without any change in its configuration for CY 2010.) Our claims data show that, in most cases patients furnished the pulmonary therapy services reported by HCPCS codes G0237, G0238, and G0239 in the HOPD on a single date of service received some individual and some group services (approximately 2.4 units of service per day) and, less often, associated assessments and tests. We found approximately 19,000 days of care for patients who had diagnoses consistent with moderate to very severe COPD, consistent with the coverage requirements for PR in CY 2010, and that included both an individual and group service on the same day. The claims for these days of care, for which we calculated a “per session” median cost of approximately $50, were similar to the time duration and services that will be reported under new HCPCS code G0424 in CY 2010.
Specifically, to simulate the “per session” median cost of new HCPCS code G0424 from claims data for existing services, we used only claims that contained at least one unit of HCPCS code G0239, the group code that is without limitation on time duration, and one unit of HCPCS code G0237 or G0238, the individual, face-to-face codes that report 15 minutes of service, on the same date of service. We reasoned that patients in a PR program would typically receive individual and group services in each session of approximately 1 hour in duration. The approach was consistent with the public comments that suggested that PR is often provided in group sessions in the HOPD, although patients commonly require additional one-on-one care in order to fully participate in the program. We note that our use of “per session” claims reporting one unit of HCPCS code G0237 or G0238 and one unit of HCPCS code G0239 in this simulation methodology was also consistent with our overall finding of approximately 2.4 service units of the HCPCS G-codes per day on a single date of service, usually consisting of both individual and group services, for patients receiving pulmonary therapy services in the HOPD based upon CY 2008 claims. We concluded that the typical session of PR would be 1 hour based on public comments that indicated that a session of PR is typically 1 hour and based on our findings that the most commonly reported HCPCS code for pulmonary treatment is HCPCS code G0239, which has no time definition for this group service.
We included all costs of the related tests and assessment services (CPT codes 94620, 94664, and 94667 and all CPT codes for established patient clinic visits) on the same date of service as the HCPCS G-codes in the claims we used to simulate the median cost for HCPCS code G0424. After identifying these “per session” claims, which we believe to represent 1 hour of care, we summed the costs on them and calculated the median cost for the set of selected claims. In light of the cost and clinical similarities of PR and the existing services described by HCPCS codes G0237, G0238, and G0239 and the CPT codes for related assessments and tests, and the significant number of “per session” hospital claims we found, we are confident that the simulated median cost for HCPCS code G0424 is a valid Start Printed Page 60570 estimate of the expected hospital cost of a PR session. Since there is no existing clinical APC to which HCPCS code G0424 could be appropriately assigned based on considerations of the clinical and resource characteristics of PR services, we are creating new APC 0102 (Level II Pulmonary Treatment) for CY 2010. Existing APC 0078 (Level II Pulmonary Treatment) has been renamed “Level III Pulmonary Treatment” for CY 2010, with no change in its configuration.
We also are adding the phrase “per hour” to the new HCPCS code G0424 descriptor to conform the descriptor of the code to the basis for the payment being made for one unit of the code and to enable providers to determine when one session of PR ends and the second session begins. Because we are modifying our final policy to cover up to 2 sessions of PR per day in response to public comments that we should permit more than 1 session of PR on the same date, it became necessary to add a time to the definition of HCPCS code G0424 so that providers could determine when they could report a second session. Moreover, when we based the payment for new APC 0102 on the per session costs derived from the OPPS claims data for HCPCS codes G0237, G0238, and G0239, we assumed that a session represented 1 hour of care; and therefore, adding “per hour” to the descriptor for the new HCPCS G-code for PR conforms the HCPCS G-code to the payment we are establishing.
We do not agree with the commenters' suggestion that we permit hospitals to report and be paid for PR services under the existing HCPCS codes G0237, G0238, and G0239 and the CPT codes for assessments and tests because PR is a comprehensive program that consists of multiple component items and services as specified in the statute and discussed in the CY 2010 MPFS final rule with comment period. These three HCPCS G-codes were developed principally to describe services furnished by respiratory therapists in CORFs, and the services consist of therapeutic procedures to increase the strength and endurance of respiratory muscles or to improve respiratory function. When the HCPCS G-codes were established, we indicated that these were developed to provide more specific information about the services being delivered since those services were not previously clearly described by existing CPT codes (66 FR 55311). We also noted that there was no respiratory or pulmonary rehabilitation benefit (67 FR 79999).
The existing HCPCS G-codes do not represent the full scope of services in a comprehensive PR program as now authorized by section 144(a)(1) of Public Law 100–275. We want to ensure that when hospitals bill and are paid for PR services, they attest to meeting all requirements of the comprehensive PR program by the reporting of a HCPCS G-code specific to a PR session. As discussed above in the context of therapy services, patients in PR, CR, or ICR programs must receive the full complement of care defined under the benefit, including assessments and individualized treatments in accordance with their PR treatment plan. When hospitals furnish these evaluations and individualized treatment services under a PR treatment plan, we would not expect the component services of PR, CR, and ICR programs to be unbundled and billed separately. Instead, the services must be billed by the hospital as PR services under the new Level II HCPCS G-code for PR services, not the existing HCPCS codes G0237, G0238, and G0239 for respiratory treatment services, the CPT codes for the individual assessment services discussed above, or HCPCS codes for physical therapy or other services. Furthermore, we also expect that it would be uncommon for a patient receiving care under a PR treatment plan to also be receiving services under a separate plan of care to improve their respiratory strength and function, such as physical therapy or respiratory treatment. Only those patients whose medical needs for treatment to improve their respiratory strength and function are outside the treatment plan appropriate for PR should receive medically necessary services specific to those other needs. However, we would not expect this to be the norm.
As discussed above, a single period of care can only be billed as one type of treatment service, so hospitals could never bill services reported by HCPCS codes G0237, G0238, or G0239 and PR services for the same time period for the same patient (for example, an hour session from 10:00 a.m. to 11:00 a.m. on a single date of service). We plan to monitor claims data for PR services, as well as any additional claims for these other services. If we detect patterns of care that are inconsistent with our stated expectations for PR services, we may encourage Medicare contractors to review cases in which a hospital reports both types of services for the same patient during the same span of time (for example, over a period of several months) or we may propose changes to our payment methodologies for these services.
In addition, we note that a specific code for PR services allows us to administratively account for the limit on the number of covered sessions. Furthermore, we are not creating different HCPCS codes for PR sessions provided with and without assessments because we continue to believe PR is a comprehensive program and all sessions should be reported and paid through a single HCPCS G-code. We believe that it is most appropriate to pay for all of the assessments and tests that may be furnished in the program as required for coverage through our single per-session PR payment, and we took the hospital costs of these related services into consideration in establishing the CY 2010 OPPS payment rate for HCPCS code G0424. We also do not agree with the commenters' recommendation to assign the new PR per session HCPCS code to APC 0078 because the median cost of APC 0078 is almost twice the simulated “per session” median cost of HCPCS code G0424 that we calculated based on historical hospital claims data for existing, similar HCPCS codes through our “per session” methodology as described above. Finally, we do not agree with the commenters' suggestion to establish a payment rate for PR HCPCS code G0424 at the rate of 4 times the payment for APC 0077 because that also would pay for PR services at more than twice the simulated median cost for a typical session of PR as reported in our claims data.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, with modification, for OPPS payment for PR services furnished as a part of the comprehensive PR program benefit for CY 2010. We are adopting new HCPCS code G0424 (Pulmonary rehabilitation, including exercise (includes monitoring), per hour, per session) and are assigning the G-code to new APC 0102 (Level II Pulmonary Treatment), with a simulated “per-session” median cost of approximately $50. As discussed in the CY 2010 MPFS final rule with comment period, PR is covered for up to 36 one-hour sessions, with a maximum of 2 sessions per day, and with contractor discretion to approve up to 72 sessions.
3. Payment for Services Furnished to Hospital Outpatients Under a Cardiac Rehabilitation or an Intensive Cardiac Rehabilitation Program
Currently, CR services furnished by hospitals are reported using CPT codes 93797 and 93798. In the CY 2010 MPFS proposed rule (74 FR 33607), we proposed that each day CR items and services are furnished to a patient, aerobic exercises along with other exercises must be included (that is, a Start Printed Page 60571 patient must exercise aerobically every day he or she attends a CR session). In addition, we proposed that each session must be a minimum of 60 minutes and patients must participate in a minimum of two CR sessions a week, with a maximum of two CR sessions a day.
With respect to ICR services, section 1861(eee)(4)(C) of the Act, states that “an intensive cardiac rehabilitation program may be provided in a series of 72 one-hour sessions (as defined in section 1848(b)(5)), up to 6 sessions per day, over a period of up to 18 weeks.” In the CY 2010 OPPS/ASC proposed rule (74 FR 35361), for the CY 2010 OPPS, we proposed to create two new Level II HCPCS codes to report the services of an ICR program that are furnished to hospital outpatients, consistent with the provisions of section 1861(eee)(4)(C) of the Act: HCPCS code GXX28 (Intensive cardiac rehabilitation; with or without continuous ECG monitoring with exercise, per session) and HCPCS code GXX29 (Intensive cardiac rehabilitation; with or without continuous ECG monitoring; without exercise, per session). These proposed new HCPCS G-codes would be used to report ICR services furnished by hospitals that have an ICR program that has received a designation as a qualified ICR program. Consistent with the proposal in the CY 2010 MPFS proposed rule, we proposed that each session of ICR must be a minimum of 60 minutes and that each day ICR items and services are provided to a patient, aerobic exercises along with other exercises must be included (that is, a patient must exercise aerobically every day he or she attends a ICR session).
For the CY 2010 OPPS, we proposed to assign HCPCS codes GXX28 and GXX29 to APC 0095 (Cardiac Rehabilitation) with a status indicator of “S.” The proposed median cost of APC 0095 for CY 2010 was approximately $39. This proposed median cost reflected historical hospital cost data for one session of general CR services reported with CPT code 93797 or 93798. Both CR and ICR programs consist of exercise, cardiac risk factor modification, psychosocial assessment, outcomes assessment, and other services, as described in the CY 2010 MPFS proposed rule (74 CR 33607). Although more sessions per day for a beneficiary may be provided in an ICR program than a CR program, in the CY 2010 OPPS/ASC proposed rule (74 FR 35361) we noted our belief the hospital costs for a single session would be similar, and OPPS payment for CR and ICR services would be provided on a per session basis. Therefore, because CR and ICR services are similar from both clinical and resource perspectives, we believed that it would be appropriate to assign the two proposed new Level II HCPCS codes for ICR services to APC 0095 while we collect cost information from hospitals specific to ICR. We proposed to make a single payment through APC 0095 for each session of ICR reported on hospital outpatient claims.
Comment: Some commenters supported the proposal to create two new HCPCS G-codes for ICR services and to pay them at the same rate as CR services through APC 0095. Other commenters objected to setting the payment for ICR services at the same level as CR services on the basis that ICR services should be more costly than CR services because of their intensive nature. Some commenters were concerned about basing payment for CR on historical hospital costs because these costs do not include the new level of physician and clinical staff work required for coverage of CR and ICR, in particular, the psychosocial and outcome assessments that are required by section 144(a)(1) of Public Law 100–275. Under CMS' proposal, these assessments would not be paid separately but, instead, would be paid through payment for the per session CR CPT codes or ICR HCPCS codes.
Some commenters believed the proposed median cost for APC 0095 was too low on the basis that the July 2008 RTI final report for CMS entitled “Refining Cost to Charge Ratios for Calculating APC and DRG Relative Payment Weights” indicated that the current payment calculations for CR are significantly flawed because hospitals misclassify CR costs and that, therefore, the resulting APC 0095 median cost understates the cost of CR. The commenters indicated that the study showed that the cost of CR is approximately $100 per session when an appropriate CCR, based on the costs and charges for CR and not on the application of a hospital-specific overall ancillary CCR, is applied to the charges for CR services. The commenters were concerned about CMS' stated delay in implementing a specific nonstandard cost center for CR until 2011 because the true cost of CR would not be captured by the OPPS for ratesetting until CY 2013 or later. The commenters encouraged CMS to correct the underpayment of CR services for CY 2010 in order to ensure the availability of CR programs for Medicare beneficiaries.
Response: We have no reason to believe that ICR services as defined in the coverage criteria are more costly to furnish per session than CR services. We note that one of the major differences between CR and ICR services is that ICR may be furnished in up to six sessions per day, in comparison with the two sessions per day that are covered for CR. In the case of a Medicare beneficiary who receives six ICR sessions in one day, payment also would be six times the payment for one session and, therefore, the total hospital payment would appropriately pay for the additional costs of more services in a day.
With regard to the comment that our claims data do not reflect the costs of the additional assessments that are now required for CR and ICR coverage, we analyzed the per session cost of CR in our historical hospital claims data and incorporated the costs of hospital outpatient visits that we believe would have been previously reported for assessments furnished on the same day to CR patients into our estimate of the median cost for APC 0095. We found that the APC median cost was essentially unchanged. We believe that psychosocial and outcomes assessments are already a part of high quality CR/ICR programs and that our per session payment for CR and ICR services through APC 0095 will appropriately provide payment for all required components of CR and ICR, including the necessary assessments.
The median cost on which the CY 2010 payment for APC 0095 is based is approximately $38, which is an increase over the median cost of approximately $36 on which the CY 2009 payment is based. As we explained in the CY 2009 OPPS/ASC final rule with comment period (74 FR 68525), we recognize that there are areas of concern with the cost report that are integral to our estimation of hospital costs for OPPS ratesetting and we are taking steps to address some of them, including adopting a nonstandard cost center for CR. This cost center will be available for use in cost reporting periods beginning on or after February 2010. We refer readers to section II.A.1.c.(2) of this final rule with comment for further discussion of the creation of this new cost center. However, as we have previously explained (74 FR 68522), modifying the cost report data from its submitted form for use in OPPS ratesetting based upon assumptions about the data typically would be contrary to our principle of using the data as submitted by hospitals. Therefore, we are not making changes or adjustments to our OPPS cost estimation for CR services for CY 2010 but, instead, will await more accurate information for future ratesetting that may be submitted to us by hospitals when the nonstandard cost center for CR services is available. Start Printed Page 60572 We currently have no reason to believe that Medicare beneficiaries have limited access to CR services or that our payment is inappropriate. We have over 2.5 million claims for CR sessions from CY 2008 claims data, and further note that the overall number of services and the number of providers furnishing CR have been stable over the past several years.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to continue to assign the CPT codes for CR, specifically CPT codes 93797 and 93798, to APC 0095. We also are finalizing our CY 2010 proposal to assign the new HCPCS G-codes for ICR, specifically G0422 (Intensive cardiac rehabilitation; with or without continuous ECG monitoring, with exercise, per hour, per session) and G0423 (Intensive cardiac rehabilitation; with or without continuous ECG monitoring, without exercise, per hour, per session) to APC 0095. We have added the phrase “per hour” to the descriptors of these codes because we expect that the OPPS cost data for CR services from the claims submitted for CPT codes 93978 and 93979 generally reflect 1 hour of CR services, in accordance with our reporting instructions for more than one session per day of CR services in Section 200.1 of Chapter 4 of the Medicare Claims Processing Manual. We believe that 1 hour of service is the standard for a session of CR. Section 1861(eee)(4)(C) of the Act provides for up to 72 one-hour sessions of ICR and hence, adding “per hour” to the two new HCPCS code descriptors for ICR services implements the statutory definition of an ICR session as being one hour of service. Moreover, we have established the payment for ICR services on the presumption that one session represents one hour of care. Therefore, we believe that it is appropriate to specify in the descriptors of the HCPCS codes for ICR services that one unit of the code represents one hour of care. The final APC median cost of APC 0095 is approximately $38. As discussed in the CY 2010 MPFS final rule with comment period, CR is covered for up to 36 one-hour sessions, with a minimum of 1 session per week and a maximum of 2 sessions per day, and Medicare contractors have the authority to approve additional sessions, up to 72 sessions, over an additional period of time. With respect to ICR, section 144(a)(1) of Public Law 100–275 authorizes coverage of ICR programs in a series of 72 one-hour sessions, up to 6 sessions per day, over a period of 18 weeks.
We also note that as discussed in section II.G.9. of the CY 2010 MPFS final rule with comment period, we are requiring that all ICR programs be approved through the NCD process. Once we have approved an ICR program or programs through the NCD process, individual sites wishing to furnish ICR items and services via an approved ICR program may enroll with their local Medicare contractor to become an ICR program supplier as outlined in § 424.510. This enrollment is designed to ensure that the specific sites meet the specific statutory and regulatory requirements to furnish these services and will provide a mechanism to appeal a disapproval of a prospective ICR program site. With regards to billing and payment for CR and ICR services, hospital providers will continue to use their CMS Certification Number (CCN or provider number) and appeals related to the payment of claims will follow those established processes. CMS will provide further instructions for the NCD and individual site enrollment processes.
4. Physician Supervision for Pulmonary Rehabilitation, Cardiac Rehabilitation, and Intensive Cardiac Rehabilitation Services
Section 144 of Public Law 110–275 includes requirements for immediate and ongoing physician availability and accessibility for both medical consultations and medical emergencies at all times items and services are being furnished under CR, ICR, and PR programs. In section II.G.8. of the CY 2010 MPFS proposed rule (74 FR 33606), we proposed that these requirements would be met through existing definitions for direct physician supervision in physicians' offices and hospital outpatient departments at § 410.26(a)(2) (defined through cross reference to § 410.32(b)(3)(ii)) and § 410.27, respectively). We noted that direct supervision, as defined in the regulations, is consistent with the requirements of Public Law 110–275 because the physician must be present and immediately available where the services are being furnished. The physician must also be able to furnish assistance and direction throughout the performance of the services, which would include medical consultations and medical emergencies.
For CR, ICR, and PR services provided to hospital outpatients, direct physician supervision is the standard set forth in the April 7, 2000 OPPS final rule with comment period (68 FR 18524 through 18526) for supervision of hospital outpatient therapeutic services covered and paid by Medicare in hospitals and provider-based departments of hospitals. We noted in the discussions of CR and PR in the CY 2010 MPFS proposed rule (74 FR 33609 and 33614) that if we were to propose future changes to the physician office or hospital outpatient policies for direct physician supervision, we would provide our assessment of the implications of those proposals for the supervision of CR and PR services at that time.
As discussed in more detail in the CY 2010 OPPS/ASC proposed rule (74 FR 35362), we proposed to refine the definition of the direct supervision of hospital outpatient therapeutic services for those services provided in the hospital and in an on-campus PBD of the hospital. For services, including CR, ICR, and PR services, provided in the hospital and in an on-campus PBD of the hospital, direct supervision would mean that the physician must be present on the same campus, in the hospital or the on-campus PBD of the hospital, as defined in § 413.65, and immediately available to furnish assistance and direction throughout the performance of the procedure. We also proposed to define “in the hospital” in proposed new paragraph § 410.27(g) to mean areas in the main building(s) of the hospital that are under the ownership, financial, and administrative control of the hospital; are operated as part of the hospital; and for which the hospital bills the services furnished under the hospital's CCN. We did not propose significant change to the definition or requirements for direct supervision of hospital outpatient therapeutic services provided in off-campus PBDs of a hospital. Thus, with respect to CR, ICR, and PR services furnished in off-campus PBDs of the hospital, direct supervision would continue to mean that the physician must be in the off-campus PBD and immediately available to furnish assistance and direction throughout the performance of the procedure. We believe that direct supervision, as defined in the proposed regulations for hospital outpatient therapeutic services, continues to be consistent with the requirements of Pub. L. 110–275 for CR, ICR, and PR services because the physician must be present and immediately available where the services are being furnished. The physician must also be able to furnish assistance and direction throughout the performance of the services, which would include medical consultations and medical emergencies. For a complete discussion of the current and proposed requirements for the direct Start Printed Page 60573 supervision of hospital outpatient therapeutic services, we refer readers to section XII.D. of the CY 2010 OPPS/ASC proposed rule (74 FR 35362 through 35368).
Section 144 of Public Law 110–275 also states that in the case of items and services furnished under such a CR, ICR, or PR program in a hospital, physician availability shall be presumed. As we have stated in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68702 through 68704), the longstanding presumption of direct physician supervision for hospital outpatient services means that direct physician supervision is the standard for supervision of hospital outpatient therapeutic services covered and paid by Medicare in hospitals and PBDs of hospitals, and we expect that hospitals are providing services in accordance with this standard.
We note that in the CY 2010 OPPS/ASC proposed rule (74 FR 35362), we also proposed that nonphysician practitioners, defined for the purpose of proposed revised § 410.27 of the regulations as clinical psychologists, physician assistants, nurse practitioners, clinical nurse specialists, and certified nurse-midwives, may directly supervise all hospital outpatient therapeutic services that they may perform themselves within their State scope of practice and hospital-granted privileges, provided that they meet all additional requirements, including any collaboration or supervision requirements as specified in §§ 410.71, 410.74, 410.75, 410.76, and 410.77. However, in the CY 2010 MPFS proposed rule and in the corresponding proposed regulation text (74 FR 33674 and 33675, respectively), we proposed a different requirement for the direct supervision of CR, ICR, and PR services. We proposed that services provided in CR, ICR, and PR programs must be supervised by a doctor of medicine or osteopathy, as defined in section 1861(r)(1) of the Act. In addition, we proposed specific requirements for the expertise and licensure of physicians supervising CR and ICR services. It would not be in accordance with the proposed regulations for a nonphysician practitioner to supervise services furnished in a CR, ICR, or PR program. The physician supervision and expertise requirements proposed in the coverage policy and regulations for CR, ICR, and PR services must be met for those services to be covered and, therefore, paid by Medicare in hospital outpatient settings.
Comment: One commenter supported the proposed requirements for physician supervision of CR programs and requested that CMS confirm that the proposed definition of “direct supervision” that would apply to therapeutic services in the HOPD would also apply to CR services. Many commenters objected to the requirement that a physician must be present when PR or CR/ICR services are furnished. They indicated that the law presumes that physician supervision exists in hospitals and that, therefore, the same rules that apply to other hospital outpatient therapeutic services provided in hospitals should apply to PR, CR, and ICR services. The commenters also asked that CMS permit physician assistants, nurse practitioners, clinical nurse specialists, and certified nurse midwives who are functioning within their State licensure and scope of practice and who are permitted to supervise the services under the hospital bylaws to supervise PR, CR, and ICR services. The commenters expressed concern that the CY 2010 proposals in the MPFS proposed rule did not include a justification for why it would be necessary to impose physician-only direct supervision for PR, CR, and ICR services in HOPDs than for other outpatient services. Some commenters explained that rural providers have great difficulty securing physician services and rely heavily on nonphysician practitioners to furnish care in hospitals. They argued that to require physician-only supervision would mean that some PR, CR, and ICR programs in rural areas would have to close for lack of physician supervision and that there would be no access to the PR, CR, and ICR services for beneficiaries in those communities.
Response: We understand the reasoning of the commenters that PR, CR, and ICR services should require direct supervision by physicians and certain nonphysician practitioners, as we proposed for other hospital outpatient therapeutic services, given that PR, CR and ICR services are similar to other hospital outpatient therapeutic services. However, we are unable to revise the regulations to permit nonphysician practitioners to supervise PR, CR, and ICR services. We do not believe that the law provides the flexibility for us to permit anyone other than a physician to supervise hospital outpatient PR, CR, and ICR services because nonphysician practitioners are not physicians as defined in section 1861(r)(1) of the Act. The statutory language of section 144(a)(1) of Public Law 100–275 defines PR, CR and ICR programs as “physician-supervised.” More specifically, it establishes in section 1861(eee)(2)(B) of the Act that for PR, CR and ICR programs, “a physician is immediately available and accessible for medical consultation and medical emergencies at all times items and services are being furnished under the program, except that, in the case of items and service furnished under such a program in a hospital, such availability shall be presumed * * *.” The text of the statute uses the word “physician” and does not include nonphysician practitioners. Also, as we explained in the CY 2009 OPPS/ASC proposed rule and final rule with comment period (73 FR 41518 through 41519 and 73 FR 68702 through 68704, referencing the April 7, 2000 OPPS final rule (65 FR 18525)), the “presumption” or “assumption” of direct supervision means that direct physician supervision is the standard for all hospital outpatient therapeutic services. We have assumed this requirement is met on hospital premises (meaning we have expected that hospitals are meeting this requirement) because staff physicians would always be nearby in the hospital. In other words, the requirement is not negated by a presumption that the requirement is being met. Hence, unlike the standards for the direct supervision of other hospital outpatient therapeutic services, which we have established through regulation based on section 1861(s) of the Act, in the case of PR, CR, and ICR services, the authorizing provision of the Act at section 1861(eee)(2)(B) explicitly requires direct physician supervision of these services. While we have some flexibility to determine the type of practitioner who may supervise other hospital outpatient therapeutic services, as discussed in XII.D.3. of this final rule with comment period, in the case of PR, CR, and ICR services specifically, the statutory language of section 144(a)(1) of Public Law 100–275 does not provide such flexibility. Instead, the statute imposes strict requirements, describing the direct physician supervision standard for PR, CR, and ICR services, and gives us no flexibility to modify the requirement to allow for other supervisory practitioners.
After consideration of the public comments we received, and in accordance with the final policies set forth in sections II.G.8. and II.G.9. of the CY 2010 MPFS final rule with comment period, we are finalizing our CY 2010 proposal, without modification, to require the direct physician supervision (by a doctor of medicine or doctor of osteopathy) of PR, CR, and ICR services that are furnished to hospital outpatients. We note that we define “direct supervision” with regard to Start Printed Page 60574 what it means to be immediately available and accessible for medical consultation and medical emergencies in the same manner for PR, CR, and ICR programs as we do for other therapeutic services furnished in HOPDs. The final CY 2010 definitions of direct supervision for hospital outpatient therapeutic services provided on the campus and in off-campus provider-based departments also apply. These definitions are discussed in detail in section XII.D.3. of this final rule with comment period, including the new and revised regulations.
C. Stem Cell Transplant
Stem cell transplantation is a treatment in which stem cells that are harvested from either a patient's or a donor's bone marrow or peripheral blood are later infused into that patient to treat an illness. Autologous stem cell transplantation is a technique for providing additional stem cells using the patient's own previously harvested stem cells. Allogeneic stem cell transplantation is a procedure in which stem cells from a healthy donor are acquired and prepared to provide a patient with new stem cells.
We recently revised section 90.3.3 of Chapter 3 of the Medicare Claims Processing Manual (Pub. 100–04) and created new section 231.10 of Chapter 4 of the Medicare Claims Processing Manual in order to clarify billing under Medicare for autologous and allogeneic stem cell transplant services. As stated in the cited new and revised manual sections, autologous stem cell transplants performed on Medicare beneficiaries may be provided on an inpatient or an outpatient basis. Hospitals are instructed to bill and show all charges for autologous stem cell harvesting, processing, and transplant procedures based on the status of the patient (that is, inpatient or outpatient) when the individual services are furnished. The CPT codes describing these services may be billed and are separately payable under the OPPS when the services are provided in the hospital outpatient setting.
In contrast, we stated in the CY 2010 OPPS/ASC proposed rule (74 FR 35362) that we believe allogeneic stem cell transplants performed on Medicare beneficiaries are provided on an inpatient basis only, and all services related to acquiring the stem cells from a donor (whether performed on an inpatient or outpatient basis) are billed and are payable under Medicare Part A through the IPPS MS–DRG payment for the stem cell transplant. In addition to payment for the stem cell transplant procedure itself, the MS–DRG payment for the stem cell transplant includes payment for stem cell acquisition services, which include, but are not limited to, National Marrow Donor Program fees for stem cells from an unrelated donor (if applicable); tissue typing of a donor and a recipient; donor evaluation; physician pre-admission/pre-procedure donor evaluation services; costs associated with the harvesting procedure; post-operative/post-procedure evaluation of a donor; and preparation and processing of stem cells. While certain acquisition services, such as donor harvesting procedures, may be performed in the hospital outpatient setting, hospitals are instructed to include the charges for these services in the recipient's inpatient transplant bill as acquisition services and not to bill them under the OPPS.
In order to be consistent with the revised Section 90.3.3 and the new Section 231.10 of the Medicare Claims Processing Manual cited earlier, which reflect what we believed at the time to be the current clinical practice of performing allogeneic stem cell transplants on Medicare beneficiaries on an inpatient basis only, in the CY 2010 OPPS/ASC proposed rule (74 FR 35362), we proposed to revise the status indicator assignments of certain stem cell transplant-related CPT codes under the OPPS. Specifically, we proposed to change the status indicator for CPT code 38205 (Blood-derived hematopoietic progenitor cell harvesting for transplantation, per collection; allogenic) from “S” to “E” for the CY 2010 OPPS to reflect that, while an allogeneic stem cell harvesting procedure performed on the donor may take place in the HOPD, payment for the service is made through the IPPS MS–DRG payment for the associated transplant procedure performed on the recipient. We also proposed to change the status indicators for CPT code 38240 (Bone marrow or blood-derived peripheral stem cell transplantation; allogenic) and CPT code 38242 (Bone marrow or blood-derived peripheral stem cell transplantation; allogeneic donor lymphocyte infusions) from “S” to “C” for the CY 2010 OPPS to reflect that these allogeneic stem cell transplant procedures are payable by Medicare as inpatient procedures only.
At its August 2009 meeting, the APC Panel heard from presenters who stated that reduced intensity conditioning (RIC) regimens have made outpatient allogeneic stem cell transplants feasible for some Medicare beneficiaries. The presenters stated that revising the status indicators for CPT codes 38205, 38240, and 38242 to make them nonpayable on outpatient claims would impede the current clinical practice of providing allogeneic stem cell transplant and harvesting procedures on an outpatient basis, which, according to public commenters on the proposed rule, would lower costs, provide greater patient comfort, optimize hospital resources, and decrease the risk of nosocomial infections. The APC Panel agreed with the presenters and recommended that CMS maintain the CY 2009 APC assignments and status indicators for CPT codes 38205 and 38242, which are both currently assigned to APC 0111 (Blood Product Exchange), and for CPT code 38240, which is currently assigned to APC 0112 (Apheresis and Stem Cell Procedures) for CY 2009.
Comment: Several commenters on the CY 2010 proposed rule disagreed with CMS' assertion that allogeneic stem cell transplants performed on Medicare beneficiaries are provided on an inpatient basis only. According to the commenters, allogeneic stem cell transplants are currently performed in both the inpatient and outpatient settings and are considered safe and clinically appropriate for many Medicare patients. The commenters argued that CMS' proposal would create strong financial incentives for hospitals to unnecessarily admit patients who could have otherwise been treated in the outpatient setting and requested that CMS maintain the current CY 2009 status indicators and APC assignments of CPT codes 38205, 38240, and 38242 for CY 2010. The commenters urged CMS to continue to pay for CPT code 38205 separately under the OPPS, regardless of where the transplant ultimately occurs, because CMS' policy of bundling payment for stem cell harvesting procedures into the payment for the transplant procedure is overly complicated and burdensome, particularly when the harvesting procedure is performed in a different hospital from the hospital where the transplant is performed. The commenters also requested that CMS revise its manual guidance to reflect that hospitals should report all allogeneic stem cell harvesting, processing, and transplant services and their associated charges based on the status of the patient (that is, inpatient or outpatient) when the individual services are furnished.
Some commenters summarized other billing and payment issues related to allogeneic stem cell transplants for which they are seeking further direction and policy development from CMS, such as how hospitals could be paid for search and procurement costs related to Start Printed Page 60575 allogeneic stem cell transplants that do not occur due to a change in the patient's health status and the potential creation of separate MS–DRGs for allogeneic and autologous stem cell transplant services. The commenters recognized that greater analysis of these complex and unique issues would be required before CMS could address them fully.
Response: We appreciate the information on current clinical practice for allogeneic stem cell transplants provided by commenters. Upon further review, we agree with the commenters and the APC Panel that the allogeneic stem cell transplant procedures described by CPT codes 38240 and 38242 can be safely and appropriately performed on some Medicare beneficiaries on an outpatient basis. Therefore, we are not adopting our CY 2010 proposal to change the status indicators for CPT codes 38240 and 38242 from “S” to “C” in order to indicate that Medicare would only pay for these procedures when furnished in the hospital inpatient setting. Rather, we are continuing to assign CPT code 38240 to APC 0112 and CPT code 38242 to APC 0111 for CY 2010 for purposes of hospital outpatient payment, and are maintaining the assignment of status indicator “S” to both CPT codes that describe these procedures. CPT codes 38240 and 38242 are assigned to these same APCs for CY 2009, and we believe the allogeneic stem cell transplant-related procedures they describe share the clinical and resource characteristics of other procedures assigned to those APCs for CY 2010.
We do not agree with the public commenters and the APC Panel that the allogeneic stem cell harvesting procedure described by CPT code 38205 should be separately payable with status indicator “S” under the OPPS for CY 2010 because hospitals may bill and receive payment only for services provided to the Medicare beneficiary who is the recipient of the stem cell transplant and whose illness is being treated with the stem cell transplant. We do not agree with the commenters that we should pay for allogeneic stem cell harvesting services separately because these services are not directly furnished to beneficiaries. Instead, we believe that it continues to be appropriate to pay for these services through payment for the associated stem cell transplant procedure. The hospital should report all allogeneic stem cell acquisition charges, including costs associated with the harvesting procedure, on the recipient's inpatient or outpatient transplant bill under revenue code 0819. Payment for the allogeneic stem cell harvesting procedure performed on the donor and reported under revenue code 0819 is packaged into the IPPS MS–DRG payment or the OPPS APC payment for the associated transplant procedure performed on the recipient, depending on whether the transplant procedure is performed in the inpatient setting or the outpatient setting. Therefore, we are modifying our CY 2010 proposal to change the status indicator for CPT code 38205 from “S” to “E” for the CY 2010 OPPS. Instead, we are assigning status indicator “B” to CPT code 38205 for CY 2010 to reflect that the code is not recognized by OPPS when submitted on an outpatient hospital Part B bill type. We will update section 90.3.3 of Chapter 3 and section 231.10 of Chapter 4 of the Medicare Claims Processing Manual to comport with these billing and payment changes for allogeneic stem cell transplants and related services as described in this section.
We appreciate the commenters' summaries of other billing and payment issues related to allogeneic stem cell transplants for which they sought further direction and policy development from CMS. While we consider these issues to be outside of the scope of the proposed rule, we will consider the commenters' suggestions as we explore this policy area more broadly in the future. We note that current guidance in Section 90.3.3 of Chapter 3 of the Medicare Claims Processing Manual instructs providers to include on the Medicare cost report any costs associated with acquisition services for allogeneic stem cell acquisition services in cases that do not result in transplant due to death of the intended recipient or other causes.
After consideration of the public comments we received, we are modifying our CY 2010 proposal for allogeneic stem cell transplant procedures. Specifically, for CY 2010, we are accepting the APC Panel's recommendation and continuing to assign CPT code 38240 to APC 0112 and CPT code 38242 to APC 0111, which is consistent with their CY 2009 assignments. The final APC median costs of APC 0112 and APC 0111 are approximately $2,225 and $798, respectively, for CY 2010. We are maintaining status indicator “S” for the procedures described by both of these CPT codes. In addition, we are not adopting the APC Panel's recommendation or the public commenters' suggestion to maintain the CY 2009 status indicator and APC assignment for CPT code 38205. Instead, we are assigning status indicator “B” to CPT code 38205 for CY 2010 to reflect that the code is not recognized by OPPS when submitted on an outpatient hospital Part B bill type because payment is made for allogeneic stem cell harvesting through payment for the recipient's transplant procedure, whether the transplant is provided in the hospital inpatient setting or the outpatient setting.
D. Physician Supervision
1. Background
In the CY 2009 OPPS/ASC proposed rule and final rule with comment period (73 FR 41518 through 41519 and 73 FR 68702 through 68704, respectively), we provided a restatement and clarification of the requirements for physician supervision of hospital outpatient diagnostic and therapeutic services that were set forth in the April 2000 OPPS final rule with comment period (65 FR 18524 through 18526). As we stated in those rules, section 1861(s)(2)(C) of the Act authorizes payment for diagnostic services that are furnished to a hospital outpatient for the purpose of diagnostic study. We have further defined the requirements for diagnostic services furnished to hospital outpatients, including requirements for physician supervision of diagnostic services, in §§ 410.28 and 410.32 of our regulations. Section 410.28(e) states that Medicare Part B makes payment for diagnostic services furnished at provider-based departments (PBDs) of hospitals “only when the diagnostic services are furnished under the appropriate level of physician supervision specified by CMS in accordance with the definitions in §§ 410.32(b)(3)(i), (b)(3)(ii), and (b)(3)(iii).” In addition, in the April 2000 OPPS final rule with comment period (65 FR 18526), we stated that our model for the requirement was the requirement for physician supervision of diagnostic tests payable under the MPFS that was set forth in the CY 1998 MPFS final rule (62 FR 59048). In 2000, we also explained with respect to the supervision requirements for individual diagnostic tests that we intended to instruct hospitals and fiscal intermediaries to use the MPFS as a guide pending issuance of updated requirements. For diagnostic services not listed in the MPFS, we stated that fiscal intermediaries, in consultation with their medical directors, would define appropriate supervision levels in order to determine whether claims for these services are reasonable and necessary. Since 2000, we have continued to follow the supervision requirements for individual diagnostic tests as listed in the MPFS Relative Value File. The file is updated quarterly and is available on the CMS Web site at: Start Printed Page 60576 http://www.cms.hhs.gov/PhysicianFeeSched/.
In the CY 2009 OPPS/ASC proposed rule and final rule with comment period (73 FR 41518 through 41519 and 73 FR 68702 through 68704, respectively), we also reiterated that direct physician supervision is the standard for physician supervision as set forth in the April 2000 OPPS final rule with comment period for supervision of hospital outpatient therapeutic services covered and paid by Medicare in hospitals and PBDs of hospitals. We noted that section 1861(s)(2)(B) of the Act authorizes payment for hospital services “incident to physicians' services rendered to outpatients.” We have further defined the supervision requirements for hospital outpatient therapeutic services and supplies “incident to” a physician's service in § 410.27 of our regulations. More specifically, § 410.27(f) states: “Services furnished at a department of a provider, as defined in § 413.65(a)(2) of this subchapter, that has provider-based status in relation to a hospital under § 413.65 of this subchapter, must be under the direct supervision of a physician. `Direct supervision' means the physician must be present and on the premises of the location and immediately available to furnish assistance and direction throughout the performance of the procedure. It does not mean that the physician must be present in the room when the procedure is performed.” This language makes no distinction between on-campus and off-campus PBDs.
In the preamble of the April 2000 OPPS final rule with comment period (65 FR 18525), we further discussed the requirement for physician supervision and the finalization of the proposed regulation text. In that discussion, we stated that the language of § 410.27(f) “applies to services furnished at an entity that is located off the campus of a hospital that we designate as having provider-based status as a department of a hospital in accordance with § 413.65.” We also stated that, for services furnished in a department of a hospital that is located on the campus of a hospital, “we assume the direct supervision requirement to be met as we explain in Section 3112.4(a) of the Intermediary Manual.” We further stated that “we assume the physician supervision requirement is met on hospital premises because staff physicians would always be nearby within the hospital.”
In the CY 2009 OPPS/ASC proposed rule and final rule with comment period (73 FR 41518 through 41519 and 73 FR 68702 through 68704, respectively), we restated the existing physician supervision policy for hospital outpatient therapeutic services because we were concerned that some stakeholders may have misunderstood our use of the term “assume” in the April 2000 OPPS final rule with comment period, believing that our statement meant that we do not require any supervision in the hospital or in an on-campus PBD for hospital outpatient therapeutic services, or that we only require general supervision for those services. This is not the case. It has been our expectation that hospital outpatient therapeutic services are provided under the direct supervision of physicians in the hospital and in all PBDs of the hospital, specifically, both on-campus and off-campus departments of the hospital. The expectation that a physician would always be nearby predates the OPPS and is related to the statutory authority for payment of hospital outpatient services—that Medicare makes payment for hospital outpatient services “incident to” the services of physicians in the treatment of patients as described in section 1861(s)(2)(B) of the Act. Section 410.27(a)(1)(ii) of the regulations states that Medicare Part B pays for hospital services and supplies furnished incident to a physician service to outpatients if they are provided “as an integral though incidental part of a physician's services.” In addition, we have stated in Section 20 of Chapter 6 of the Medicare Benefit Policy Manual (Pub. 100–2) that hospitals provide two distinct types of services to outpatients: Services that are diagnostic in nature, and other services that aid the physician in the treatment of the patient. We further defined these therapeutic services and supplies in Section 20.5.1 of the Medicare Benefit Policy Manual, stating “therapeutic services and supplies which hospitals provide on an outpatient basis are those services and supplies (including the use of hospital facilities) which are incident to the services of physicians in the treatment of patients.” We also provide in Section 20.5.1 that services and supplies must be furnished on a physician's order and delivered under physician supervision. However, the manual indicates further that each occasion of a service by a nonphysician does not need to also be the occasion of the actual rendition of a personal professional service by the physician responsible for the care of the patient. Nevertheless, as stipulated in that same section of the manual “during any course of treatment rendered by auxiliary personnel, the physician must personally see the patient periodically and sufficiently often enough to assess the course of treatment and the patient's progress and, where necessary, to change the treatment regimen.”
The expectation that a physician would always be nearby within the hospital also dates back to a time when hospital inpatient services provided in a single hospital building represented the majority of hospital payments by Medicare. Since that time, advances in medical technology, changes in the patterns of health care delivery, and changes in the organizational structure of hospitals have led to the development of extensive hospital campuses, sometimes spanning several city blocks, as well as off-campus and satellite provider-based campuses at different locations. In the April 2000 OPPS final rule with comment period (65 FR 18525), we described the focus of the direct physician supervision requirement for off-campus PBDs. In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68703), we stated that we do expect direct physician supervision of all hospital outpatient therapeutic services, regardless of their on-campus or off-campus location, but that we would continue to emphasize the physician supervision requirement for off-campus PBDs. However, we also noted that if there were problems with outpatient care in a hospital or in an on-campus PBD where direct supervision was not in place (that is, the expectation of direct physician supervision was not met), we would consider that to be a quality concern. We noted that we want to ensure that payment is made for high quality hospital outpatient services provided to beneficiaries in a safe and effective manner and consistent with Medicare requirements.
Finally, we noted that the definition of direct supervision in § 410.27(f) for PBDs requires that the physician must be present and on the premises of the location and immediately available to furnish assistance and direction throughout the performance of the procedure. In the April 2000 OPPS final rule with comment period (65 FR 18525), we further distinguished “on the premises of the location” by stating “* * * a physician must be present on the premises of the entity accorded status as a department of the hospital and therefore, immediately available to furnish assistance and direction for as long as patients are being treated at the site.” We also stated that this characterization does not mean that the physician must be physically in the room where a procedure or service is furnished. We noted in the CY 2009 OPPS/ASC final rule with comment Start Printed Page 60577 period (73 FR 68703) that although we have not further defined the term “immediately available” for this specific context, the lack of timely physician response to a problem in the HOPD would represent a quality concern from our perspective that hospitals should consider in structuring their provision of services in ways that meet the direct physician supervision requirement for HOPD services.
In response to a comment requesting clarification, we also discussed in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68703 through 68704) that a nonphysician practitioner may not provide the physician supervision in a PBD, even if a nurse practitioner's or a physician assistant's professional service was being billed as a nurse practitioner or a physician assistant service and not a physician service. We noted that section 1861(r) of the Act defines a physician as follows: “[t]he term `physician', when used in connection with the performance of any function or action, means (1) a doctor of medicine or osteopathy legally authorized to practice medicine and surgery by the State in which he performs such function or action * * *; (2) a doctor of dental surgery or of dental medicine [legally authorized to practice in the State and acting within the scope of his license]; (3) a doctor of podiatric medicine [for certain purposes and to the extent authorized by the State]; (4) a doctor of optometry [for certain purposes and to the extent legally authorized by the State]; or (5) a chiropractor [for certain purposes and to the extent legally authorized by the State and consistent with the Secretary's standards].” In addition, we pointed out that the conditions of participation for hospitals under § 482.12(c)(1)(i) through (c)(1)(vi) of our regulations require that every Medicare hospital patient is under the care of a doctor of medicine or osteopathy, a doctor of dental surgery or dental medicine, a doctor of podiatric medicine, a doctor of optometry, a chiropractor, or a clinical psychologist; each practicing within the extent of the Act, the Federal regulations, and State law. Further, § 482.12(c)(4) of our regulations requires that a doctor of medicine or osteopathy must be responsible for the care of each Medicare patient with respect to any medical or psychiatric condition that is present on admission or develops during hospitalization and is not specifically within the scope of practice of one of the other practitioners listed in § 482.12(c)(1)(ii) through (c)(1)(vi).
Moreover, section 1861(s)(2)(B) of the Act authorizes payment for hospital services “incident to physicians' services rendered to outpatients.” We have further defined the requirements for hospital outpatient therapeutic services and supplies “incident to” a physician's service in § 410.27 of our regulations. Section 410.27(a)(1)(ii) describes payment for hospital outpatient services when they are “an integral though incidental part of a physician's services.” Also, § 410.27(f) requires that hospital outpatient services provided in PBDs must be under the direct supervision of a physician. We stated that the language of the statute and regulations does not include nonphysician practitioners other than clinical psychologists. Therefore, it would not be in accordance with the law and regulations for a nonphysician practitioner other than a clinical psychologist to be providing the physician supervision in a PBD, even if a nurse practitioner's or a physician assistant's professional service was being billed as a nurse practitioner or a physician assistant service and not a physician service.
2. Issues Regarding the Physician Supervision of Hospital Outpatient Services Raised by Hospitals and Other Stakeholders
Although we received a few public comments on the discussion of physician supervision in the CY 2009 OPPS/ASC proposed rule, since publication of the CY 2009 OPPS/ASC final rule with comment period on November 18, 2008, we have received many questions and concerns about the current policies from hospitals and other stakeholders, as we discussed in the CY 2010 OPPS/ASC proposed rule (74 FR 35364). Some stakeholders expressed appreciation for the CMS clarification, stating that the supervision policies were clear and represented needed safeguards for beneficiaries. On the other hand, we have received numerous questions about the application of the policies to hospital outpatient therapeutic services furnished in areas of the hospital that some stakeholders believe have not clearly been discussed, such as the application of direct supervision to hospital outpatient therapeutic services furnished within the main buildings of the hospital that may not be PBDs of the hospital. Some hospitals expressed difficulty in determining whether certain areas of their hospitals were considered provider-based. Other stakeholders cited the direct supervision policy as first articulated in 2000 as problematic because they believe that CMS failed to consider hospitals' current organizational structures. Some hospitals and other stakeholders inquired about a physician's qualifications for providing supervision or questioned whether physician supervision must be provided by a physician in a particular medical specialty. A number of stakeholders challenged the current policy that nonphysician practitioners cannot provide direct supervision for those hospital outpatient therapeutic services they may personally perform or that they may order to be provided by other hospital staff incident to the nonphysician practitioner's services. In addition, numerous stakeholders, especially rural hospitals, raised budgetary and patient access concerns related to ensuring adequate physician staffing, especially because nonphysician practitioners may not directly supervise the delivery of hospital outpatient therapeutic services. Furthermore, rural hospitals and CAHs raised concerns regarding the inconsistency of the direct supervision requirements for CAHs with other CAH policies, pointing out that the Medicare conditions of participation for CAHs allow nurse practitioners and physician assistants to be responsible for the care of Medicare patients in CAHs. Some stakeholders specifically questioned whether § 410.27(f) applied to CAHs because the term “CAH” is not in the heading of § 410.27, which currently reads “Outpatient hospital services and supplies incident to a physician service: Conditions.” Other stakeholders complained about the significant burden on hospitals to provide direct physician supervision because they believe there is no clear clinical need for such supervision, particularly a uniform level of supervision for all types of hospital outpatient therapeutic services. Some stakeholders challenged the applicability of the direct supervision requirements to specific types of hospital outpatient services, such as partial hospitalization or chemotherapy administration services.
Similar to the issues related to direct supervision of hospital outpatient therapeutic services raised by hospitals and other stakeholders, we have received questions since publication of the CY 2009 OPPS/ASC final rule with comment period regarding the application of physician supervision policies for hospital outpatient diagnostic services, especially with respect to services provided within the main buildings of the hospital that are not PBDs. In addition, some stakeholders have pointed out that there is no site-of-service requirement for hospital outpatient diagnostic services, and that, therefore, hospitals may send Start Printed Page 60578 patients to independent diagnostic testing facilities (IDTFs) or other entities to receive diagnostic services under arrangement. They added that although these facilities are not PBDs, the hospital would bill for these services as hospital outpatient services in accordance with the hospital bundling rules. Some of these stakeholders have asked what type of physician supervision is required for diagnostic services provided under arrangement.
A number of stakeholders urged CMS to withdraw or delay the physician supervision policies discussed in the CY 2009 OPPS/ASC final rule with comment period, arguing that this rule included policy changes rather than clarification and, therefore, sufficient opportunity for public notice and comment was not provided. Some further argued that CMS should suspend enforcement of these policies while CMS gathers additional public input and considered alternatives. These stakeholders suggested a variety of additional approaches to soliciting full feedback from the hospital and physician communities on the supervision policies and their impact, including holding an open door forum or town hall meeting and reopening the discussion during the CY 2010 OPPS rulemaking process.
As discussed in the CY 2010 OPPS/ASC proposed rule (74 FR 35365), we provided a restatement and clarification of existing policy in the CY 2009 OPPS/ASC proposed rule (73 FR 41518 through 41519), citing numerous existing statutory, regulatory, manual, and prior rule preamble statements in section XII.A. of that rule specifically titled, “Physician Supervision of HOPD Services.” The CY 2009 OPPS/ASC proposed rule provided for a 60-day comment period. We stated that we continue to believe that the CY 2009 restatement and clarification made no change to longstanding hospital outpatient physician supervision policies as incorporated in prior statements of policy, including the codified Federal regulations. In addition, we provided for public notice and comment regarding these physician supervision polices through the CY 2009 OPPS/ASC proposed rule in which, as noted above, we discussed physician supervision in a distinct section of the proposed rule. However, we received only a few public comments on that section. We also noted that the physician supervision policies for hospital outpatient diagnostic and therapeutic services as described in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68702 through 68704) continue to be in effect for CY 2009. In the CY 2010 OPPS/ASC proposed rule (74 FR 35365), we stated that we have not instructed contractors to delay initiation of enforcement actions or to discontinue pursuing pending enforcement actions regarding the physician supervision of hospital outpatient services.
However, while we did not propose to withdraw the longstanding physician supervision policies for hospital outpatient services in CY 2009, we stated in the CY 2010 OPPS/ASC proposed rule (74 FR 35365) that we had extensively considered the many questions and concerns on this topic raised to us by stakeholders in the course of developing the proposed rule in order to assess whether proposed changes to the existing policies should be considered. We appreciated the many detailed comments and suggestions interested stakeholders have raised in the first few months since publication of the CY 2009 OPPS/ASC final rule with comment period. We considered a wide variety of potential modifications to our physician supervision policies in response to this information about current health care delivery practices and challenges. The dialogue with interested stakeholders provided us with sufficient information to develop proposals for certain changes to the supervision policies for hospital outpatient services for CY 2010 in order to take into full consideration current clinical practice and patterns of care, the need to ensure patient access, the associated hospital and physician responsibilities, consistency among requirements for different sites of services, and other important factors. We indicated our belief that the proposals we were making for CY 2010 would address many of the concerns and questions regarding our existing policies that had been raised to us by stakeholders over the first 6 months of CY 2009. In the CY 2010 OPPS/ASC proposed rule (74 FR 35365), we welcomed robust public comments regarding our CY 2010 proposals for physician supervision in order to inform our decisions regarding final policies for CY 2010.
In considering the questions and concerns that had been raised over the first 6 months of CY 2009, we identified three areas within our existing hospital outpatient physician supervision policies for which we stated our belief that proposals of policy changes were appropriate for CY 2010, two related to the supervision of therapeutic services and one related to the supervision of diagnostic services. Our specific CY 2010 proposals, including the proposed changes to our regulations to conform to these proposals, the public comments on the proposals and our responses, and our final CY 2010 supervision policies for hospital outpatient therapeutic and diagnostic services are discussed below.
3. Policies for Direct Supervision of Hospital and CAH Outpatient Therapeutic Services
In the CY 2010 OPPS/ASC proposed rule (74 FR 35365), we proposed that nonphysician practitioners, specifically physician assistants, nurse practitioners, clinical nurse specialists, and certified nurse-midwives, may directly supervise all hospital outpatient therapeutic services that they may perform themselves in accordance with their State law and scope of practice and hospital-granted privileges, provided that they continue to meet all additional requirements, including any collaboration or supervision requirements as specified in the regulations at §§ 410.74 through 410.77. Clinical psychologists may already provide direct supervision, as we mentioned in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68703 through 68704) because they, along with physicians (as defined in section 1861(r)(1) of the Act), may be responsible for the care of a hospital patient, as discussed in the Medicare conditions of participation for hospitals in § 482.12(c) of our regulations. We stated our belief that allowing certain nonphysician practitioners (nurse practitioners, physician assistants, clinical nurse specialists, and certified nurse-midwives) to provide direct supervision of certain hospital outpatient therapeutic services is appropriate because, even though these practitioners are not physicians, they are recognized in statute and regulation as providing services that are analogous to physicians' services. Medicare Part B covers the professional services of clinical psychologists, nurse practitioners, physician assistants, clinical nurse specialists, and certified nurse-midwives when the services would be physicians' services if furnished by a physician (a doctor of medicine or osteopathy, as set forth in section 1861(r)(1) of the Act). Their services are described in §§ 410.71(a), 410.74(a), 410.75(a) and (c), 410.76(a) and (c), and 410.77(a), respectively, of our regulations. Medicare also makes payment for services provided incident to the services of these nonphysician practitioners as specified in §§ 410.71(a)(2)(iii), 410.74(b), 410.75(d), 410.76(d), and 410.77(c), respectively.
We also noted that section 1861(r) of the Act does not include clinical psychologists, nurse practitioners, Start Printed Page 60579 physician assistants, clinical nurse specialists, or certified nurse-midwives in the definition of a physician. However, as previously mentioned, the conditions of participation for hospitals at § 482.12(c)(1)(vi) of our regulations do include clinical psychologists as practitioners who may be responsible for the care of Medicare patients. The conditions of participation at §§ 482.12(c)(1)(i) through (c)(1)(vi) require that every Medicare hospital patient be under the care of a doctor of medicine or osteopathy, a doctor of dental surgery or dental medicine, a doctor of podiatric medicine, a doctor of optometry, a chiropractor, or a clinical psychologist; each practicing in accordance with the Act, Federal regulations, and State law. Further, § 482.12(c)(4) of our regulations requires that a doctor of medicine or osteopathy must be responsible for the care of each Medicare patient with respect to any medical or psychiatric condition that is present on admission or develops during hospitalization and is not specifically within the scope of practice of one of the other practitioners listed in §§ 482.12(c)(1)(ii) through (c)(1)(vi). Also, as permitted by State law, certain nonphysician practitioners may admit individuals to a hospital or CAH and order and provide therapeutic services to them. Since 1998, we have allowed payment for the professional services of these nonphysician practitioners in addition to payment for physicians' services when the nonphysician practitioner's professional services are furnished in an HOPD. We also have made outpatient facility payments to the hospital for those facility services provided incident to the professional services of these nonphysician practitioners (63 FR 58873). In addition, the conditions of participation for CAHs at § 485.631 require that a doctor of medicine or osteopathy, a nurse practitioner, a physician assistant, or a clinical nurse specialist be available to furnish patient care services at all times the CAH operates. A doctor of medicine or osteopathy must be present for sufficient periods of time to provide medical direction, medical care services, consultation and supervision as described in the conditions of participation and must be available through radio or telephone contact for assistance with medical emergencies or patient referral.
Taking into consideration the totality of these existing conditions and requirements, we proposed to revise § 410.27 of the regulations to make clear that Medicare Part B payment may be made for hospital outpatient services and supplies furnished incident to the services of a physician, clinical psychologist, nurse practitioner, physician assistant, clinical nurse specialist, or certified nurse-midwife service; and to add that, effective January 1, 2010, clinical psychologists, nurse practitioners, physician assistants, clinical nurse specialists, or certified nurse-midwives may provide direct supervision for hospital outpatient therapeutic services that they may perform themselves under State law and within their scope of practice and hospital-granted privileges in the context of the existing requirements in §§ 410.71, 410.74, 410.75, 410.76, and 410.77. However, we note that, in the CY 2010 OPPS/ASC proposed rule (74 FR 35366), we proposed that the direct supervision of CR, ICR, and PR services must be furnished by a doctor of medicine or osteopathy, as specified in the proposed coverage policy and regulations for CR, ICR, and PR services. We also noted that Medicare does not make a payment to a physician under the MPFS when the physician solely provides the direct physician supervision of hospital outpatient therapeutic services but furnishes no direct professional services to a patient. This also would apply to the supervision of hospital outpatient therapeutic services provided by nonphysician practitioners.
We also indicated that we did not propose to modify requirements relating to physician supervision or collaboration for these nonphysician practitioners. In regard to the supervision of physician assistants, § 410.74(a)(iv) requires that physician assistants perform services under the general supervision of a physician. We have further defined this general supervision in section 190(c) of Chapter 15 of the Medicare Benefit Policy Manual. Section 190(c) states that “the PA's physician supervisor (or a physician designated by the supervising physician or employer as provided under State law or regulations) is primarily responsible for the overall direction and management of the physician assistant's professional activities and for assuring that the services provided are medically appropriate for the patient. The physician supervisor (or physician designee) need not be physically present with the physician assistant when a service is being furnished to a patient and may be contacted by telephone, if necessary, unless State law or regulations require otherwise.”
The requirements for collaboration of nurse practitioners are defined in § 410.75(c)(3) of the regulations and Section 200(D) of Chapter 15 of the Medicare Benefit Policy Manual. The requirements for clinical nurse specialists are located in § 410.76(c)(3) of the regulations and Section 210(D) of Chapter 15 of the Medicare Benefit Policy Manual. These sections define collaboration as a process in which the nurse practitioner or the clinical nurse specialist works with one or more physicians (doctors of medicine or osteopathy) to deliver health care services within the scope of the practitioner's expertise, with medical direction and appropriate supervision as required by the law of the State in which the services are being furnished. In the absence of more stringent State law requirements governing collaboration, collaboration is to be evidenced by the nurse practitioner or the clinical nurse specialist documenting his or her scope of practice and indicating the relationships that he or she has with physicians to deal with issues outside their scope of practice. The collaborating physician does not need to be present with the nurse practitioner or clinical nurse specialist when the services are furnished or to make an independent evaluation of each patient who is seen by the nurse practitioner or clinical nurse specialist.
In addition, for CY 2010, we proposed to refine the definition of direct supervision of hospital outpatient therapeutic services for those services furnished in a hospital and in on-campus PBDs of a hospital. For services furnished on a hospital's main campus, we proposed that direct supervision means that the supervisory physician or nonphysician practitioner must be present on the same campus, in the hospital or the on-campus PBD of the hospital as defined in § 413.65, and immediately available to furnish assistance and direction throughout the performance of the procedure. We proposed to add a new paragraph (a)(1)(iv)(A) to § 410.27 to reflect this requirement. We also proposed to define “in the hospital” in new paragraph § 410.27(g) as meaning areas in the main building(s) of a hospital that are under the ownership, financial, and administrative control of the hospital; that are operated as part of the hospital; and for which the hospital bills the services furnished under the hospital's CCN. Therefore, to be present in the hospital or the on-campus PBD of the hospital and immediately available requires that the physician or nonphysician practitioner must be physically present in areas on the campus of the hospital that are part of Start Printed Page 60580 the hospital, including on-campus PBDs, that are operated by the hospital, and where services furnished in those areas are billed under the hospital's CCN. Under the CY 2010 proposal, the supervisory physician or nonphysician practitioner of the hospital's outpatient therapeutic services may not be located in any other entity, such as a physician's office, IDTF, co-located hospital, or hospital-operated provider or supplier such as a skilled nursing facility (SNF), end-stage renal disease (ESRD) facility, or home health agency (HHA), or any other nonhospital space that may be co-located on the hospital's campus, as “hospital campus” is defined in § 413.65(a)(2) of the regulations.
We stated that while we have not previously specified in policy guidance a definition for the term “immediately available” with respect to services provided in areas of the hospital on its main campus that are not PBDs, we believe that the existing definitions of direct supervision in §§ 410.27(f) and 410.32(b)(3)(ii) that apply to PBDs and physician office settings indicate that the physician must be physically present in order to provide direct supervision of services. With regard to services provided in PBDs of hospitals or physicians' offices, these regulations specify that the physician must be present in the PBD or in the office suite, respectively. Thus, we have previously established that direct supervision requires immediate physical presence. While we also have not specifically defined the word “immediate” for direct supervision in terms of time or distance, we noted that the general definition of the word means “without interval of time.” Therefore, the supervisory physician or nonphysician practitioner could not be immediately available while, for example, performing another procedure or service that he or she could not interrupt. In addition, we stated that we understand that advances in medical technology, changes in the patterns of health care delivery, and changes in the organizational structure of hospitals have led to the development of extensive hospital campuses, sometimes spanning several city blocks. However, in the context of direct physician or nonphysician practitioner supervision, we believed that it would be neither appropriate nor “immediate” for the supervisory physician or nonphysician practitioner to be so physically far away on the main campus from the location where hospital outpatient services are being furnished that he or she could not intervene right away. As we stated in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68703), if there were problems with outpatient care in a hospital or in an on-campus PBD where the requirement for direct supervision was not met, we would consider that to be a quality concern. Appropriate supervision is a key aspect of the delivery of safe and high quality hospital outpatient services that are paid under Medicare.
In addition, the definition of direct supervision in existing § 410.27(f) has included and would continue to specify under our CY 2010 proposal that the physician or nonphysician practitioner must be available to furnish assistance and direction throughout the performance of the procedure. This means that the physician or nonphysician practitioner must be prepared to step in and perform the service, not just to respond to an emergency. This includes the ability to take over performance of a procedure and, as appropriate to both the supervisory physician or nonphysician practitioner and the patient, to change a procedure or the course of treatment being provided to a particular patient. We originally stated in the April 2000 OPPS final rule (65 FR 18525) that the physician does not “necessarily need to be of the same specialty as the procedure or service that is being performed.” We also have stated in manual guidance that hospital medical staff that supervises the services “need not be in the same department as the ordering physician” (Section 20.5.1 of Chapter 6 of the Medicare Benefits Policy Manual). However, in order to furnish appropriate assistance and direction for any given service or procedure, we believed the supervisory physician or nonphysician practitioner must have, within his or her State scope of practice and hospital-granted privileges, the ability to perform the service or procedure.
We did not propose significant changes to the definition or requirements for direct supervision in off-campus PBDs of the hospital other than to allow nonphysician practitioners to provide direct supervision in these PBDs for the services that these practitioners may perform. With respect to off-campus PBDs of hospitals, direct supervision would continue to mean that the physician or nonphysician practitioner must be in the off-campus PBD and immediately available to furnish assistance and direction throughout the performance of the procedure. We proposed to revise existing § 410.27(f) and include the revised language as § 410.27(a)(1)(iv) and make a technical change in § 410.27(a)(i)(iv)(B) to clarify the current language by removing “present and on the premises of the location” and replacing it with “present in the off-campus provider-based department.” While the meaning of this provision is the same, we believed that this proposed modification to the language defining direct supervision is more consistent with the language of the other proposed changes to § 410.27. As we clarified in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68704), the supervisory physician for hospital outpatient therapeutic services must be in each PBD of a particular off-campus outpatient location, but that does not mean that the physician must be in the room when the procedure is performed. In the April 2000 OPPS final rule (65 FR 18525), we responded to public commenters who asserted that requiring a physician to be onsite at a PBD throughout the performance of all “incident to” (therapeutic) services would be burdensome and costly for hospitals where there are a limited number of physicians available to provide coverage, particularly in rural settings. We disagreed then that the supervision requirement was unnecessary and burdensome because hospitals, prior to 2000, were already required to “meet a direct supervision of `incident to' services requirement that is unrelated to the provider-based rules. That is, we require that hospital services and supplies furnished to outpatients that are incident to physician services be furnished on a physician's order by hospital personnel and under a physician's supervision” (Section 3112.4 of the Medicare Intermediary Manual). In addition, when we discussed the “assumption” or expectation that the physician supervision requirement is met on the hospital's main campus in the April 2000 OPPS final rule (65 FR 18525), we specifically did not extend that assumption to off-campus departments of the hospital. We stated that we continue to believe that it would be inappropriate to allow one physician or nonphysician practitioner to supervise all services being provided in all PBDs at a particular off-campus outpatient location. Since first allowing off-campus sites to be considered PBDs of hospitals, we have placed particular emphasis on ensuring the quality and safety of the services provided in these locations, which can be many miles from the main hospital campus, through both additional provider-based requirements in § 413.65(e) and our emphasis on direct physician supervision under existing § 410.27(f). In addition, because Start Printed Page 60581 the physician or nonphysician practitioner must be immediately available and have, within his or her State scope of practice and hospital-granted privileges, the ability to perform the services being supervised, we believed it would be highly unlikely that one physician or nonphysician practitioner would be both immediately available at all times that therapeutic services are being provided and would have the knowledge and ability to adequately supervise all services being performed at once in multiple off-campus PBDs at a single location.
To reflect these proposed changes for the provision of direct supervision of therapeutic services provided to hospital outpatients in our regulations, we proposed to revise the language of the existing § 410.27(f) and include the revised language in a new paragraph (a)(1)(iv) of § 410.27 to specify that direct physician or nonphysician practitioner supervision of hospital outpatient therapeutic services is required for Medicare Part B payment. We proposed to add a new paragraph (a)(1)(iv)(A) to § 410.27 to state that, for services provided on the hospital's main campus, direct supervision means that the physician or nonphysician practitioner must be present on the same campus, in the hospital or on-campus PBD of the hospital, as defined in § 413.65, and immediately available to furnish assistance and direction throughout the performance of the procedure. It does not mean that the physician or nonphysician practitioner must be in the room when the procedure is performed. We also proposed to add new paragraph (a)(1)(iv)(B) to § 410.27 to reflect that, for off-campus PBDs of hospitals, the physician or nonphysician practitioner must be present in the off-campus PBD, as defined in § 413.65, and immediately available to furnish assistance and direction throughout the performance of the procedure. It does not mean that the physician or nonphysician practitioner must be in the room when the procedure is performed. As we stated previously, the proposed language of paragraph (a)(1)(iv)(B) is similar to existing § 410.27(f) that we proposed to revise. Furthermore, we proposed to make a technical change to clarify the language in this paragraph to remove “present and on the premises of the location” and replace it with “present in the off-campus provider-based department.” Also, in the CY 2010 OPPS/ASC proposed rule (74 FR 35368) and as proposed in the CY 2010 MPFS proposed rule (74 FR 33674), we proposed that the direct supervision of CR, ICR, and PR services must be furnished by a doctor of medicine or osteopathy, as specified in proposed §§ 410.47 and 410.49, respectively. We proposed to include this exception in proposed paragraphs (a)(1)(iv)(A) and (a)(1)(iv)(B) in § 410.27. In addition, we proposed to add a new paragraph (f) to § 410.27 to define a nonphysician practitioner for purposes of § 410.27 as a clinical psychologist, a physician assistant, a nurse practitioner, a clinical nurse specialist, or a certified nurse-midwife. Proposed new § 410.27(a)(1)(iv) would provide that these nonphysician practitioners may directly supervise services that they could furnish themselves in accordance with State law and within their scope of practice and hospital-granted privileges, as long as all requirements for coverage, including the physician supervision or collaboration for these nonphysician practitioners, are met in accordance with §§ 410.71, 410.74, 410.75, 410.76, and 410.77, respectively. We also proposed to define “in the hospital” in new paragraph § 410.27(g) to mean areas in the main building(s) of the hospital that are under the ownership, financial, and administrative control of the hospital; that are operated as part of the hospital; and for which the hospital bills the services furnished under the hospital's CCN. Finally, we proposed to make a technical correction to the title of § 410.27 to read, “Outpatient hospital or CAH services and supplies incident to a physician service: Conditions” to clarify in the title that the requirements for payment of hospital outpatient therapeutic services incident to a physician or nonphysician practitioner service in that section apply to both hospitals and CAHs. Similarly, we proposed to include the phrase “hospital or CAH” throughout the text of § 410.27 wherever the text currently refers just to “hospital.” The omission of the term “CAH” from § 410.27 was a drafting oversight. However, we have applied the requirements of § 410.27, including “incident to” requirements such as the site-of-service requirement and physician supervision, as well as other hospital policies, such as the bundling rules, to CAHs, just as we have in 42 CFR Part 409 (Subparts A through D and F through H) and § 410.28 and § 413.65 of the regulations where CAHs are explicitly mentioned.
Comment: All commenters who addressed the proposed direct supervision by nonphysician practitioners (specifically physician assistants, nurse practitioners, clinical nurse specialists, and certified nurse-midwives) supported the proposal. Some commenters also requested that CMS include licensed clinical social workers in the list of nonphysician practitioners who are permitted to supervise outpatient psychiatric services and partial hospitalization program (PHP) services. Other commenters requested that pharmacists be permitted to supervise medication therapy management services and that specialty certified registered nurses, such as wound care nurses, also be able to provide the direct supervision of hospital outpatient therapeutic services. One commenter stated that because physicians do not furnish nursing services or the services of other ancillary health professionals, they should not be expected to supervise those services and it would be inappropriate to expect physicians to accept responsibility for care that they have not personally furnished.
Response: We appreciate the commenters' support for our proposal to revise § 410.27 of the regulations to allow certain nonphysician practitioners to directly supervise services that they may perform themselves under their State scope of practice and hospital-granted privileges in the context of the existing requirements in §§ 410.71, 410.74, 410.75, 410.76 and 410.77. We agree with the commenters that we should add licensed clinical social workers to the group of nonphysician practitioners permitted to provide direct supervision for hospital outpatient therapeutic services. We believe this is appropriate because licensed clinical social workers are recognized under the Medicare program as independent practitioners who may furnish services for the diagnosis and treatment of mental illness that they are legally authorized to perform under State law of the State in which the services are performed. We further agree with the commenters that the inclusion of licensed clinical social workers would help to ensure continued access to mental health services, especially in rural hospitals and CAHs where other types of practitioners may be less available. We emphasize though, that licensed clinical social workers, like other nonphysician practitioners, may only supervise those therapeutic services within their own scope of practice and hospital-granted privileges. We are not expanding the regulations further to allow supervision by pharmacists, registered nurses, or other medical professionals. These professionals are not recognized in the Social Security Act as providing services that would be physicians' services if performed by a physician and Start Printed Page 60582 they may not enroll in Medicare as independent practitioners and receive payment directly for their professional services.
With regard to the comments about physician scope of practice and supervision, we remind hospitals that the only statutory basis for payment of hospital outpatient therapeutic services is incident to the services of a physician, meaning the services are ordered by the physician or qualified practitioner and furnished as an integral though incidental part of his or her services. It follows, then, that a qualified physician or nonphysician practitioner would supervise the provision of those services to ensure the service or procedure is being furnished appropriately.
Comment: One commenter supported the proposed requirements for the direct physician supervision of CR programs and requested that CMS confirm that the proposed definition of “direct supervision” that would apply to therapeutic services in the HOPD would also apply to CR services. However, many commenters, especially CAHs and rural hospitals, asked that CMS permit physician assistants, nurse practitioners, clinical nurse specialists, and certified nurse-midwives who are functioning within their State licensure and scope of practice and who are permitted to supervise the services under the hospital or CAH bylaws to supervise CR, ICR, and PR services.
Response: As discussed in detail in sections sections II.G.8. and II.G.9. of the CY 2010 MPFS final rule with comment period and in section XII.B.4. of this final rule with comment period, section 144(a)(1) of Public Law 100–275 imposes strict requirements for the direct physician supervision of PR, CR, and ICR services and gives us no flexibility to modify the requirement. Therefore, we are finalizing our CY 2010 proposal, without modification, to require the direct physician supervision (by a doctor of medicine or a doctor of osteopathy) of PR, CR, and ICR services that are furnished to hospital outpatients. We note that we define “direct supervision” with regard to what it means to be immediately available and accessible for medical consultation and medical emergencies in the same manner for PR, CR, and ICR services as we do for other therapeutic services furnished in HOPDs, as discussed below. Also, the final definitions of direct supervision in § 410.27 for therapeutic services provided on campus and in off-campus PBDs also apply. These definitions and the final regulation text of §§ 410.27(a)(1)(iv)(A) and (B) are discussed in detail below.
Comment: With respect to the proposed definition of direct supervision of hospital outpatient therapeutic services, some commenters fully supported the proposals as appropriate and necessary for ensuring that Medicare beneficiaries receive safe and high quality care. Many commenters acknowledged that the proposal to broaden the location of the supervising physician from in the department on campus to “in the hospital” would give hospitals significantly more flexibility. However, the commenters stated that, while the proposal would be more flexible, it would still limit access to care and would cost hospitals and the Medicare program. Other commenters questioned why CMS has a supervision requirement at all, stating that there is no such specified requirement for hospital inpatient services. Many commenters believed that the proposals would not help CAHs and rural hospitals, where physicians often maintain offices off-campus and qualified nonphysician practitioners may not be readily available to provide services in the hospital. The commenters claimed that the proposed definition of direct supervision would mean that CAHs and rural hospitals would be required to hire staff solely to supervise services and that this extra cost would force these hospitals and CAHs to eliminate services. These commenters requested that CMS not apply the “incident to” requirements of § 410.27 to CAHs.
Response: We appreciate the commenters' support and the acknowledgement that we are striving to provide more flexibility for hospitals, while maintaining appropriate supervision of hospital outpatient therapeutic services that helps to ensure safe, high quality care and providing Medicare payment that is consistent with the statutory requirements for coverage. We have received numerous comments and questions since publication of the CY 2009 OPPS/ASC final rule with comment period, met with interested stakeholders to hear their questions and concerns throughout the year, and reviewed many thoughtful, wide-ranging comments on the CY 2010 OPPS/ASC proposed rule. In considering all of this information, we have taken into full consideration current clinical practice and patterns of care, the need to ensure patient access, the associated hospital and physician responsibilities, consistency among requirements for different sites of services, and other important factors. We believe that our final policies address many of the concerns and questions regarding our proposals. Through the expansion of supervisory authority to nonphysician practitioners and a modification to the requirement for direct supervision of on-campus therapeutic services, discussed in more detail below, we believe our final policies allow more flexibility for hospitals and CAHs and help ensure access to care for Medicare beneficiaries while maintaining our standards for safe, high quality care and consistent interpretation of longstanding regulations.
We disagree with the commenters who suggested that there should be no supervision requirements for hospital outpatient services because there are not similar codified supervision requirements for hospital inpatient services. Hospitals provide a wide variety of complex services to their outpatients who may or may not have an established relationship with the supervising physician or nonphysician practitioner and hospital staff on the day the hospital outpatient services are furnished. A treating physician or nonpractitioner in the community may not even be aware that ordered outpatient services are being furnished by the hospital on a given day. Therefore, we believe it is appropriate for CMS to set requirements for the safe and effective diagnosis and treatment of Medicare beneficiaries, including standards for the appropriate supervision of hospital outpatient services by a physician or nonphysician practitioner, in accordance with the statutory basis for payment of hospital outpatient services in section 1861(s)(2)(B) of the Act, which is “incident to physicians' services rendered to outpatients.”
We set the standard for direct supervision of hospital outpatient therapeutic services that we have held since the implementation of the OPPS in 2000, and we have assumed that this standard was being met because we assumed that a physician would always be nearby in the hospital. Given that hospital inpatients generally have medically complex conditions requiring a high level of acute care, we have not established explicit supervision requirements in regulations because we believe hospitals would have physicians or other qualified practitioners available at all times that complex hospital inpatient services are being furnished. If this is not the case, we may consider addressing the supervision of hospital inpatient services in the future.
In regard to hospital and CAH concerns about hiring physician and nonphysician staff solely for Start Printed Page 60583 supervisory services, we reiterate that the supervisor need only be available when outpatient therapeutic services and procedures are being furnished, meaning that many services or departments would not require 24 hour per day, 7 days per week direct supervision, as some commenters believed. We also believe that allowing the supervisory physician or nonphysician practitioner to be located anywhere on the hospital campus, as discussed more fully below, should alleviate this concern for many hospitals and CAHs. In addition, we remind CAHs that the conditions of participation for CAHs at § 485.631 require that a doctor of medicine or osteopathy, a nurse practitioner, a physician assistant, or a clinical nurse specialist is available “ to furnish patient care services at all times the CAH operates” (emphasis added). It follows then that this physician or nonphysician practitioner who is available at all times the CAH operates would be directly furnishing services that are within his or her State scope of practice and CAH-granted privileges and that the CAH would not be furnishing services that are not within this practitioner's scope.
Comment: Many commenters stated that it is overly restrictive and arbitrary to not allow direct supervision by practitioners located in other entities on-campus and to require the immediate physical presence of the physician. Several commenters pointed out that, because of the varying ways that hospitals have structured their services and campuses, a physician's office may be next door or closer to the HOPD in which services that he or she would be supervising are furnished, than a practitioner located in another HOPD. Other commenters stated that the proposal would preclude physicians from taking a lunch break, patronizing the retail establishments in the hospital, or going to other areas such as parking lots.
The commenters were particularly concerned about specialized services such as chemotherapy, blood transfusions, and radiation therapy services. Several hospital associations and other commenters requested that CMS remove the phrase “immediately available to furnish assistance and direction throughout the performance of the procedure.” They instead recommended that CMS redefine direct supervision for therapeutic services to mean that the physician may be on or in close proximity to the campus and able to respond in a timely manner according to hospital's policies and bylaws. The commenters also believed that “immediate availability” does not and should not mean immediate physical presence and that requiring physical presence in every instance is impractical. Instead, the commenters believed the supervising practitioner should be able to directly supervise services and procedures remotely by telephone, radio, robotic device, or electronic media.
Response: We appreciate the many public comments that we received on the proposed definition of direct supervision for hospital outpatient therapeutic services provided on the campus of the hospital. We acknowledge the comments related to hospital building and campus structures and the physical proximity of physicians' offices and other entities to the hospital department where a particular hospital outpatient service is furnished. We agree with the commenters that allowing the supervising physician to be in nonhospital space on the campus could make it easier for a supervising physician or nonphysician practitioner to respond immediately. Therefore, we believe it would be appropriate to allow the supervising physician or nonphysician practitioner to be located anywhere on the same campus of the hospital, as long as he or she was immediately available to furnish assistance and direction throughout the performance of the procedure.
This is consistent with our longstanding definition of “direct supervision” as it has been applied across settings in terms of the immediate physical presence of the physician and what it means to “furnish assistance and direction throughout the performance of the procedure.” However, we continue to believe that the supervisory physician or nonphysician practitioner could not be immediately available while, for example, performing another procedure or service that he or she could not interrupt. It also would be neither appropriate nor “immediate” for the supervisory physician or nonphysician practitioner to be so physically far away on the main campus from the location where hospital outpatient services are being furnished that he or she could not intervene right away.
As we stated in the CY 2010 OPPS/ASC proposed rule (74 FR 35367), we believe that the existing definitions of direct supervision in §§ 410.27(f) and 410.32(b)(3)(ii) that apply to PBDs and physicians' office settings indicate that the physician must be physically present in order to provide direct supervision of services. With regard to services provided in PBDs of hospitals or physicians' offices, for at least 9 years these regulations have specified that the physician must be present in the PBD or in the office suite, respectively. Thus, we have previously established that direct supervision requires immediate physical presence. Medicare only covers hospital outpatient therapeutic services furnished incident to physicians' services, yet a hospital service ordered by a physician may be furnished on a day when the beneficiary does not receive services directly from a physician. Therefore, we believe it is important to retain direct supervision as the standard.
With regard to the commenters' recommendations that CMS redefine direct supervision for therapeutic services to mean that the physician may be “on or in close proximity to the campus” and “able to respond in a timely manner” according to hospital's policies and bylaws, we believe that the suggested new definition of direct supervision for on-campus hospital outpatient therapeutic services would be wholly inconsistent with the definition of the term as previously codified in at least two Medicare sections of the Code of Federal Regulations. We disagree with the commenters that describing immediate availability as without lapse of time would be read so narrowly as to require that the supervising physician or nonphysician practitioner must be standing in the room next to the nonphysician personnel furnishing the service. A similar argument could be made that the phrase “able to respond in a timely manner” is so vague that a supervising physician or nonphysician practitioner could interpret it to mean that arriving within an hour or hours would be reasonable. In addition, we note that the definition proposed by commenters is virtually identical in interpretation to the current existing definition of general supervision. Section 410.32(b)(3)(i) of the regulations defines general supervision to mean that “the procedure is furnished under the physician's overall direction and control, but the physician's presence is not required during the performance of the procedure.” We have historically interpreted this to mean that the physician may be available by telephone or other electronic device to supervise and direct the nonphysician personnel furnishing the service. This lower standard of general supervision would not ensure the immediate presence of a qualified practitioner in the hospital to furnish assistance and direction to hospital personnel providing a wide array of complex therapeutic services to hospital outpatients. Several of the Start Printed Page 60584 commenters requesting this relaxed definition of direct supervision also asked that certain services be designated as requiring only general supervision. We are unclear about the commenters' understanding of the definition of general supervision if their suggested definition of direct supervision requires no physical presence and only specifies availability in a reasonable period of time by telephone, radio, robotics, or telemedicine.
We have set direct supervision as a minimum standard for supervision of all Medicare hospital outpatient therapeutic services. We do not believe that this standard or the definition, as codified, precludes a hospital from developing bylaws, credentialing procedures, and policies that it believes are appropriate to ensure that all hospital patients receive high quality services in a safe and effective manner. We believe that hospitals take quality of care and patient safety very seriously, and we understand that hospitals are subject to accreditation requirements. Considering that hospitals provide a wide array of very complex services and procedures, including surgeries, we would expect that hospitals already have the leadership, credentialing procedures, bylaws, and other policies in place to ensure that services furnished to Medicare beneficiaries are being provided only by qualified practitioners in accordance with all applicable laws, regulations, and coding guidance. For services not furnished directly by a physician, we would expect that these bylaws and policies would ensure that the services are being supervised in a manner commensurate with the complexity of the service, including personal supervision where appropriate.
Comment: Many commenters disagreed with statements in the proposed rule that the supervising physician should have, within his or her scope of practice and hospital-granted privileges, the knowledge, ability, and hospital privileges to perform the services being supervised. Some commenters stated that the supervisor should be required to provide only medical consultation and attend to medical emergencies, but should not be expected to intervene or change the course of treatment because this usurps the responsibility of treating physician. Other commenters stated that the supervisor should be “clinically appropriate” to supervise the outpatient therapeutic services.
Response: As we explained in the CY 2010 OPPS/ASC proposed rule (74 FR 35367), we believe the supervising physician or nonphysician practitioner must be prepared to step in and perform the service, not just to respond to an emergency. This includes the ability to take over performance of a procedure and, as appropriate to both the supervisory physician or nonphysician practitioner and the patient, to change a procedure or the course of treatment being provided to a particular patient. We originally stated in the April 2000 OPPS final rule (65 FR 18525) that the physician does not “necessarily need to be of the same specialty as the procedure or service that is being performed.” We also have stated in manual guidance that hospital medical staff that supervises the services “need not be in the same department as the ordering physician” (Section 20.5.1 of Chapter 6 of the Medicare Benefits Policy Manual). We understand hospitals' concerns, and note that we would not expect that a supervising physician would operate in a vacuum, making all decisions without informing or consulting the patient's treating physician or nonphysician practitioner. This would be illogical and inappropriate for good medical practice. However, in order to furnish appropriate assistance and direction for any given service or procedure, we continue to believe the supervisory physician or nonphysician practitioner must have, within his or her State scope of practice and hospital-granted privileges, the ability to perform the service or procedure. We believe that our interpretation of the requirement means that the supervisor must be a person who is “clinically appropriate” to supervise the service or procedure. We believe it is inappropriate for a supervisory physician or nonphysician practitioner to be responsible for patients, hospital staff, and services that are outside the scope of their knowledge, skills, licensure, or hospital-granted privileges.
This interpretation of the previously codified language is consistent with our longstanding application of direct supervision across settings in terms of the physical presence of the physician and what it means to “furnish assistance and direction throughout the performance of the procedure.” We do not believe that allowing a supervisor to be responsible for emergencies only would satisfy the standard to “furnish assistance and direction throughout the performance of the procedure” as the language has historically been interpreted for physicians' offices and PBDs. We disagree with commenters who stated that the historical intent of direct supervision has been for a supervising physician to provide guidance and direction without expecting that professional to be able to perform the service or procedure and that performance of the procedure applies only to personal supervision. It would be unreasonable to think that a physician or nonphysician practitioner could competently assist and direct a procedure for which they do not have sufficient knowledge and skills to perform or redirect the procedure or service.
Comment: Several commenters requested additional guidance as to what CMS considers hospital outpatient “incident to” services. One commenter requested clarification on the applicability of “incident to” to emergency department services. The commenter believed that the “incident to” provision for hospital outpatient services may not be applicable to emergency department services because the emergency department physician would be immediately available in the area to care for patients, but would not have previously seen and established a relationship with the patient, as this commenter believed is required. Other commenters believed that the requirements for supervision of hospital outpatient therapeutic services should be specific to each clinical service and should be designated as either general or direct, as for diagnostics. Some commenters asked for clarification of how to report services in a Condition Code 44 situation when the patient received care as an inpatient, with no direct supervision, and the hospital then changed the patient's status to outpatient.
Response: As previously stated in this discussion, § 410.27(a)(1)(ii) of the regulations states that Medicare Part B pays for hospital services and supplies furnished incident to a physician service to outpatients if they are provided “as an integral though incidental part of a physician's services.” In addition, we have stated in Section 20 of Chapter 6 of the Medicare Benefit Policy Manual that hospitals provide two distinct types of services to outpatients: services that are diagnostic in nature and other services that aid the physician in the treatment of the patient. We further defined these therapeutic services and supplies in Section 20.5.1 of Chapter 6 of the Medicare Benefit Policy Manual, stating “therapeutic services and supplies which hospitals provide on an outpatient basis are those services and supplies (including the use of hospital facilities) which are incident to the services of physicians in the treatment of patients.” In essence, all hospital outpatient services that are not Start Printed Page 60585 diagnostic are services that aid the physician in the treatment of the patient, and are called therapeutic services. These are the services for which Medicare makes a hospital facility payment only when they are provided “incident to” the services of a physician.
We also provide in Section 20.5.1 that services and supplies must be furnished on a physician's order and delivered under physician supervision. However, the manual indicates further that each occasion of a service by a nonphysician does not need to also be the occasion of the actual rendition of a personal professional service by the physician responsible for the care of the patient. Nevertheless, as stipulated in that same section of the manual “during any course of treatment rendered by auxiliary personnel, the physician must personally see the patient periodically and sufficiently often enough to assess the course of treatment and the patient's progress and, where necessary, to change the treatment regimen.” Section 20.5.1 also explicitly includes clinic and emergency room services as examples of hospital outpatient services that are provided incident to the services of a physician. We note in this section that the policies for hospital services incident to physicians' services rendered to outpatients differ in some respects from policies that pertain to “incident to” services furnished in office and physician-directed clinic settings. Those requirements are discussed in Section 60 of Chapter 15 of the Medicare Benefit Policy Manual. The commenter incorrectly believed that for hospital services to be “incident to” a physician's services, the physician must have previously seen and established a relationship with the patient. When a patient presents to an emergency department or a hospital clinic and a physician examines the patient and orders services to be provided, the provision of those services is incident to the services of that physician.
We recognize the potential benefit to hospitals of specifically designating supervision requirements for individual therapeutic services based on clinical complexity, especially for less complex services that we might deem to require general supervision. We note that the commenters requesting individual designations mentioned only defining services as requiring general or direct supervision. However, as we discussed earlier in this section, direct supervision represents a minimum standard currently applicable to all outpatient therapeutic services. If we were to designate individual supervision levels for hospital outpatient therapeutic services just as we do for diagnostic services, it would be most consistent and appropriate for CMS to make specific determinations for services that we believe may only be safely provided under the personal supervision of a physician or that must be performed only by a physician. We stated above that we expect that hospitals already have credentialing procedures, bylaws, and other policies established to ensure that services furnished to Medicare beneficiaries are being provided only by qualified practitioners in accordance with all applicable laws, regulations and coding guidance. For services not furnished directly by a physician, we would expect that these bylaws and policies would ensure that the services are being supervised in a manner commensurate with the complexity of the service, including personal supervision where appropriate.
The use of Condition Code 44 pertains to the entire patient encounter, the patient's status, and the hospital bill type submitted. Reporting of individual HCPCS codes on an outpatient claim must be consistent with all instructions and CMS guidance, including the requirements of § 410.27 of the regulations, which specify that direct supervision is required for hospital outpatient therapeutic services.
Comment: One commenter requested clarification of the meaning of the phrase “performance of the procedure” in the definition of direct supervision. The commenter was unclear whether this phrase specifically applied only to surgical procedures or whether it was a general term for services and procedures. The commenter also asked if the direct supervision requirement would apply to recovery room services following a surgical procedure performed by the physician.
Response: The use of the term “procedure” is intended to encompass all services and procedures and includes all components of a service or procedure furnished by a hospital to an outpatient, including recovery room services, and covered and paid by Medicare, regardless of whether separate payment is made for those component services. This is how we have applied the term within the codified definitions of the levels of supervision (general, direct, and personal) in §§ 410.27 and 410.32. While each supervision definition uses the phrase “performance of the procedure,” the term “service” may be substituted for the word “procedure” each time “procedure” appears in the regulations.
Comment: Several commenters raised questions about the proposed definition of the phrase “in the hospital.” Some requested clarification of the meaning of “on the same campus.” Several commenters suggested that the word “ownership” in the definition seems to unintentionally exclude areas that are operated and controlled by the hospital under a lease agreement or a written operations agreement. These commenters suggested either removing this term from the definition of “in the hospital” or clarifying in this final rule with comment period where CMS is referring to the business operation aspect of ownership rather than the physical building.
Response: “On the same campus” was used to denote that the supervising physician or nonphysician practitioner must be physically located on the same campus as the services being furnished by the hospital because it is possible for some hospitals to have more than one main campus, as well as off-campus PBDs.
We appreciate the public comments that raised the questions about the term “ownership.” We agree with the commenters that a narrow interpretation of the word “ownership” would exclude spaces that the hospital leases or operates under another type of operations agreement. This was not our intention. The commenters correctly pointed out that the word “ownership” in this context applies to the actual business operation, not solely a physical building. However, we also believe that the rest of the definition includes these aspects. If the definition is read as a whole, instead of parsing individual clauses out of context, then the spirit of the regulation is understood. Because the phrase “in the hospital or CAH” applies broadly to “incident to” requirements such as the site of service requirement for therapeutic services provided by the hospital directly and under arrangement, we are finalizing the proposed definition of “in the hospital” in new paragraph (g) of § 410.27 as meaning areas in the main building(s) of a hospital that are under the ownership, financial, and administrative control of the hospital; that are operated as part of the hospital; and for which the hospital bills the services furnished under the hospital's CCN.
Comment: One commenter requested that CMS reiterate that the “incident to” and physician supervision requirements of § 410.27 do not apply to physical therapy, occupational therapy, and speech-language pathology services furnished under a therapy plan of care.
Response: Section 1833(t) of the Act excludes physical therapy, occupational Start Printed Page 60586 therapy, and speech-language pathology from hospital outpatient services paid under the OPPS. In addition, in the April 2000 OPPS final rule (65 FR 18525), we stated in response to a comment about physical therapy services that the coverage provision in section 1861(s)(2)(D) of the Act does not require that physical therapy services be provided incident to the services of a physician. Finally, in Section 20 (Hospital Outpatient Services) of Chapter 6 of the Medicare Benefit Policy Manual, we state, “[t]he following rules pertaining to the coverage of outpatient hospital services are not applicable to physical therapy, speech-language pathology, occupational therapy, or end stage renal disease (ESRD) services furnished by hospitals to outpatients.” This section instructs readers to consult sections 220 and 230 of Chapter 15 of the Medicare Benefit Policy Manual for rules on the coverage of outpatient physical therapy, occupational therapy, and speech-language pathology furnished by a hospital.
Comment: Several commenters from CMHCs and hospital-based PHPs questioned whether the direct physician supervision clarifications and the “incident to” requirements of § 410.27 apply to those programs. Several hospital-based PHP stakeholders noted the discrepancy in the physician supervision requirements for PHP services furnished by CMHCs and hospitals.
Response: Medicare makes payment for hospital outpatient therapeutic services only when provided “incident to” the services of a physician. Outpatient psychiatric and hospital-based PHP services are outpatient hospital services paid under the OPPS and, therefore, must meet all conditions of § 410.27, including the requirement for direct supervision by a physician or qualified nonphysician practitioner.
Because CMHCs are paid under the OPPS for PHP services, the “incident to” requirements of § 410.27 also apply to CMHCs, with the exception of direct supervision for outpatient PHP services. Currently, CMHCs have a different physician supervision standard to meet. On February 11, 1994, CMS issued the Partial Hospitalization Services in Community Mental Health Centers (CMHCs) interim final rule with comment period (59 FR 6570), developing and implementing the coverage criteria and payment methodology for CMHCs under Medicare. At the time when the CY 1994 CMHC rule was published, the decision was made to permit general supervision in CMHCs. As implemented in § 410.110(a), services provided in a CMHC PHP must be prescribed by a physician and furnished under the general supervision of a physician. General supervision means that a physician must be at least available by telephone, but is not required to be on the premises of the CMHC at all times (59 FR 6573). We recognized that a direct supervision requirement could cause hardship to CMHCs because some of these entities were unable to employ physicians on a full-time basis due to the expense involved. In the CY 1994 interim final rule with comment, we explained that because we believed that less than direct supervision by a full-time physician in a CMHC would not jeopardize a patient's health or treatment program, in combination with our belief that there would be a number of professionals involved in the care of the patient who are authorized to furnish services that would otherwise be furnished by a physician, we required general physician supervision.
On August 1, 2000 (65 FR 18434), the OPPS was implemented and provided payment for PHP services provided in two settings: hospital outpatient departments to their outpatients and CMHCs. Although PHP is one benefit and both provider types receive the same payment for services rendered, CMHCs have been permitted to furnish PHP services under general supervision; and the OPPS has, since 2000, held a standard of direct supervision for hospital outpatient therapeutic services, including PHP services (42 CFR 410.110 (a)). While the policy was clearly codified for PBDs of hospitals in the CY 2009 OPPS/ASC proposed rule and final rule with comment period, CMS restated and clarified that the policy also applies to hospital outpatient services not provided in PBDs due to questions referencing the assumption of physician supervision in the April 2000 OPPS final rule (73 FR 68702 through 68704 referencing 65 FR 18525). Even though there are different physician supervision standards for CMHCs and hospital-based PHPs, the certification, recertification, and plan of treatment requirements at § 424.24(e) and section 1835(2)(F) of the Act continue to apply to both provider types. The physician would certify and recertify (where services are furnished over a period of time) that the individual would require inpatient psychiatric care in the absence of such services; an individualized, written plan for furnishing such services has been established by a physician and is reviewed periodically by a physician; and such services are or were furnished while the individual is or was under the care of a physician. The physician recertification must be signed by a physician who is treating the patient and has knowledge of the patient's response to treatment.
In order to unify the benefit and create more equity and consistency, we are exploring the possibility of extending the same physician supervision requirements to both provider types. Specifically, we are concerned whether the current policy of requiring only general physician supervision in CMHCs continues to be appropriate, taking into account the differences among provider settings. We also plan to analyze PHP claims data from the past several years and assess whether our current policies and payment structures are working. Therefore, we will evaluate the policies and the possibility of extending the same physician supervision requirement to PHP services furnished in both CMHCs and HOPDs in future rulemaking.
With that in mind, we are requesting comments on this final rule with comment period that address: (1) What types of practitioners currently provide the supervision of PHP services in CMHCs; (2) what is the expertise of supervising practitioners in CMHCs and what is the expectation for their availability; (3) based on the final CY 2010 supervision requirements for hospital outpatient therapeutic services (including PHP services furnished in HOPDs), under what circumstances would direct supervision of PHP services furnished in CMHCs not be occurring, according to the applicable definitions for direct supervision of on-campus and off-campus therapeutic services; and (4) what would be the rationale for maintaining different supervision requirements for PHP services furnished in CMHCs and HOPDs, given that all PHP services are paid under the OPPS.
Comment: A few commenters stated that the provisions for off-campus PBDs should not require the supervising practitioner to be in the department because the existing policy is burdensome and costly, especially for rural providers. The commenters requested that a supervising physician or nonphysician practitioner be able to supervise services being furnished in more than one off-campus PBD at a time. Some commenters stated that they know of off-campus PBDs in current operation that operate with a supervisor nearby, for example, in a physician's office, but not in the PBD.
Response: We first note that the requirement for direct supervision of hospital outpatient therapeutic services furnished in PBDs of the hospital was Start Printed Page 60587 codified in § 410.27(f) of the regulations in the April 2000 OPPS final rule. In that rule, we explicitly stated that “on the premises of the location” means that the supervisor must be on the premises of the PBD (65 FR 18525). We also responded to public commenters who asserted that requiring a physician to be onsite at a PBD throughout the performance of all “incident to” therapeutic services would be burdensome and costly for hospitals where there are a limited number of physicians available to provide coverage, particularly in rural settings. We disagreed then that the supervision requirement was unnecessary and burdensome because hospitals, prior to 2000, were already required to “meet a direct supervision of `incident to' services requirement that is unrelated to the provider-based rules.” We continue to believe that it would be inappropriate to allow one physician or nonphysician practitioner to supervise all services being provided in all PBDs at a particular off-campus outpatient location. Since first allowing off-campus sites to be considered PBDs of hospitals, we have placed particular emphasis on ensuring the quality and safety of the services provided in these locations, which can be many miles from the main hospital campus, through both additional provider-based requirements in § 413.65(e) and our emphasis on direct physician supervision under § 410.27(f). In addition, because the physician or nonphysician practitioner must be immediately available and have, within his or her State scope of practice and hospital-granted privileges, the ability to perform the services being supervised, we believe it would be highly unlikely that one physician or nonphysician practitioner would be both immediately available at all times that therapeutic services are being provided and would have the knowledge and ability to adequately supervise all services being performed at once in multiple off-campus PBDs.
Comment: Many commenters maintained that the CY 2009 OPPS/ASC final rule with comment period statements on supervision were not a “restatement or clarification” but a significant change in policy. They argued that any enforcement should be prospective beginning in CY 2010 only, with no enforcement regarding prior years' hospital outpatient therapeutic services. The commenters believed that the policy prior to CY 2009 at best was unclear, and that the regulations were not comprehensive, and therefore, they concluded that hospitals should not be held accountable to the policy stated in the CY 2009 OPPS/ASC final rule with comment period for hospital outpatient therapeutic services furnished in 2000 through CY 2008. The commenters stated that CMS should also not enforce the policy as clarified in the CY 2009 OPPS/ASC final rule with comment period for CY 2009 hospital outpatient therapeutic services because sufficient opportunity for public notice and comment was not provided. Many hospitals were unaware that the policy had been discussed in the CY 2000 OPPS rulemaking process, and the commenters argued that hospitals may not have had enough time to meet these requirements for CY 2009. Furthermore, hospitals expressed concern about their potential liability due to qui tam litigation.
Response: We provided a restatement and clarification of existing policy in the CY 2009 OPPS/ASC proposed rule (73 FR 41518 through 41519), citing numerous existing statutory, regulatory, manual, and prior rule preamble statements in section XII.A. of that rule, specifically titled, “Physician Supervision of HOPD Services.” The CY 2009 OPPS/ASC proposed rule provided for a 60-day comment period. We continue to believe that the CY 2009 restatement and clarification made no change to longstanding hospital outpatient physician direct supervision policies as incorporated in prior statements of policy, including the regulations. In addition, we provided for public notice and comment regarding these physician supervision policies through the CY 2009 OPPS/ASC proposed rule in which, as noted above, we discussed physician supervision explicitly in a distinct section of the proposed rule, and we responded in the final rule to the few public comments we received on the supervision discussion. Therefore, we believe that the usual enforcement practices of Medicare contractors are appropriate for services furnished in CY 2009. Likewise, the final supervision policies described in this CY 2010 final rule with comment period for hospital outpatient therapeutic services are effective and, therefore, subject to enforcement beginning January 1, 2010.
In regard to hospital outpatient therapeutic services provided in CY 2000 through CY 2008, in CY 2009 we recognized the need for clarification of the direct supervision policy. CMS was relatively silent on this topic between 2000 and 2008. Furthermore, the existing regulations at § 410.27(f) only specify the supervision requirements for hospital outpatient therapeutic services furnished in PBDs but do not address services furnished in areas of the hospital that may not be PBDs. However, we note that the details of the direct supervision policy for hospital outpatient therapeutic services furnished in off-campus PBDs were clearly and consistently stated in the April 2000 OPPS final rule discussion and the regulations, including the requirement that the supervising practitioner be physically present in an off-campus PBD when such services were being furnished. As stated earlier in this section, we have placed and will continue to place particular emphasis on ensuring the quality and safety of the services provided in these locations and will continue to do so through our enforcement and other efforts. However, in the case of hospital outpatient therapeutic services furnished on the hospital's campus in 2000 through 2008, we plan to exercise our discretion and decline to enforce in situations involving claims where the hospital's noncompliance with the direct physician supervision policy resulted from error or mistake.
After consideration of the public comments we received, we are finalizing our proposal to allow, in addition to clinical psychologists, certain other nonphysician practitioners to directly supervise services that they may perform themselves under their State license and scope of practice and hospital-granted or CAH-granted privileges, with one modification. In addition to physician assistants, nurse practitioners, clinical nurse specialists, and certified nurse-midwives, in this final rule with comment period, we are allowing licensed clinical social workers to provide direct supervision. Specifically, we are modifying the final text of revised § 410.27(f) to include licensed clinical social workers among the listing of nonphysician practitioners who may directly supervise the provision of hospital outpatient therapeutic services. These nonphysician practitioners may directly supervise services that they may personally furnish in accordance with State law and all additional requirements, including those specified in §§ 410.71, 410.73, 410.74, 410.75, 410.76, and 410.77, respectively. Accordingly, we also are adding the cross-reference to § 410.73 (Clinical social worker services) in the final revision of paragraph (a)(1)(iv) of § 410.27 (which indicates that services must be furnished under the direct supervision of a physician or a nonphysician practitioner as specified in paragraph (f)).
We are finalizing the proposed direct physician supervision requirements for PR, CR, and ICR services furnished in Start Printed Page 60588 the HOPD that would require the direct supervision to be provided by a doctor of medicine or osteopathy. Accordingly, we are finalizing the relevant language in proposed §§ 410.27(a)(1)(iv)(A) and (B) which indicates that, for PR, CR, and ICR services, direct supervision must be furnished by a doctor of medicine or osteopathy, as specified in §§ 410.47 and 410.49, respectively.
For services furnished on a hospital's main campus, we are finalizing a modification of our proposed definition of “direct supervision” in new paragraph (a)(1)(iv)(A) of § 410.27 that allows for the supervisory physician or nonphysician practitioner to be anywhere on the hospital campus, including a physician's office, an on-campus SNF, RHC, or other nonhospital space. Therefore, direct supervision means that the supervisory physician or nonphysician practitioner must be present on the same campus and immediately available to furnish assistance and direction throughout the performance of the procedure.
Because the term “in the hospital or CAH” applies broadly to “incident to” requirements such as the site of service requirement for therapeutic services provided by the hospital directly and under arrangement, we also are finalizing the definition of “in the hospital” in new paragraph § 410.27(g) as meaning areas in the main building(s) of a hospital or CAH that are under the ownership, financial, and administrative control of the hospital or CAH; that are operated as part of the hospital; and for which the hospital bills the services furnished under the hospital's or CAH's CCN.
We are finalizing, without modification, the addition of new paragraph (a)(1)(iv)(B) to § 410.27 to reflect that, for off-campus PBDs of hospitals, the physician or nonphysician practitioner must be present in the off-campus PBD, as defined in § 413.65, and immediately available to furnish assistance and direction throughout the performance of the procedure. It does not mean that the physician or nonphysician practitioner must be in the room when the procedure is performed. As we stated previously, the language of paragraph (a)(1)(iv)(B) is similar to existing § 410.27(f) that we are revising and relocating. Furthermore, we are finalizing the proposed technical change to clarify the language in this paragraph by removing the phrase “present and on the premises of the location” and replacing it with the phrase “present in the off-campus provider-based department.”
Additionally, we are finalizing the proposal to make a technical correction to the title of § 410.27 to read, “Outpatient hospital or CAH services and supplies incident to a physician service: Conditions” to clarify in the title that the requirements for payment of hospital outpatient therapeutic services incident to a physician or nonphysician practitioner service in that section apply to both hospitals and CAHs. Similarly, we are including the phrase “hospital or CAH” throughout the text of § 410.27 wherever the text currently refers just to “hospital.”
4. Policies for Direct Supervision of Hospital and CAH Outpatient Diagnostic Services
As we discussed in detail in the CY 2010 OPPS/ASC proposed rule (74 FR 35368), with respect to the physician supervision requirements for individual diagnostic tests, we have continued since the April 2000 OPPS final rule discussion (65 FR 18526) to instruct hospitals that, for diagnostic services furnished in PBDs of hospitals, hospitals should follow the supervision requirements for individual diagnostic tests as listed in the MPFS Relative Value File. For diagnostic services not listed in the MPFS file, Medicare contractors, in consultation with their medical directors, define appropriate supervision levels in order to determine whether claims for these services are reasonable and necessary. To further specify the supervision policy across service settings and to provide consistency for all hospital outpatient diagnostic services, for CY 2010 we proposed to require that all hospital outpatient diagnostic services that are provided directly or under arrangement, whether provided in the main buildings of the hospital, in a PBD of a hospital, or at a nonhospital location, follow the physician supervision requirements for individual tests as listed in the MPFS Relative Value File. We also proposed that the definitions of general, direct, and personal supervision as defined in §§ 410.32(b)(3)(i) through (b)(3)(iii) would also apply. In the case of direct supervision of diagnostic services furnished directly by the hospital or under arrangement in the main hospital buildings or on-campus in a PBD, we proposed that the definition of direct supervision would be the same as the definition we proposed for therapeutic services provided on-campus as discussed in the CY 2010 OPPS/ASC proposed rule (74 FR 35369), meaning that the physician would be present on the same campus, in the hospital or the on-campus PBD of the hospital, as defined in § 413.65, and immediately available to furnish assistance and direction throughout the performance of the procedure. In addition, the definition of “in the hospital or CAH” as defined in proposed § 410.27(g), discussed above, would apply. In the CY 2010 OPPS/ASC proposed rule, we explained that this means that the supervisory physician may not be located in any entity such as a physician's office, co-located hospital, IDTF, or hospital-operated provider or supplier such as a SNF, ESRD facility, or HHA, or any other nonhospital space that may be co-located on the hospital's campus, as campus is defined in § 413.65(a)(2).
Similarly, in the case of direct physician supervision of diagnostic services furnished directly or under arrangement in an off-campus PBD, we proposed that the definition of direct supervision would be the same as the current definition for therapeutic services provided in an off-campus PBD as discussed in the CY 2010 OPPS/ASC proposed rule (74 FR 35369), meaning the physician must be present in the off-campus PBD, as defined in § 413.65 and immediately available to furnish assistance and direction throughout the performance of the procedure. As we discussed in the April 2000 OPPS final rule (65 FR 18524 through 18525) and the CY 2009 OPPS/ASC final rule with comment period (73 FR 68702 through 68704), we have long made the analogy of the PBD to the physician's office suite, as described in the definition of direct supervision in § 410.32(b)(3)(ii).
In addition to providing diagnostic services directly or under arrangement in the hospital, including PBDs of the hospital, a hospital may also send its outpatients to another entity, such as an IDTF, to furnish these services under arrangement for the hospital. For example, in the April 2000 OPPS final rule (65 FR 185440 through 185441), in a discussion of the hospital bundling rules, we discussed that an entity, like an IDTF, may be located in the main buildings of a hospital or on the hospital campus but operated independently of the hospital. In addition, these suppliers, providers, or other entities may be located elsewhere, not on a hospital's main campus or other hospital property. These entities, like IDTFs and physicians' offices, may provide services to their own patients (not hospital outpatients) and to hospital outpatients under arrangements with the hospital. They follow the physician supervision requirements of the MPFS and § 410.32 when providing services to Medicare beneficiaries who are not hospital outpatients. For consistency, we proposed for CY 2010 Start Printed Page 60589 that all diagnostic services provided to hospital outpatients under arrangement in nonhospital entities, whether those entities are located on the main campus of the hospital or elsewhere, would also follow the requirements as described in § 410.32(b)(3)(i) through (iii). When hospitals contract with other entities to provide services under arrangement, the hospital must exercise professional responsibility over the arrangement for services, in accordance with the guidance provided in Section 10.3 (Under Arrangements) of Chapter 5 of the Medicare General Information, Eligibility and Entitlement Manual. This means that for the hospital to receive payment, it is responsible for ensuring that all applicable requirements in §§ 410.28 and 410.32 are met. In the case of hospital outpatient diagnostic services provided under arrangement at nonhospital locations, such as IDTFs, we believe that the term “office suite” used in § 410.32(b)(3)(ii) is directly applicable because these facilities usually also provide diagnostic services to their own patients and, therefore, would be able to apply the direct supervision requirement in § 410.32(b)(3)(ii) without further definition.
We stated in the CY 2010 OPPS/ASC proposed rule (74 FR 35369) that physician assistants, nurse practitioners, clinical nurse specialists, and certified nurse-midwives who operate within the scope of practice under State law may order and perform diagnostic tests, as discussed under § 410.32(a)(3) and in the corresponding manual guidance in section 80 (Requirements for Diagnostic X–Ray, Diagnostic Laboratory, and Other Diagnostic Tests) of Chapter 15 of the Medicare Benefit Policy Manual. However, this manual guidance and the regulations at § 410.32(b)(1) also state that diagnostic x-ray and other diagnostic tests must be furnished under the appropriate level of supervision by a physician as defined in section 1861(r) of the Act. Thus, physician assistants, nurse practitioners, clinical nurse specialists, and certified nurse-midwives may not function as supervisory physicians for the purposes of diagnostic tests. In accordance with these existing requirements, we did not propose to allow physician assistants, nurse practitioners, clinical nurse specialists, and certified nurse-midwives to provide the supervision of diagnostic tests provided to hospital outpatients. Clinical psychologists may supervise only diagnostic psychological and neuropsychological testing services as described in an exception to the basic rule at § 410.32(b)(2)(iii) for diagnostic psychological and neuropsychological testing services, when these services are personally furnished by a clinical psychologist or an independently practicing psychologist or when they are furnished under the general supervision of a physician or clinical psychologist.
To reflect these proposed changes for the provision of direct supervision of diagnostic services provided to hospital outpatients in the regulations, we proposed to revise existing § 410.28(e). First, we proposed to specify that the provisions of proposed revised paragraph (e) apply to diagnostic services furnished by the hospital, directly or under arrangement, consistent with our proposal to apply the existing diagnostic services supervision requirement for PBDs to diagnostic services provided directly by the hospital or under arrangement. We would continue to specify that the definitions of general and personal physician supervision included in § 410.32(b)(3)(i) and (b)(3)(iii) apply to these levels of supervision of hospital outpatient diagnostic services. Furthermore, we proposed to add new paragraph (e)(1) to § 410.28 to indicate that, for services furnished directly or under arrangement, in the hospital or in an on-campus department of a provider, as defined in § 413.65, direct supervision means that the physician must be present on the same campus, in the hospital or PBD of the hospital as defined in § 413.65, and immediately available to furnish assistance and direction throughout the performance of the procedure. We also would continue to provide that direct supervision does not mean that the physician must be in the room when the procedure is performed. As discussed above, we would apply the definition of “in the hospital” as proposed in § 410.27(g) of the regulations. In addition, we proposed to add new paragraph (e)(2) to § 410.28 to reflect that, for the direct physician supervision of diagnostic services furnished directly or under arrangement in off-campus PBDs of hospitals, the physician must present in the off-campus PBD, as defined in § 413.65, and immediately available to furnish assistance and direction throughout the performance of the procedure. We would continue to provide that direct supervision does not mean that the physician must be in the room when the procedure is performed. Finally, we proposed to add new paragraph (e)(3) to specify that for the direct supervision of hospital outpatient services provided under arrangement in physicians' offices and other nonhospital locations, the definition of direct supervision in § 410.32(b)(3)(ii) applies.
Comment: Some commenters fully supported the proposal for hospital outpatient diagnostic services, agreeing with CMS that it is appropriate to apply the requirements for physician supervision consistently across all sites of service. Other commenters, including major hospitals associations and commenters representing rural hospitals, believed the proposal is unnecessary, unrealistic, costly, and would reduce access to diagnostic services. Several commenters stated that CMS' interpretation of “furnish assistance and direction throughout the performance of the procedure” would mean that the physician would have to be able to operate sophisticated equipment, replacing the technicians who are trained to operate the equipment. Some commenters indicated that rural hospitals would not be able to meet the proposed requirements. Other commenters requested that CMS add a column to Addendum B specifying the required level of supervision for diagnostic tests to make it easier for hospitals to comply with the requirements.
Response: We disagree with the commenters that requiring hospitals to follow the MPFS levels of supervision for individual diagnostic tests would create additional hospital burden for many services because CMS has already applied the lowest level of supervision (general supervision) to numerous diagnostic services. Additionally, in the April 2000 OPPS final rule (65 FR 18536), we codified § 410.28(e) of the regulations to apply this requirement to all on and off campus PBDs of hospitals. It is appropriate to apply the same requirements to diagnostic services that are provided “in the hospital” as those that have been long established for on-campus and off-campus PBDs of the hospital, including rural hospitals. We also believe that, in the interest of safe and high quality care for beneficiaries, it is appropriate for hospitals to follow the same requirements for supervision as physicians and IDTFs when furnishing more complex diagnostic tests that we have specifically identified as requiring direct or personal supervision, or should be performed only by the physician. In addition, since hospitals may contract with other entities to have diagnostic services provided under arrangement, it is also appropriate to ensure that those entities are consistently following the supervision levels that we have identified for both their own patients as well as hospital outpatients. We recognize that specially trained Start Printed Page 60590 ancillary staff and technicians are the primary operators of some specialized diagnostic testing equipment. However, we also believe it is reasonable for the physician that supervises the provision of the services to be knowledgeable about those tests.
Therefore, we are finalizing our CY 2010 proposal that all hospital outpatient diagnostic services that are provided directly or under arrangement, whether provided in the main buildings of the hospital, in a PBD of the hospital, or at a nonhospital location, follow the physician supervision requirements for individual tests as listed in the MPFS Relative Value File. The definitions of general, direct, and personal supervision as defined in §§ 410.32(b)(3)(i) through (b)(3)(iii) also apply. In the case of direct supervision of diagnostic services furnished directly by the hospital or under arrangement in the main hospital buildings or on-campus in a PBD, the definition of direct supervision is the same as the modified definition that we are finalizing for therapeutic services provided on-campus, as discussed in section XII.D.3. of this final rule with comment period, meaning that the physician would be present on the same campus and immediately available to furnish assistance and direction throughout the performance of the procedure. We would continue to specify that direct supervision does not mean that the physician must be in the room when the procedure is performed. As discussed above, we are applying the definition of “in the hospital” as proposed and finalized in § 410.27(g) of the regulations. While the definition of “in the hospital” is no longer a component of the definition of direct supervision, it remains applicable to describe areas operated as part of the hospital that are not PBDs for other purposes, such as services provided under arrangement. It is also appropriate to apply the definition of that term consistently for both diagnostic and therapeutic hospital outpatient services. In addition, we are finalizing our proposal to add new paragraph (e)(2) to § 410.28 to reflect that, for the direct physician supervision of diagnostic services furnished directly or under arrangement in off-campus PBDs of hospitals, the physician must be present in the off-campus PBD of the hospital, as defined in § 413.65, and immediately available to furnish assistance and direction throughout the performance of the procedure. We would continue to specify that direct supervision does not mean that the physician must be in the room when the procedure is performed. Also, we are finalizing our proposal to add new paragraph (e)(3) to specify that for the direct supervision of hospital outpatient services provided under arrangement in physicians' offices and other nonhospital locations, the definition of direct supervision in § 410.32(b)(3)(ii) applies.
We acknowledge the commenters' request to publish the diagnostic supervision levels in Addendum B. Addendum B currently specifies information related directly to the payment for services described by HCPCS codes, including relative weights, payment rates, and copayments. We do not believe it is necessary to include the supervision levels for diagnostic services in Addendum B that are not directly relevant to the payment rates for those services. These supervision levels are readily available on the CMS Web site in the MPFS RVU File, and, because we make both the MPFS RVU File and Addendum B available in spreadsheet format, an interested hospital can easily modify Addendum B to add whatever code-specific information the hospital believes would be most useful to incorporate in a single electronic file for reference purposes.
Comment: Several commenters asserted that nonphysician practitioners should be able to supervise diagnostic tests because they may order and perform diagnostic tests that are within their scope of practice under State law.
Response: We acknowledged in the CY 2010 OPPS/ASC proposed rule (74 FR 35369) that physician assistants, nurse practitioners, clinical nurse specialists, and certified nurse-midwives who operate within the scope practice under State law may order and perform diagnostic tests, as discussed in § 410.32(a)(3) and corresponding manual guidance in Section 80 of Chapter 15 of the Medicare Benefit Policy Manual. However, we noted that this manual guidance and the long established regulation at § 410.32(b)(1) also state that diagnostic x-ray and other diagnostic tests must be furnished under the appropriate level of supervision by a physician as defined in section 1861(r) of the Act. Thus, CMS historically has not permitted physician assistants, nurse practitioners, clinical nurse specialists, and certified nurse-midwives to function as supervisory “physicians” for the purposes of diagnostic tests. In accordance with these existing requirements, we did not propose to allow physician assistants, nurse practitioners, clinical nurse specialists, and certified nurse-midwives to provide the supervision of diagnostic tests provided to hospital outpatients. Because we establish the physician supervision levels in the MPFS Relative Value File based on the policy that only a physician may provide the supervision, we believe it continues to be most appropriate to allow only physicians to provide the supervision of hospital outpatient diagnostic services.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to require that all hospital outpatient diagnostic services that are provided directly or under arrangement, whether provided in the main buildings of the hospital, in a PBD of a hospital, or at a nonhospital location, follow the physician supervision requirements for individual tests as listed in the MPFS RVU File. The definitions of general, direct, and personal supervision as defined in §§ 410.32(b)(3)(i) through (b)(3)(iii) also apply. In the case of direct supervision of diagnostic services furnished directly by the hospital or under arrangement in the main hospital buildings or on-campus in a PBD of a hospital, the definition of direct supervision is the same as the modified definition that we are finalizing for therapeutic services provided on-campus as discussed in section XII.D.3. of this final rule with comment period, meaning that the physician must be present on the same campus and immediately available to furnish assistance and direction throughout the performance of the procedure. We continue to provide that direct supervision does not mean that the physician must be in the room when the procedure is performed. As discussed above, we are applying the definition of “in the hospital” as specified in new § 410.27(g) of the regulations. While the definition of in the hospital is no longer a component of the definition of direct supervision, it remains applicable to describe areas operated as part of the hospital that are not PBDs for other purposes, such as services provided under arrangement. It is also appropriate to apply the definition of that term consistently for both diagnostic and therapeutic hospital outpatient services. In addition, we are finalizing our CY 2010 proposal, without modification, to add new paragraph (e)(2) to § 410.28 to reflect that, for the direct physician supervision of diagnostic services furnished directly or under arrangement in off-campus PBDs of hospitals, the physician must present in the off-campus PBD, as defined in § 413.65, and immediately available to furnish Start Printed Page 60591 assistance and direction throughout the performance of the procedure. We continue to provide that direct supervision does not mean that the physician must be in the room when the procedure is performed. Also, we are finalizing the CY 2010 proposal, without modification, to add new paragraph (e)(3) to specify that, for the direct supervision of hospital outpatient services provided under arrangement in physicians' offices and other nonhospital locations, the definition of direct supervision in § 410.32(b)(3)(ii) applies. We did not propose to allow physician assistants, nurse practitioners, clinical nurse specialists, and certified nurse-midwives to provide the supervision of diagnostic tests provided to hospital outpatients and we are finalizing this policy.
5. Summary of CY 2010 Physician Supervision Final Policy
In summary, for CY 2010, nonphysician practitioners who are specified under § 410.27 of the final regulations as clinical psychologists, licensed clinical social workers, physician assistants, nurse practitioners, clinical nurse specialists, and certified nurse-midwives, may directly supervise all hospital outpatient therapeutic services that they may perform themselves within their State scope of practice and hospital-granted privileges, provided that they meet all additional requirements, including any collaboration or supervision requirements as specified in §§ 410.71, 410.73, 410.74, 410.75, 410.76, and 410.77. We are finalizing the proposed direct physician supervision requirements for PR, CR, and ICR services furnished in the HOPD that would require the supervision to be provided by a doctor of medicine or osteopathy. Accordingly, we are finalizing proposed §§ 410.27(a)(1)(iv)(A) and (B) which indicate that, for PR, CR, and ICR services, direct supervision must be furnished by a doctor of medicine or osteopathy, as specified in §§ 410.47 and 410.49, respectively.
We also are refining the definition of the direct supervision of hospital outpatient therapeutic services for those services provided in the hospital or on-campus PBD of the hospital. For services provided in the hospital or on-campus PBD of the hospital, direct supervision would mean that the physician or nonphysician practitioner must be present on the same campus and immediately available to furnish assistance and direction throughout the performance of the procedure. In addition, we are finalizing the definition of “in the hospital” in new paragraph § 410.27(g) to mean areas in the main building(s) of a hospital or CAH that are under the ownership, financial, and administrative control of the hospital or CAH; that are operated as part of the hospital or CAH; and for which the hospital or CAH bills the services furnished under the hospital's or CAH's CCN.
We are not making any significant changes to the definition or requirements for direct supervision of hospital outpatient therapeutic services provided in off-campus PBDs of a hospital or CAH, other than to allow nonphysician practitioners to provide direct supervision for the services that they may perform themselves in those locations. Therefore, we are finalizing, without modification, the addition of new paragraph (a)(1)(iv)(B) to § 410.27 to reflect that, for off-campus PBDs of hospitals or CAHs, the physician or nonphysician practitioner must be present in the off-campus PBD, as defined in § 413.65, and immediately available to furnish assistance and direction throughout the performance of the procedure. We state that this requirement does not mean that the physician or nonphysician practitioner must be in the room when the procedure is performed.
Additionally, we are finalizing the proposal to make a technical correction to the title of § 410.27 and the text of § 410.27 to clarify throughout that the requirements for payment of hospital outpatient therapeutic services incident to a physician or nonphysician practitioner service in that section apply to both hospitals and CAHs.
For CY 2010, we are finalizing the proposal to require that all hospital outpatient diagnostic services provided directly or under arrangement, whether provided in the hospital, in a PBD of a hospital, or at a nonhospital location, follow the physician supervision requirements for individual tests as listed in the MPFS Relative Value File. The existing definitions of general and personal supervision as defined in §§ 410.32(b)(3)(i) and (b)(3)(iii) also apply. For services furnished directly or under arrangement in the hospital or on-campus PBD, direct supervision means that the physician must be present on the same campus and immediately available to furnish assistance and direction throughout the performance of the procedure. For the purposes of § 410.28, as for the general purposes of § 410.27, the definition of “in the hospital,” as defined in § 410.27(g), applies. For diagnostic services furnished directly or under arrangement off-campus in a PBD of the hospital, direct supervision continues to mean that the physician must be present in the off-campus PBD and immediately available to furnish assistance and direction throughout the performance of the procedures. For all hospital outpatient diagnostic services provided under arrangement in nonhospital locations, such as IDTFs and physicians' offices, the existing definition of direct supervision under § 410.32(b)(3)(ii) applies. We are revising § 410.28 of the regulations to reflect these changes.
E. Direct Referral for Observation Services
Since CY 2003, hospitals have reported a Level II HCPCS code for Medicare billing purposes for a “direct admission” to a hospital for outpatient observation services. In section 290 of Chapter 4 of the Medicare Claims Processing Manual (Pub. 100–4), we define a “direct admission” as the direct referral of a patient by a community physician to a hospital for observation services without an associated emergency room visit, hospital outpatient clinic visit, critical care service, or hospital outpatient surgical procedure (that is, a status indicator “T” procedure) on the day of the initiation of observation services. Since CY 2006, we have instructed hospitals to report a “direct admission” referred for observation services using HCPCS code G0379 (Direct admission of patient for hospital observation care) (70 FR 68688 through 68691).
Observation care is a hospital outpatient service that is reported using HCPCS code G0378 (Hospital observation services, per hour). Hospitals report outpatient observation services, which are commonly provided in association with a hospital clinic visit, emergency department visit, or other major service, on hospital outpatient claims, just like other outpatient services. Physicians order observation care, defined as clinically appropriate services, including ongoing short-term treatment, assessment, and reassessment furnished in order for the physician to determine whether the beneficiary will require further treatment as an inpatient or whether the beneficiary may be safely discharged from the hospital.
We have become aware that, because the word “admission” is generally used in reference to inpatient hospital care, our historical use of the phrase “direct admission” in the code descriptor for HCPCS code G0379 and the use of the phrase “observation status” in the Medicare Claims Processing Manual (Chapter 4, Section 290) and the Medicare Benefit Policy Manual Start Printed Page 60592 (Chapter 6, Section 20) may be contributing to confusion for hospitals and beneficiaries related to a beneficiary's status as an inpatient or an outpatient when he or she is receiving observation services. For Medicare payment purposes, there is no patient status termed “observation status.” Hospitals may only bill for items and services furnished to inpatients, outpatients, or nonpatients. We believe that using terminology such as “observation status” or “admission to observation” may be confusing for physicians, hospitals, and beneficiaries. Therefore, in the CY 2010 OPPS/ASC proposed rule (74 FR 35370 through 35371), we proposed to modify the code descriptor for HCPCS code G0379 to remove the reference to the word “admission” and to replace it with “referral.” The proposed long code descriptor for HCPCS code G0379 would be “Direct referral for hospital observation care.” We proposed this change to more accurately reflect that the physician in the community has referred the beneficiary to the hospital for observation services as a hospital outpatient. In addition to the proposed CY 2010 change to the code descriptor for HCPCS code G0379, we modified the Medicare Claims Processing Manual and the Medicare Benefit Policy Manual to remove most references related to “admission” for observation services or “observation status.” We refer readers to Transmittal 1760 dated June 23, 2009 (which rescinded and replaced Transmittal 1745, dated May 22, 2009) and Transmittal 107 dated May 22, 2009 (both issued under Change Request 6492), for more information regarding the specific changes incorporated in the manuals.
We did not propose to change the status indicator or payment methodology for HCPCS code G0379 for CY 2010. Instead, we proposed to continue the payment policy that was finalized for the CY 2009 OPPS (73 FR 68554). In the CY 2010 OPPS/ASC proposed rule (74 FR 35370 through 35371), we proposed to continue to assign HCPCS code G0379 status indicator “Q3,” indicating that it would be eligible for payment through APC 8002 (Level I Extended Assessment & Management Composite) when certain criteria are met or through APC 0604 (Level I Hospital Clinic Visits) when other criteria are met; otherwise, its payment would be packaged into payment for other separately payable services in the same encounter. The established criteria for payment of HCPCS code G0379 under either composite APC 8002, as part of the extended assessment and management composite service, or APC 0604, as a separately payable individual service that we would continue for CY 2010 are: (1) both HCPCS codes G0378 and G0379 are reported with the same date of service; and (2) no service with a status indicator of “T” or “V” or Critical Care (APC 0617) is provided on the same date of service as HCPCS code G0379. If either of the above criteria is not met, HCPCS code G0379 is assigned status indicator “N” and its payment is packaged into the payment for other separately payable services provided in the same encounter.
We did not receive any public comments related to our CY 2010 proposal to revise the code descriptor for HCPCS code G0379 to read “Direct referral for hospital observation care,” or on our proposal to continue the CY 2009 status indicator assignment and payment methodology for HCPCS code G0379 for CY 2010. Therefore, we are finalizing these CY 2010 proposals, without modification.
XIII. OPPS Payment Status and Comment Indicators
A. OPPS Payment Status Indicator Definitions
Payment status indicators (SIs) that we assign to HCPCS codes and APCs play an important role in determining payment for services under the OPPS. They indicate whether a service represented by a HCPCS code is payable under the OPPS or another payment system and also whether particular OPPS policies apply to the code. The final CY 2010 status indicator assignments for APCs and HCPCS codes are shown in Addendum A and Addendum B, respectively, to this final rule with comment period.
As we proposed in the CY 2010 OPPS/ASC proposed rule (74 FR 35371), in this final rule with comment period, we are changing the definitions of status indicators “H” and “K.” We did not propose to make any changes to the other status indicators that were listed in Addendum D1 of the CY 2009 OPPS/ASC final rule with comment period. The final status indicators are listed in the tables under sections XIII.A.1., 2., 3., and 4. of this final rule with comment period.
1. Payment Status Indicators to Designate Services That Are Paid Under the OPPS
| Indicator | Item/code/service | OPPS payment status |
|---|---|---|
| G | Pass-Through Drugs and Biologicals | Paid under OPPS; separate APC payment. |
| H | Pass-Through Device Categories | Separate cost-based pass-through payment; not subject to copayment. |
| K | Nonpass-Through Drugs and Nonimplantable Biologicals, including Therapeutic Radiopharmaceuticals | Paid under OPPS; separate APC payment. |
| N | Items and Services Packaged into APC Rates | Paid under OPPS; payment is packaged into payment for other services. Therefore, there is no separate APC payment. |
| P | Partial Hospitalization | Paid under OPPS; per diem APC payment. |
| Q1 | STVX–Packaged Codes | Paid under OPPS; Addendum B displays APC assignments when services are separately payable. (1) Packaged APC payment if billed on the same date of service as a HCPCS code assigned status indicator “S,” “T,” “V,” or “X.” (2) In all other circumstances, payment is made through a separate APC payment. |
| Q2 | T–Packaged Codes | Paid under OPPS; Addendum B displays APC assignments when services are separately payable. (1) Packaged APC payment if billed on the same date of service as a HCPCS code assigned status indicator “T.” (2) In all other circumstances, payment is made through a separate APC payment. |
| Start Printed Page 60593 | ||
| Q3 | Codes that may be paid through a composite APC | Paid under OPPS; Addendum B displays APC assignments when services are separately payable. Addendum M displays composite APC assignments when codes are paid through a composite APC. (1) Composite APC payment based on OPPS composite-specific payment criteria. Payment is packaged into a single payment for specific combinations of service. (2) In all other circumstances, payment is made through a separate APC payment or packaged into payment for other services. |
| R | Blood and Blood Products | Paid under OPPS; separate APC payment. |
| S | Significant Procedure, Not Discounted When Multiple | Paid under OPPS; separate APC payment. |
| T | Significant Procedure, Multiple Reduction Applies | Paid under OPPS; separate APC payment. |
| U | Brachytherapy Sources | Paid under OPPS; separate APC payment. |
| V | Clinic or Emergency Department Visit | Paid under OPPS; separate APC payment. |
| X | Ancillary Services | Paid under OPPS; separate APC payment. |
Section 142 of Public Law 110–275 (MIPPA) required CMS to pay for therapeutic radiopharmaceuticals for the period of July 1, 2008, through December 31, 2009, at hospitals' charges adjusted to the costs. The status indicator “H” was assigned to therapeutic radiopharmaceuticals to indicate that an item was paid at charges adjusted to cost during CY 2009. In the CY 2010 OPPS/ASC proposed rule (74 FR 35373), we proposed to pay prospectively and separately for therapeutic radiopharmaceuticals with average per day costs greater than the proposed CY 2010 drug packaging threshold of $65 under the OPPS. Therefore, we proposed to change the status indicator for HCPCS codes used to report separately payable therapeutic radiopharmaceuticals from “H” to “K,” which indicates that an item is separately paid under the OPPS at the APC payment rate established for the item. We refer readers to section V.B.5. of the proposed rule (74 FR 35333 through 36336) and this final rule with comment period for discussion of the proposed and final CY 2010 changes to our payment policy for therapeutic radiopharmaceuticals.
We received many comments on our proposal to establish prospective payment rates for therapeutic radiopharmaceuticals. We respond to these comments and discuss our final policy in section V. B. 5. of this final rule with comment period. However, we did not receive any public comments related to our proposal to change the definitions of status indicators “H” and “K,” to reflect this change in policy. Therefore, we are changing the definitions of status indicators “H” and “K” as proposed, without modification, to reflect our final therapeutic radiopharmaceutical payment policy, and we are finalizing assignment of status indicator “K” to therapeutic radiopharmaceuticals with average per day costs greater than the final CY 2010 drug packaging threshold of $65 under the OPPS.
As we discussed in detail in section V.A.4. of the CY 2010 OPPS/ASC proposed rule (74 FR 35311 through 35314), we proposed to consider implantable biologicals that are not on pass-through status as a biological before January 1, 2010, as devices for pass-through evaluation and payment beginning in CY 2010. Therefore, as devices, pass-through implantable biologicals would be assigned a status indicator of “H,” while nonpass-through implantable biologicals would be assigned a status indicator of “N” beginning in CY 2010. Those implantable biologicals that have been granted pass-through status under the drug and biological criteria prior to January 1, 2010, would continue to be assigned a status indicator of “G” until they are proposed for expiration from pass-through status during our annual rulemaking cycle. In the proposed rule (74 FR 35373), we proposed to assign status indicator “K” to nonimplantable biologicals and to adjust the definition of status indicator “K” accordingly.
We received numerous comments on our proposal to treat implantable biologicals as devices and we respond to them in section V.A.4. of this final rule with comment period. We did not receive any public comments with regard to the proposed changes to status indicator “K” to reflect the implantable biological pass-through payment policy. Therefore, we are finalizing our proposal, without modification, to assign status indicator “K” to nonimplantable biologicals and to adjust the definition of status indicator “K” accordingly.
The final CY 2010 status indicators are displayed in the table above, as well as in Addendum D1 to this final rule with comment period.
2. Payment Status Indicators to Designate Services That Are Paid Under a Payment System Other Than the OPPS
We did not propose any changes to the status indicators listed below for the CY 2010 OPPS.
| Indicator | Item/code/service | OPPS payment status |
|---|---|---|
| A | Services furnished to a hospital outpatient that are paid under a fee schedule or payment system other than OPPS, for example: | Not paid under OPPS. Paid by fiscal intermediaries/MACs under a fee schedule or payment system other than OPPS. |
| • Ambulance Services | ||
| • Clinical Diagnostic Laboratory Services | Not subject to deductible or coinsurance. | |
| • Non-Implantable Prosthetic and Orthotic Devices | ||
| • EPO for ESRD Patients | ||
| • Physical, Occupational, and Speech Therapy | ||
| • Routine Dialysis Services for ESRD Patients Provided in a Certified Dialysis Unit of a Hospital | ||
| • Diagnostic Mammography | ||
| • Screening Mammography | Not subject to deductible. | |
| C | Inpatient Procedures | Not paid under OPPS. Admit patient. Bill as inpatient. |
| Start Printed Page 60594 | ||
| F | Corneal Tissue Acquisition; Certain CRNA Services; and Hepatitis B Vaccines | Not paid under OPPS. Paid at reasonable cost. |
| L | Influenza Vaccine; Pneumococcal Pneumonia Vaccine | Not paid under OPPS. Paid at reasonable cost; not subject to deductible or coinsurance. |
| M | Items and Services Not Billable to the Fiscal Intermediary/MAC | Not paid under OPPS. |
| Y | Non-Implantable Durable Medical Equipment | Not paid under OPPS. All institutional providers other than home health agencies bill to DMERC. |
We did not receive any public comments regarding the status indicators that designate services that are paid under a payment system other than the OPPS. Therefore, we are finalizing our CY 2010 proposal, without modification. The final CY 2010 status indicators are displayed in the table above, as well as in Addendum D1 to this final rule with comment period.
3. Payment Status Indicators To Designate Services That are Not Recognized Under the OPPS but That May Be Recognized by Other Institutional Providers
We did not propose any changes to the status indicators listed below for the CY 2010 OPPS.
| Indicator | Item/code/service | OPPS payment status |
|---|---|---|
| B | Codes that are not recognized by OPPS when submitted on an outpatient hospital Part B bill type (12x and13x) | Not paid under OPPS. • May be paid by fiscal intermediaries/MACs when submitted on a different bill type, for example, 75x (CORF), but not paid under OPPS. |
| • An alternate code that is recognized by OPPS when submitted on an outpatient hospital Part B bill type (12x and 13x) may be available. |
We did not receive any public comments regarding the status indicators that designate services that are not recognized under the OPPS but that may be recognized for payment to other institutional providers. Therefore, we are finalizing our CY 2010 proposal, without modification. The final status indicators are displayed in the table above, as well as in Addendum D1 to this final rule with comment period.
4. Payment Status Indicators To Designate Services That Are Not Payable by Medicare on Outpatient Claims
We did not propose any changes to the payment status indicators listed below for the CY 2010 OPPS.
| Indicator | Item/code/service | OPPS payment status |
|---|---|---|
| D | Discontinued Codes | Not paid under OPPS or any other Medicare payment system. |
| E | Items, Codes, and Services: • That are not covered by any Medicare outpatient benefit based on statutory exclusion | Not paid by Medicare when submitted on outpatient claims (any outpatient bill type). |
| • That are not covered by any Medicare outpatient benefit for reasons other than statutory exclusion | ||
| • That are not recognized by Medicare for outpatient claims; alternate code for the same item or service may be available | ||
| • For which separate payment is not provided on outpatient claims |
We did not receive any public comments related to payment status indicators that designate services that are not payable by Medicare on outpatient claims and, therefore, we are finalizing our CY 2010 proposal, without modification. The final status indicators are displayed in the table above, as well as in Addendum D1 to this final rule with comment period.
Addendum B, with a complete listing of HCPCS codes that includes their payment status indicators and APC assignments for CY 2010 is available electronically on the CMS Web site under supporting documentation for this final rule with comment period at: http://www.cms.hhs.gov/HospitalOutpatientPPS/HORD/list.asp#TopOfPage.
B. Comment Indicator Definitions
In the CY 2010 OPPS/ASC proposed rule (74 FR35374), we proposed to use the same two comment indicators that are in effect for the CY 2009 OPPS.
- “CH”—Active HCPCS codes in current and next calendar year; status indicator and/or APC assignment have changed or active HCPCS code that will be discontinued at the end of the current calendar year.
- “NI”—New code for the next calendar year or existing code with substantial revision to its code descriptor in the next calendar year as compared to current calendar year, interim APC assignment; comments will be accepted on the interim APC assignment for the new code.
We proposed to use the “CH” comment indicator in this CY 2010 OPPS/ASC final rule with comment period to indicate HCPCS codes for which the status indicator or APC assignment, or both, would change in CY 2010 compared to their assignment as of December 31, 2009.
We believe that using the “CH” indicator in this CY 2010 OPPS/ASC final rule with comment period will Start Printed Page 60595 help facilitate the public's review of the changes that we are finalizing for CY 2010. The use of the comment indicator “CH” in association with a composite APC indicates that the configuration of the composite APC is changed in this CY 2010 OPPS/ASC final rule with comment period.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35374 through 35375), we did not propose any changes to the definitions of the OPPS comment indicators for CY 2010 and we did not receive any public comments on the comment indicators. However, we want to clarify our policy regarding the use of comment indicator “NI” in this CY 2010 OPPS/ASC final rule with comment period to describe a new code. There are numerous instances in which the descriptor of an existing Category I CPT code is substantially revised for CY 2010 so that it describes a new service or procedure that could have been assigned a new code number by the CPT Editorial Panel and that new code number would then have been assigned the “NI” comment indicator. Because, for CY 2010, not all new services or procedures will be assigned a new CPT code number, but instead will be described by an existing CPT code number with a substantially revised code descriptor, we are assigning the comment indicator “NI” to these codes in order to allow for comment on these substantially revised codes. Therefore, for this final rule with comment period, we have expanded the definition of comment indicator “NI” to include an existing code with a substantial revision to its code descriptor in the next calendar year as compared to the current calendar year to indicate that the code's CY 2010 OPPS treatment is open to public comment on this final rule with comment period. Like all codes labeled with comment indicator “NI,” we will respond to public comments and finalize their OPPS treatment in the CY 2011 OPPS/ASC final rule with comment period. In accordance with our usual practice, CPT and Level II HCPCS code numbers that are new for CY 2010 are also labeled with comment indicator “NI” in Addendum B to this final rule with comment period.
Only HCPCS codes with comment indicator “NI” in this CY 2010 OPPS/ASC final rule with comment period are subject to comment. HCPCS codes that do not appear with comment indicator “NI” in this CY 2010 OPPS/ASC final rule with comment period are not open to public comment, unless we specifically have requested additional comments elsewhere in this final rule with comment period. The CY 2010 treatment of HCPCS codes that appears in this CY 2010 OPPS/ASC final rule with comment period to which comment indicator “NI” is not appended was open to public comment during the comment period for the CY 2010 OPPS/ASC proposed rule, and we are responding to those comments in this final rule with comment period.
We did not receive any public comments regarding comment indicators. Therefore, we are finalizing our proposal, without modification, and we are continuing to use the two comment indicators, “CH” and “NI,” for CY 2010. Their definitions are listed in Addendum D2 to this final rule with comment period.
XIV. OPPS Policy and Payment Recommendations
A. MedPAC Recommendations
MedPAC was established under section 1805 of the Act to advise the U.S. Congress on issues affecting the Medicare program. As required under the statute, MedPAC submits reports to Congress not later than March and June of each year that present its Medicare payment policy recommendations. The following section describes recent recommendations relevant to the OPPS that have been made by MedPAC.
The March 2009 MedPAC “Report to Congress: Medicare Payment Policy” included the following recommendation relating specifically to the Medicare hospital OPPS:
Recommendation 2A–1: The Congress should increase payment rates for the acute inpatient and outpatient prospective payment systems in 2010 by the projected rate of increase in the hospital market basket index, concurrent with implementation of a quality incentive payment program.
CMS Response: As proposed in the CY 2010 OPPS/ASC proposed rule (74 FR 35375), in this final rule with comment period, we are increasing payment rates for the CY 2010 OPPS by the projected rate of increase in the hospital market basket through adjustment of the full CY 2010 conversion factor. Simultaneously, for CY 2010, we are continuing to reduce the annual update factor by 2.0 percentage points for hospitals that are defined under section 1886(d)(1)(B) of the Act and that do not meet the hospital outpatient quality data reporting required by section 1833(t)(17) of the Act. Specifically, we have calculated two conversion factors: A full conversion factor based on the full hospital market basket increase and a reduced conversion factor that reflects the 2.0 percentage point reduction to the hospital market basket. We discuss our update of the conversion factor and our adoption and implementation of the reduced conversion factor that will apply to hospitals that fail their quality reporting requirements for the full CY 2010 OPPS update in section XVI of this final rule with comment period.
The full March 2009 MedPAC report can be downloaded from MedPAC's Web site at: http://www.medpac.gov/documents/Mar09_EntireReport.pdf.
We note that MedPAC also submitted comments on the CY 2010 OPPS/ASC proposed rule. The specific issues that were the subject of MedPAC's comments and the sections of this final rule with comment period where they are addressed are listed below:
- Pharmacy overhead costs and setting payments for separately paid drugs: Section V.B.3.
- Payment rates for brachytherapy sources and therapeutic radiopharmaceuticals: Sections VII. and V.B.5.
- Collection of quality data through clinical registries and electronic health records (EHRs): Section XVI.I.
- Collection of quality data from ASCs: Section XVI.H.
- Collection of cost data from ASCs: Section XV.G.
- Payment policy for healthcare-associated conditions: Section XVII.
B. APC Panel Recommendations
Recommendations made by the APC Panel at its February 2009 and August 2009 meetings are discussed in the sections of this final rule with comment period that correspond to topics addressed by the APC Panel. The report and recommendations from the APC Panel's February 18–19, 2009 and August 5–6, 2009 meetings are available on the CMS Web site at: http://www.cms.hhs.gov/FACA/05_AdvisoryPanelonAmbulatoryPaymentClassificationGroups.asp.
C. OIG Recommendations
The mission of the Office of the Inspector General (OIG), as mandated by Public Law 95–452, as amended, is to protect the integrity of the U.S. Department of Health and Human Services (HHS) programs as well as the health and welfare of beneficiaries served by those programs. This statutory mission is carried out through a nationwide network of audits, investigations, and inspections. In June 2007, the OIG released a report, entitled “Impact of Not Retroactively Adjusting Outpatient Outlier Payments,” that described the OIG's research into sources of errors in CMHC outlier payments. The OIG report included the Start Printed Page 60596 following two recommendations relating specifically to the hospital OPPS under which payment is made for outpatient services provided by CMHCs.
Recommendation 1: The OIG recommended that CMS require adjustments of outpatient outlier payments at final cost report settlement, retroactive to the beginning of the cost report period.
Recommendation 2: The OIG recommended that CMS require retroactive adjustments of outpatient outlier payments when an error caused by the fiscal intermediary or provider is identified after a cost report is settled.
We addressed both of these recommendations in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68594). We noted in that final rule that the OIG's findings were based largely on information from the OPPS' early implementation period, between CY 2000 and CY 2003, and that we believed we had taken several steps since that time in order to improve the accuracy and frequency of the Medicare contractors' CCR calculations, including updating our instructions for calculating CCRs, increasing the frequency of CCR calculation, and conducting an annual review of CMHC CCRs.
However, taking into account these OIG recommendations, we proposed and finalized a policy to provide for reconciliation of outlier payments under the OPPS at final cost report settlement as recommended by the OIG, beginning in CY 2009. We discuss our rationale for this policy in detail in section II.F.4. of the CY 2009 OPPS/ASC final rule with comment period (73 FR 68594 through 68599).
Other than the June 2007 recommendations, there have been no other recent OIG recommendations pertaining to the OPPS.
XV. Updates to the Ambulatory Surgical Center (ASC) Payment System
A. Background
1. Legislative Authority for the ASC Payment System
Section 1832(a)(2)(F)(i) of the Act provides that benefits under Medicare Part B include payment for facility services furnished in connection with surgical procedures specified by the Secretary that are performed in an ASC. To participate in the Medicare program as an ASC, a facility must meet the standards specified in section 1832(a)(2)(F)(i) of the Act, which are set forth in 42 CFR part 416, subpart B and Subpart C of our regulations. The regulations at 42 CFR part 416, subpart B describe the general conditions and requirements for ASCs, and the regulations at subpart C explain the specific conditions for coverage for ASCs.
Section 141(b) of the Social Security Act Amendments of 1994, Public Law 103–432, required establishment of a process for reviewing the appropriateness of the payment amount provided under section 1833(i)(2)(A)(iii) of the Act for intraocular lenses (IOLs) that belong to a class of new technology intraocular lenses (NTIOLs). That process was the subject of a final rule entitled “Adjustment in Payment Amounts for New Technology Intraocular Lenses Furnished by Ambulatory Surgical Centers,” published on June 16, 1999, in the Federal Register (64 FR 32198).
Section 626(b) of the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (MMA), Public Law 108–173, added subparagraph (D) to section 1833(i)(2) of the Act, which required the Secretary to implement a revised ASC payment system to be effective not later than January 1, 2008. Section 626(c) of the MMA amended section 1833(a)(1) of the Act by adding new subparagraph (G), which requires that, beginning with implementation of the revised ASC payment system, payment for surgical procedures furnished in ASCs shall be 80 percent of the lesser of the actual charge for the services or the amount determined by the Secretary under the revised payment system.
Section 5103 of the Deficit Reduction Act of 2005 (DRA), Public Law 109–171, amended section 1833(i)(2) of the Act by adding a new subparagraph (E) to place a limitation on payment amounts for surgical procedures furnished in ASCs on or after January 1, 2007, but before the effective date of the revised ASC payment system (that is, January 1, 2008). Section 1833(i)(2)(E) of the Act provides that if the standard overhead amount under section 1833(i)(2)(A) of the Act for an ASC facility service for such surgical procedures, without application of any geographic adjustment, exceeds the Medicare payment amount under the hospital OPPS for the service for that year, without application of any geographic adjustment, the Secretary shall substitute the OPPS payment amount for the ASC standard overhead amount.
Section 109(b) of the Medicare Improvements and Extension Act of 2006 of the Tax Relief and Health Care Act of 2006 (MIEA–TRHCA), Public Law 109–432, amended section 1833(i) of the Act, in part, by redesignating clause (iv) as clause (v) and adding a new clause (iv) to paragraph (2)(D) and adding paragraph (7)(A), which provide the Secretary the authority to require ASCs to submit data on quality measures and to reduce the annual update by 2 percentage points for an ASC that fails to submit data as required by the Secretary on selected quality measures. Section 109(b) of the MIEA–TRHCA also amended section 1833(i) of the Act by adding new paragraph (7)(B), which requires that, to the extent the Secretary establishes such an ASC quality reporting program, certain quality of care reporting requirements mandated for hospitals paid under the OPPS, under section 109(a) of the MIEA–TRHCA, be applied in a similar manner to ASCs unless otherwise specified by the Secretary.
For a detailed discussion of the legislative history related to ASCs, we refer readers to the June 12, 1998 proposed rule (63 FR 32291 through 32292).
2. Prior Rulemaking
On August 2, 2007, we published in the Federal Register (72 FR 42470) the final rule for the revised ASC payment system, effective January 1, 2008 (the “August 2, 2007 final rule”). We revised our criteria for identifying surgical procedures that are eligible for Medicare payment when furnished in ASCs and adopted the method we would use to set payment rates for ASC covered surgical procedures and covered ancillary services furnished in association with those covered surgical procedures beginning in CY 2008. In that final rule, we also established a policy for updating on an annual calendar year basis the ASC conversion factor, the relative payment weights, the ASC payment rates, and the list of procedures for which Medicare would not make an ASC payment. We also established a policy for treating new and revised HCPCS and CPT codes under the ASC payment system. This policy is consistent with the OPPS to the extent possible (72 FR 42533).
In the CY 2008 OPPS/ASC final rule with comment period (72 FR 66827), we updated and finalized the CY 2008 ASC rates and lists of covered surgical procedures and covered ancillary services. We also made regulatory changes to 42 CFR parts 411, 414, and 416 related to our final policies to provide payments to physicians who perform noncovered ASC procedures in ASCs based on the facility practice expense (PE) relative value units (RVUs), to exclude covered ancillary radiology services and covered ancillary drugs and biologicals from the categories of designated health services (DHS) that are subject to the physician self-referral prohibition, and to reduce Start Printed Page 60597 ASC payments for surgical procedures when the ASC receives full or partial credit toward the cost of the implantable device. In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68722), we updated and finalized the CY 2009 ASC rates and lists of covered surgical procedures and covered ancillary services.
3. Policies Governing Changes to the Lists of Codes and Payment Rates for ASC Covered Surgical Procedures and Covered Ancillary Services
The August 2, 2007 final rule established our policies for determining which procedures are ASC covered surgical procedures and covered ancillary services. Under §§ 416.2 and 416.166 of the regulations, subject to certain exclusions, covered surgical procedures are surgical procedures that are separately paid under the OPPS, that would not be expected to pose a significant risk to beneficiary safety when performed in an ASC, and that would not be expected to require active medical monitoring and care at midnight following the procedure (“overnight stay”). We adopted this standard for defining which surgical procedures are covered surgical procedures under the ASC payment system as an indicator of the complexity of the procedure and its appropriateness for Medicare payment in ASCs. We use this standard only for purposes of evaluating procedures to determine whether or not they are appropriate for Medicare beneficiaries in ASCs. We define surgical procedures as those described by Category I CPT codes in the surgical range from 10000 through 69999, as well as those Category III CPT codes and Level II HCPCS codes that crosswalk or are clinically similar to ASC covered surgical procedures (72 FR 42478). We note that we added over 800 surgical procedures to the list of covered surgical procedures for ASC payment in CY 2008, the first year of the revised ASC payment system, based on the criteria for payment that we adopted in the August 2, 2007 final rule as described above in this section. Patient safety and health outcomes continue to be important to us as more health care moves to the ambulatory care setting. Therefore, as we gain additional experience with the ASC payment system, we are interested in any information the public may have regarding the comparative patient outcomes of surgical care provided in ambulatory settings, including HOPDs, ASCs, and physicians' offices, particularly with regard to the Medicare population.
In the August 2, 2007 final rule, we also established our policy to make separate ASC payments for the following ancillary items and services when they are provided integral to ASC covered surgical procedures: Brachytherapy sources; certain implantable items that have pass-through status under the OPPS; certain items and services that we designate as contractor-priced, including, but not limited to, procurement of corneal tissue; certain drugs and biologicals for which separate payment is allowed under the OPPS; and certain radiology services for which separate payment is allowed under the OPPS. These covered ancillary services are specified in § 416.164(b) and, as stated previously, are eligible for separate ASC payment (72 FR 42495). Payment for ancillary items and services that are not paid separately under the ASC payment system is packaged into the ASC payment for the covered surgical procedure.
The full CY 2009 lists of ASC covered surgical procedures and covered ancillary services are included in Addenda AA and BB, respectively, to the CY 2009 OPPS/ASC final rule with comment period (73 FR 68840 through 68933 and 69270 through 69308).
We update the lists of, and payment rates for, covered surgical procedures and covered ancillary services, in conjunction with the annual proposed and final rulemaking process to update the OPPS and ASC payment systems (§ 416.173; 72 FR 42535). In addition, because we base ASC payment policies for covered surgical procedures, drugs, biologicals, and certain other covered ancillary services on the OPPS payment policies, we also provide quarterly updates for ASC services throughout the year (January, April, July, and October), just as we do for the OPPS. The updates are to implement newly created Level II HCPCS codes and Category III CPT codes for ASC payment and to update the payment rates for separately paid drugs and biologicals based on the most recently submitted ASP data. New Category I CPT codes, except vaccine codes, are released only once a year and, therefore, are implemented through the January quarterly update. New Category I CPT vaccine codes are released twice a year and thus are implemented through the January and July quarterly updates.
In our annual updates to the ASC list of, and payment rates for, covered surgical procedures and covered ancillary services, we undertake a review of excluded surgical procedures (including all procedures newly proposed for removal from the OPPS inpatient list), new procedures, and procedures for which there is revised coding, to identify any that we believe meet the criteria for designation as ASC covered surgical procedures or covered ancillary services. Updating the lists of covered surgical procedures and covered ancillary services, as well as their payment rates, in association with the annual OPPS rulemaking cycle is particularly important because the OPPS relative payment weights and, in some cases, payment rates, are used as the basis for the payment of covered surgical procedures and covered ancillary services under the revised ASC payment system. This joint update process ensures that the ASC updates occur in a regular, predictable, and timely manner.
Comment: Several commenters provided a number of general suggestions related to the ASC list of covered surgical procedures. They contended that CMS should not restrict which procedures are payable in ASCs any more than CMS restricts which procedures are payable in HOPDs. The commenters added that if the policy to exclude procedures from the list is maintained, CMS should at least provide the exclusionary criteria for all of the payable OPPS procedures that are excluded from the ASC list so that the public can provide meaningful comments about CMS' decisions. They suggested that CMS publish an addendum to the proposed and final OPPS/ASC rules that would identify which of the criteria at § 416.166(c) triggered CMS' decision to exclude each procedure.
Some commenters urged CMS to eliminate unlisted codes from the exclusionary criteria at § 416.166(c), and other commenters requested that ASCs be allowed to use unlisted codes to bill for procedures that are from anatomic sites that could not possibly pose a potential risk to beneficiary safety. The commenters reported that unlisted codes enable surgeons to utilize innovative techniques or new technologies and are paid under the OPPS and by commercial insurers. They suggested that ASCs could provide documentation to the contractor that explains and justifies the procedure reported by an unlisted code; thus ensuring that Medicare does not make payment for a service that would otherwise be excluded from payment.
Response: We appreciate the commenters' suggestions regarding the consistency of CMS' decisions about which procedures are excluded from the ASC list. However, as we explained in the August 2, 2007 final rule (72 FR 42479), we do not believe that all Start Printed Page 60598 procedures that are appropriate for performance in HOPDs are appropriate in ASCs. HOPDs are able to provide much higher acuity care than ASCs. ASCs have neither patient safety standards consistent with those in place for hospitals, nor are they required to have the trained staff and equipment needed to provide the breadth and intensity of care that hospitals are required to maintain. Therefore, we are not modifying our policy and will continue to exclude from the ASC list of covered surgical procedures certain procedures for which payment is made in HOPDs.
We do not agree with the commenters' request that we provide specific reasons for our decisions to exclude each procedure from the ASC list other than that we believe a procedure is expected to pose a significant risk to beneficiary safety or to require an overnight stay. We believe that these reasons are sufficiently specific to enable the public to provide meaningful comments on our decisions to exclude procedures from the list of covered surgical procedures. Our decisions to exclude procedures from the ASC list are based on a number of the criteria listed at § 416.166 of the regulations, and we believe that it would be unnecessary and overly burdensome to list each and every reason for those decisions.
We also do not agree with the commenters' recommendation that we include certain unlisted codes on the list of covered procedures. Even though it may be highly unlikely that any procedures that would be expected to pose a risk to beneficiary safety or to require an overnight stay would be reported by an unlisted code from certain anatomic sites, we cannot know what surgical procedure is being reported by an unlisted code. Therefore, because we cannot evaluate any such procedure, we believe that we must exclude unlisted codes as a group from the list of covered surgical procedures.
We do not believe it is reasonable, or within the scope of our contractors' work, to accept the commenters' suggestion that ASCs could provide documentation to our Medicare contractors, upon request, in order for the contractors to make a retrospective determination about whether or not a procedure that was billed using an unlisted code represented a significant risk to beneficiary safety or would be expected to require an overnight stay.
Comment: One commenter noted that, although CMS specified in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68714) that patients may remain in ASCs up to 24 hours in order to allow adequate time for recovery following some surgical procedures, CMS did not specify the requirements for physician supervision during the recovery period. The commenter argued that CMS also failed to specify the time period during which the required post-anesthesia assessment is to be performed by requiring only that it be performed prior to discharge from the ASC. The commenter's concern was that patients in ASCs may have no physician supervision for extended periods, a policy in contrast to CMS' policy regarding the direct physician supervision required for hospital outpatient services. The commenter requested that CMS clarify why the same supervision requirements are not applied equally to hospitals and ASCs.
Response: Historically, Medicare has covered surgical procedures performed in ASCs that have relatively short recovery periods and, therefore, we have believed that physicians were always immediately available to furnish assistance and direction in the ASC while ASC services were being furnished, including during the postoperative recovery period. However, as the commenter points out, not only have we recently revised the Conditions for Coverage to allow longer stays in ASCs, we have greatly expanded the list of covered surgical procedures under the revised ASC payment system, including covering some surgical procedures that may require a prolonged recovery period. Given these two revisions, both of which enable ASCs to provide more clinically complex surgical procedures, and taking into consideration patient safety and quality of care, we believe it could be appropriate to consider establishing requirements for physician or nonphysician practitioner supervision in ASCs, similar to the requirements for the direct supervision of hospital outpatient therapeutic services that we are finalizing for HOPDs in this CY 2010 OPPS/ASC final rule with comment period. We note that, for therapeutic services furnished incident to a physician's professional service in an office setting, there also is a requirement for direct physician supervision, meaning that the physician must be in the office suite and immediately available to furnish assistance and direction throughout the procedure (§ 410.26(a)(2) and (b)(5)). In addition, we note that payment for covered ancillary services may be made to ASCs, including payment for some of the diagnostic tests that would be subject to the physician supervision requirements for hospital outpatient diagnostic services if provided in the HOPD. The final CY 2010 physician supervision requirements for hospital outpatient diagnostic and therapeutic services are discussed in detail in section XII.E. of this final rule with comment period. For diagnostic services furnished in physicians' offices, IDTFs and other Part B settings, the requirements of § 410.32 of the regulations apply, including supervision of diagnostic services.
We did not propose to adopt supervision requirements for therapeutic and diagnostic services furnished in ASCs similar to the requirements for HOPDs for CY 2010. However, given the overlap in surgical procedures that may be performed in HOPDs and ASCs and the increased breadth and complexity of ASC covered procedures, we are requesting comments on this final rule with comment period that address: (1) What types of practitioners currently provide the diagnostic and therapeutic services in ASCs, particularly during the extended postoperative recovery period; (2) what types of practitioners currently provide the supervision for the diagnostic and therapeutic services in ASCs, particularly during the extended postoperative period; (3) what is the expertise of supervising practitioners in ASCs and what is the expectation for their availability; (4) based on the final CY 2010 supervision requirements for hospital outpatient therapeutic services, under what circumstances would direct supervision of ASC services (including during the postoperative recovery period) not be occurring, according to the applicable definitions for direct supervision for HOPD services; and (5) what would be the rationale for not establishing supervision requirements for ASC services that parallel the supervision requirements in other settings, including HOPDs and physicians' offices.
After consideration of the public comments we received, we are not accepting the commenters' recommendations to not exclude all procedures reported by unlisted codes or all procedures for which Medicare payment is made to HOPDs. We will continue to exclude all procedures that we determine would be expected to pose a significant risk to beneficiary safety or require an overnight stay. Further, we are not accepting the commenters' recommendation that CMS provide more specific reasons for its decisions regarding exclusion of specific procedures from the ASC list of covered surgical procedures. In this final rule with comment period, we are soliciting public comments on the issue of physician supervision of ASC services, especially as related to extended Start Printed Page 60599 postoperative stays. In summary, we are making no changes to the final criteria for determining which procedures are excluded from the ASC list of covered surgical procedures.
B. Treatment of New Codes
1. Treatment of New Category I and Category III CPT Codes and Level II HCPCS Codes
We finalized a policy in the August 2, 2007 final rule to evaluate each year all new Category I and Category III CPT codes and Level II HCPCS codes that describe surgical procedures, and to make preliminary determinations in the annual OPPS/ASC final rule with comment period regarding whether or not they meet the criteria for payment in the ASC setting and, if so, whether they are office-based procedures (72 FR 42533). In addition, we identify new codes as ASC covered ancillary services based upon the final payment policies of the revised ASC payment system. New HCPCS codes that are released in the summer through the fall of each year, to be effective January 1, are included in the final rule with comment period updating the ASC payment system for the following calendar year. These new codes are flagged with comment indicator “NI” in Addenda AA and BB to the OPPS/ASC final rule with comment period to indicate that we are assigning a payment indicator to the codes on an interim basis. The interim payment indicators assigned to the new codes under the revised ASC payment system are subject to public comment in that final rule with comment period. These interim determinations must be made in the OPPS/ASC final rule with comment period because, in general, the new HCPCS codes and their descriptors for the upcoming calendar year are not available at the time of development of the OPPS/ASC proposed rule. We will respond to those comments in the OPPS/ASC final rule with comment period for the following calendar year. In the CY 2010 OPPS/ASC proposed rule (74 FR 35377), we proposed to continue this identification and recognition process for CY 2010.
We did not receive any public comments regarding this proposal. For CY 2010, we are continuing our established policy for recognizing new Category I and Category III CPT codes and Level II HCPCS codes.
In addition, we proposed to continue our policy of implementing through the ASC quarterly update process new mid-year CPT codes, generally Category III CPT codes, that the AMA releases in January to become effective the following July, and released in July to become effective the following January. We proposed to include in Addenda AA or BB, as appropriate, to this CY 2010 OPPS/ASC final rule with comment period the new Category III CPT codes released in January 2009 for implementation on July 1, 2009 (through the ASC quarterly update process), that we identify as ASC covered services. Similarly, we proposed to include in Addenda AA and BB to this final rule with comment period any new Category III CPT codes that the AMA released in July 2009 to be effective on January 1, 2010, that we identify as ASC covered services. However, only those new Category III CPT codes implemented effective January 1, 2010, are designated by comment indicator “NI” in the Addenda to this CY 2010 OPPS/ASC final rule with comment period to indicate that we have assigned them an interim payment indicator that is subject to public comment. The two Category III CPT codes implemented in July 2009 for ASC payment, which appeared in Table 38 of the CY 2010 OPPS/ASC proposed rule (74 FR 35378), were subject to comment through that proposed rule, and we proposed to finalize their payment indicators in this CY 2010 OPPS/ASC final rule with comment period.
We proposed to assign payment indicator “G2” (Non office-based surgical procedure added in CY 2008 or later; payment based on OPPS relative payment weight) to both of these two new codes. Because new Category III CPT codes that become effective for July are not available to CMS in time for incorporation into the Addenda to the OPPS/ASC proposed rule, our policy is to include the codes, their proposed payment indicators, and proposed payment rates in the preamble to the proposed rule but not in the Addenda to the proposed rule. These codes and their final payment indicators and rates are included in the Addenda to this CY 2010 OPPS/ASC final rule with comment period.
We did not receive any public comments regarding this proposal. For CY 2010, we are continuing our established policy for recognizing new mid-year CPT codes, and the new mid-year codes implemented in July 2009 are displayed in Table 57 below, as well as in Addendum AA to this final rule with comment period.
| CY 2010 HCPCS code | CY 2010 long descriptor | Final CY 2010 ASC payment indicator |
|---|---|---|
| 0200T | Percutaneous sacral augmentation (sacroplasty), unilateral injection(s), including the use of a balloon or mechanical device (if utilized), one or more needles | G2 |
| 0201T | Percutaneous sacral augmentation (sacroplasty), bilateral injections, including the use of a balloon or mechanical device (if utilized), two or more needles | G2 |
For CY 2010, there are numerous instances in which the descriptor of an existing Category I CPT code is substantially revised so that it describes a new service or procedure. In each such instance, revision of the code's descriptor created a more specific description of some of the services or procedures that were reported by the existing CPT code and required that at least one other code be created to describe the other services that were described by the existing code descriptor. Thus, the services or procedures that were described by the existing CPT code descriptor will be described by two new codes for CY 2010: one newly created code number and descriptor and one code with the same code number for which the code descriptor has been substantially revised. For example, CPT code 21556 (Excision tumor, soft tissue of neck or thorax; deep, subfascial, intramuscular) was revised to create two new procedures, one of which will be reported by the same CPT code number with a different description. Thus, for CY 2010, two new procedures are reported by revised CPT code 21556 (Excision, tumor, soft tissue of neck or anterior thorax, subfascial (e.g. intramuscular); less than 5 cm) and new CPT code 21554 (Excision, tumor, soft tissue of neck or anterior thorax, subfascial (e.g. intramuscular); 5 cm or Start Printed Page 60600 greater). In the past, the more common practice has been to delete an existing code number which was used to report a general description of a procedure and assign a new code number to each new, more specifically-described procedure.
Due to the practice of maintaining an existing CPT code number for a substantially revised descriptor, we have had to make changes to the payment indicators for existing code numbers that now describe different procedures. Specifically, thirty-one of the existing CPT code numbers that are used to represent new procedures in CY 2010 are currently used to report procedures that were on the ASC list of covered surgical procedures in CY 2007 and, therefore, in CY 2009 are assigned payment indicator “A2” (Surgical procedure on ASC list in CY 2007; payment based on OPPS relative payment weight based on their old descriptions). All of the newly created procedures, including the 31 procedures that will be reported by an existing CPT code number, were evaluated for appropriateness for inclusion on the ASC list of covered surgical procedures. For the procedures that we included on the ASC list, we also made an interim determination regarding whether the procedure should be designated as office-based for CY 2010. Therefore, in Addendum AA to this final rule with comment period, the same CPT code number that was assigned payment indicator “A2” for CY 2009 may be assigned payment indicators “G2” (Non office-based surgical procedure added in CY 2008 or later; payment based on OPPS relative payment weight); “P2” (Office-based surgical procedure added to ASC list in CY 2008 or later with MPFS nonfacility PE RVUs; payment based on OPPS relative payment weight); “P3” (Office-based surgical procedure added to ASC list in CY 2008 or later with MPFS nonfacility PE RVUs; payment based on MPFS nonfacility PE RVUs); or “R2” (Office-based surgical procedure added to ASC list in CY 2008 or later without MPFS nonfacility PE RVUs; payment based on OPPS relative payment weight) due to the change in the procedure assigned to the numeric code.
Any existing CPT codes with substantial revisions to their code descriptors for CY 2010 such that we consider them to describe new procedures and for which their ASC payment indicator may change are labeled with comment indicator “NI” in Addendum AA to this final rule with comment period, to indicate that we have assigned them an interim final payment status which is subject to public comment. Like all codes labeled with comment indicator “NI,” we will respond to public comments and finalize their ASC treatment in the CY 2011 OPPS/ASC final rule with comment period. In addition to assigning the “NI” indicator to these new CPT codes, in accordance with our standard practice, all new CPT and Level II HCPCS code numbers for CY 2010 are labeled with comment indicator “NI” in Addendum AA and BB to this final rule with comment period.
2. Treatment of New Level II HCPCS Codes Implemented in April and July 2009
New Level II HCPCS codes may describe covered surgical procedures or covered ancillary services. All new Level II HCPCS codes implemented in April and July 2009 for ASCs describe covered ancillary services. During the second quarter of CY 2009, we added to the list of covered ancillary services two new Level II HCPCS codes because they are drugs or biologicals for which separate payment was newly allowed under the OPPS in the same calendar quarter. The two Level II HCPCS codes added, effective April 1, 2009, were HCPCS code C9247 (Iobenguane, I–123, diagnostic, per study dose, up to 10 millicuries) and HCPCS code C9249 (Injection, certolizumab pegol, 1 mg). Although HCPCS code C9247 was created for use beginning on January 1, 2009, initially it was not paid separately under the hospital OPPS, and therefore its payment also was packaged under the ASC payment system, until April 1, 2009.
After publication of the CY 2010 OPPS/ASC proposed rule, the CMS HCPCS Workgroup created permanent HCPCS codes to replace these two HCPCS C-codes that were implemented in April 2009. We will be recognizing these HCPCS codes for payment of these drugs and biologicals under the CY 2010 ASC payment system, consistent with our general policy to use permanent HCPCS codes, if appropriate, for the reporting of drugs and biologicals. Table 58 shows the new permanent HCPCS codes that replace the HCPCS C-codes that will be deleted effective December 31, 2009.
Specifically, HCPCS code C9247 was replaced with HCPCS code A9582 (Iodine I–123 iobenguane, diagnostic, per study dose, up to 15 millicuries) and HCPCS code C9249 was replaced with HCPCS code J0718 (Injection, certolizumab pegol, 1 mg). The new HCPCS codes, effective January 1, 2010, describe the same drugs. Although HCPCS code A9582 indicates “per study dose, up to 15 millicuries” and the descriptor of its predecessor C-code designates “per study dose, up to 10 millicuries,” we believe that the reporting of one study dose would be the same in most cases under either the new permanent code or the predecessor code. The recommended dose of I–123 iobenguane is 10 millicuries for adult patients, so we expect that hospitals would report 1 unit of new HCPCS code A9582 for the typical dose in CY 2010, just as they would have reported one unit of HCPCS code C9247 previously for the typical dose and, therefore, there would be no effect on the payment indicator.
For the third quarter of CY 2009, we added 11 new Level II drug and biological HCPCS codes to the list of ASC covered ancillary services because they were newly eligible for separate payment under the OPPS effective July 1, 2009. These HCPCS codes are: C9250 (Human plasma fibrin sealant, vapor-heated, solvent-detergent (Artiss), 2 ml); C9251 (Injection, C1 esterase inhibitor (human) 10 units); C9252 (Injection, plerixafor, 1 mg); C9253 (Injection, temozolomide, 1 mg); C9360 (Dermal substitute, native, non-denatured collagen, neonatal bovine origin (SurgiMend Collagen Matrix), per 0.5 square centimeters); C9361 (Collagen matrix nerve wrap (NeuroMend Collagen Nerve Wrap), per 0.5 centimeter length); C9362 (Porous purified collagen matrix bone void filler (Integra Mozaik Osteoconductive Scaffold Strip), per 0.5 cc); C9363 (Skin substitute, Integra Meshed Bilayer Wound Matrix, per square centimeter); C9364 (Porcine implant, Permacol, per square centimeter); Q2023 (Injection, factor viii (antihemophilic factor, recombinant) (Xyntha), per i.u.); and Q4116 (Skin substitute, Alloderm, per square centimeter).
We assigned payment indicator “K2” (Drugs and biologicals paid separately when provided integral to a surgical procedure on ASC list; payment based on OPPS rate) to all of these new Level II HCPCS codes and added the codes to the list of covered ancillary services through either the April 2009 update (Transmittal 1698, Change Request 6424, dated March 13, 2009) or the July 2009 update (Transmittal 1740, Change Request 6496, dated May 22, 2009) to the CY 2009 ASC payment system. Initially, we assigned payment indicator “K2” to new HCPCS code Q4115 (Skin substitute, Alloskin, per square centimeter) for July 2009, but then changed that assignment retroactive to July 2009 to signify that this HCPCS code was not a covered ancillary service because it was not recognized for payment under the OPPS during that Start Printed Page 60601 same time period. Subsequently, in the October 2009 quarterly update CR (Transmittal 1806, Change Request 6629, dated August 28, 2009), HCPCS code Q4115 was added as payable (assigned payment indicator “K2”), effective October 1, 2009.
In the CY 2010 OPPS/ASC proposed rule (74 FR 35378), we solicited public comment on the proposed CY 2010 ASC payment indicators and payment rates for the drugs and biologicals, as listed in Tables 39 and 40 of the proposed rule (74 FR 35378 through 35379). Those HCPCS codes became payable in ASCs beginning in April 2009 or July 2009, respectively, based on the ASC rates posted for the appropriate calendar quarter on the CMS Web site at: http://www.cms.hhs.gov/ASCPayment/.
The HCPCS codes that were listed in Table 39 of the proposed rule became effective in April 2009 and were included in Addendum BB to the proposed rule. However, because HCPCS codes that become effective for July are not available to CMS in time for incorporation into the Addenda to the OPPS/ASC proposed rule, our policy is to include these HCPCS codes and their proposed payment indicators and payment rates in the preamble to the proposed rule but not in the Addenda to the proposed rule. These codes and their final payment indicators and rates are included in the appropriate Addendum to this CY 2010 OPPS/ASC final rule with comment period. Thus, the codes implemented by the July 2009 ASC quarterly update and their proposed CY 2010 payment rates (based on July 2009 ASP data) that were displayed in Table 40 of the CY 2010 OPPS/ASC proposed rule were not included in Addendum BB to the CY 2010 OPPS/ASC proposed rule. We proposed to include the services reported using the new HCPCS codes that were displayed in Tables 39 and 40 as covered ancillary services and to incorporate all of them into Addendum BB to this CY 2010 OPPS/ASC final rule with comment period, consistent with our annual update policy.
After publication of the CY 2010 OPPS/ASC proposed rule, the HCPCS Workgroup created permanent HCPCS J-codes for 4 of the 11 separately payable covered ancillary services that were displayed in Table 40 of the proposed rule. Consistent with our general policy of using permanent HCPCS codes, if appropriate, rather than HCPCS C-codes in order to streamline coding, effective for CY 2010, we are adopting the 4 permanent HCPCS J-codes to replace the HCPCS C-codes. As displayed in Table 59 below, HCPCS code C9251 is replaced with J0598 (Injection, C1 esterase inhibitor (human), 10 units); C9252 with J2562 (Injection, plerixafor, 1 mg); C9253 with J9328 (Injection, temozolomide, 1 mg); and Q2023 with J7185 (Injection, factor viii (antihemophilic factor, recombinant) (Xyntha), per i.u.). The HCPCS J-codes, effective January 1, 2010, describe the same drugs and the same dosages as the HCPCS C-codes that will be deleted December 31, 2009. Therefore, there is no effect on the services' payment indicators.
We did not receive any public comments regarding our proposals. We are adopting as final the ASC payment indicators for the new Level II HCPCS codes implemented in April and July 2009 as shown in Tables 58 and 59, respectively. Moreover, we are adopting as final the replacement HCPCS codes, specifically, A9582 and J0718, as shown in Table 58 and HCPCS codes J0598, J2562, J7185, and J9328 as displayed in Table 59 below. All of the new HCPCS codes and payment indicators also are included in Addendum BB to this final rule with comment period.
| CY 2010 HCPCS Code | CY 2009 HCPCS Code | CY 2010 Long descriptor | Final CY 2010 payment indicator |
|---|---|---|---|
| A9582 | C9247 | Iodine I–123 iobenguane, diagnostic, per study dose, up to 15 millicuries | K2 |
| J0718 | C9249 | Injection, certolizumab pegol, 1 mg | K2 |
| CY 2010 HCPCS Code | CY 2009 HCPCS Code | CY 2010 Long descriptor | Final CY 2010 ASC payment indicator |
|---|---|---|---|
| C9250 | C9250 | Human plasma fibrin sealant, vapor-heated, solvent-detergent (Artiss), 2 ml | K2 |
| J0598 | C9251 | Injection, C1 esterase inhibitor (human), 10 units | K2 |
| J2562 | C9252 | Injection, plerixafor, 1 mg | K2 |
| J9328 | C9253 | Injection, temozolomide, 1 mg | K2 |
| C9360 | C9360 | Dermal substitute, native, non-denatured collagen, neonatal bovine origin (SurgiMend Collagen Matrix), per 0.5 square centimeters | K2 |
| C9361 | C9361 | Collagen matrix nerve wrap (NeuroMend Collagen Nerve Wrap), per 0.5 centimeter length | K2 |
| C9362 | C9362 | Porous purified collagen matrix bone void filler (Integra Mozaik Osteoconductive Scaffold Strip), per 0.5 cc | K2 |
| C9363 | C9363 | Skin substitute, Integra Meshed Bilayer Wound Matrix, per square centimeter | K2 |
| C9364 | C9364 | Porcine implant, Permacol, per square centimeter | K2 |
| J7185 | Q2023 | Injection, factor viii (antihemophilic factor, recombinant) (Xyntha), per i.u. | K2 |
| Q4116 | Q4116 | Skin substitute, Alloderm, per square centimeter | K2 |
C. Update to the Lists of ASC Covered Surgical Procedures and Covered Ancillary Services
1. Covered Surgical Procedures
a. Additions to the List of ASC Covered Surgical Procedures
In the CY 2010 OPPS/ASC proposed rule (74 FR 35379), we proposed to update the ASC list of covered surgical procedures by adding 28 procedures to the list. Twenty-six of these procedures were among those excluded from the ASC list for CY 2009 because we believed they did not meet the definition of a covered surgical procedure based on our expectation that they would pose a significant safety risk to Medicare beneficiaries or would require an overnight stay if performed in ASCs. The other two procedures, specifically those described by CPT code 0200T (Percutaneous sacral augmentation (sacroplasty), unilateral injection(s), including the use of a balloon or mechanical device (if utilized), one or more needles) and CPT code 0201T (Percutaneous sacral augmentation (sacroplasty), bilateral injections, including the use of a balloon or mechanical device (if utilized), two or more needles), are new Category III CPT codes that became effective July 1, 2009, and were implemented in the July 2009 ASC update (Table 57 above). As a result of our clinical evaluation of the procedures described by the new Category III codes, we determined that these two new procedures may be appropriately provided to Medicare beneficiaries in ASCs.
In response to comments on the CY 2009 proposed rule, we stated in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68724) that, as we developed the CY 2010 proposed rule, we would perform a comprehensive review of the APCs in order to identify potentially inconsistent ASC treatment of procedures assigned to a single APC under the OPPS. Thus, we examined surgical procedures that are excluded from the current ASC list of covered surgical procedures and the APCs to which they are assigned under the OPPS. We identified for review 223 excluded surgical procedures that were assigned to the same APCs in CY 2009 as one or more ASC covered surgical procedures. Based upon our clinical review of those procedures, we determined that 26 surgical procedures may be appropriate for performance in ASCs and proposed to add them to the CY 2010 ASC list of covered surgical procedures and to assign payment indicator “G2” (Non office-based surgical procedure added in CY 2008 or later; payment based on OPPS relative payment weight) to each of them. We found that the remaining 197 excluded procedures would pose significant safety risks to beneficiaries or would be expected to require an overnight stay if provided in ASCs. Therefore, we did not propose to add those 197 procedures to the CY 2010 ASC list of covered surgical procedures.
The 28 procedures that we proposed to add to the ASC list of covered surgical procedures, including their HCPCS code short descriptors and proposed CY 2010 payment indicators, were displayed in Table 41 in the CY 2010 OPPS/ASC proposed rule (74 FR 35379 through 35380).
Among the procedures we identified as meeting the criteria for designation as a covered surgical procedure was CPT code 35475 (Transluminal balloon angioplasty, percutaneous; brachiocephalic trunk or branches, each vessel). The volume and utilization data for this procedure indicate that it is most frequently performed in outpatient settings. After review, our CMS medical advisors found that it would be appropriate to propose designation of CPT code 35475 as an ASC covered surgical procedure for CY 2010. Related to our proposal to add CPT code 35475 to the list of covered surgical procedures is our concurrent proposal to delete two Level II HCPCS codes we created effective for CY 2007, HCPCS codes G0392 (Transluminal balloon angioplasty, percutaneous; for maintenance of hemodialysis access, arteriovenous fistula or graft; arterial) and G0393 (Transluminal balloon angioplasty, percutaneous; for maintenance of hemodialysis access, arteriovenous fistula or graft; venous) to enable ASCs to receive Medicare payment for providing the angioplasty services required to maintain the arteriovenous fistulae that are important to individuals who undergo routine dialysis. We proposed to delete HCPCS codes G0392 and G0393 concurrently with the designation of CPT code 35475 as a covered surgical procedure because there no longer would be a need for the two Level II HCPCS G-codes. ASCs would be able to use CPT 35475 and CPT code 35476 (Transluminal balloon angioplasty, percutaneous; venous), which was included on the list of ASC covered surgical procedures beginning in CY 2008, to report the same procedures currently reported by HCPCS codes G0392 and G0393.
Thus, we proposed to add the 28 surgical procedures listed in Table 41 of the OPPS/ASC proposed rule to the list of covered ASC surgical procedures and to delete the HCPCS codes displayed in Table 42 of the proposed rule (74 FR 35380).
Comment: One commenter requested that CMS not finalize several of its proposed additions to the ASC list for CY 2010. The commenter believed that the procedure described by CPT code 26037 (Decompressive fasciotomy, hand) is inappropriate for the ASC setting. The commenter stated that the typical patient that requires this procedure has had a severe crush injury to the hand and/or an infection and would require at least 23 hours of medical monitoring following the surgery. The commenter also objected to adding procedures described by CPT codes 42225 (Palatoplasty for cleft palate; attachment pharyngeal flap) and 42227 (Lengthening of palate, with island flap) because those procedures are overwhelmingly performed on infants and very young children who would require at least an overnight stay to observe for postoperative swelling, airway compromise, and bleeding. The commenter believed that CMS' inclusion of these procedures on the ASC list would be inappropriate because Medicare claims data are inadequate for CMS' use in making its determination and that inclusion of the procedures on the Medicare ASC list leads to interpretation by commercial insurers that the ASC setting is appropriate for all patient populations and would result in very young patients not being able to receive care in more appropriate settings.
The commenter also reiterated a previous request (73 FR 68729) that CMS remove other cleft lip and palate reconstruction procedures from the ASC list of covered surgical procedures. Those procedures and their CPT codes are: 21215 (Graft, bone; mandible (includes obtaining graft)); 40700 (Plastic repair of cleft lip/nasal deformity; primary, partial or complete, unilateral); 40701 (Plastic repair of cleft lip/nasal deformity, primary bilateral, one stage procedure); 42200 (Palatoplasty for cleft palate, soft and/or hard palate only); 42205 (Palatoplasty for cleft palate, with closure of alveolar ridge; soft tissue only); 42210 (Palatoplasty for cleft palate, with closure of alveolar ridge; with bone graft to alveolar ridge includes obtaining graft)), 42215 (Palatoplasty for cleft palate; major revision); and 42220 (Palatoplasty for cleft palate; secondary lengthening procedure). The commenter stated that all of these procedures require general anesthesia and close postoperative monitoring and are often performed in the inpatient setting. Start Printed Page 60603
Response: Our medical advisors reviewed the three procedures described by CPT codes 26037, 42225, and 42227 that we proposed to add to the ASC list for CY 2010. As a result of that review, we continue to believe that all three of the procedures may be appropriately provided to a Medicare beneficiary in an ASC. We do not see a basis for removing the eight procedures from the ASC list as requested by the commenter. All of these procedures were on the list of covered surgical procedures even before CY 2007 and, to our knowledge, have been safely performed in ASCs for many years. We continue to believe that these 11 procedures would not pose a significant safety risk to Medicare beneficiaries and would not require an overnight stay if performed in ASCs.
As established at § 416.166(b), decisions regarding whether a surgical procedure should be excluded from the Medicare ASC list of covered surgical procedures are based on assessments of the needs of Medicare beneficiaries and not all patient populations. We include on the ASC list all procedures we believe are appropriate for some Medicare beneficiaries in order to provide physicians and patients with the greatest possible choice for sites-of-service. We expect that physicians will consider for each individual patient which site-of-service is most appropriate. We understand that the procedures on the ASC list are sometimes more appropriately performed on an inpatient basis due to the individual's age or other clinical considerations.
Comment: Several commenters supported the proposal to add 28 procedures to the list of covered surgical procedures and requested that CMS add 24 additional surgical procedures. A few commenters on the CY 2010 OPPS/ASC proposed rule and on the CY 2009 OPPS/ASC final rule with comment period requested that a total of 18 specific unlisted codes be added to the ASC list. Some commenters provided specific reasons for their requests for addition of particular procedures, but for most of the requested additions, no specific information was submitted.
The commenters who requested that the procedure reported by CPT code 50593 (Ablation, renal tumor(s) unilateral, percutaneous, cryotherapy) be added to the ASC list stated that the procedure is similar to the procedure reported by CPT code 50592 (Ablation, 1 or more renal tumor(s), percutaneous, unilateral, radiofrequency), which is already on the ASC list, and that CPT code 50593 is compatible with CMS' safety criteria. The commenters also reported that a significant number of the laminectomy procedures listed in Table 60 below already are being performed in ASCs for commercially insured patients. They stated that the most common of these laminectomy procedures are performed on the cervical and lumbar regions of the spine, take only 60 to 90 minutes to perform, and typically require only about 4 hours of recovery time. They also stated that patients are carefully screened before the ASC is selected as the appropriate site for their surgical procedures, a practice they would expect to see applied to the Medicare population as well.
The commenters who requested the addition of the procedure reported by CPT code 52649 (Laser enucleation of the prostate with morcellation, including control of postoperative bleeding, complete (vasectomy, meatotomy, cystourethroscopy, urethral calibration and/or dilation, internal urethrotomy and transurethral resection of prostate are included if performed)) requested that the procedure be added to the ASC list because it does not require an overnight stay and it is similar to benign prostatic hypertrophy treatment procedures, which are already included on the ASC list. The commenters who requested addition of the procedure described by CPT code 57310 (Closure of urethrovaginal fistula) reported that it should be added to the list because a substantially similar and more complex procedure, described by CPT code 57320 (Closure of vesicovaginal fistula; vaginal approach), is already on the ASC list.
The commenters who requested the addition of the unlisted procedure described by CPT code 19499 (Unlisted procedure, breast) stated that it should be added to the list because it is used for setting breast ductoscopy prior to some surgeries in lieu of a ductogram and because it may be used to report services described by CPT codes 0046T (Catheter lavage of a mammary duct(s) for collection of cytology specimen(s), in high risk individuals (GAIL risk scoring or prior personal history of breast cancer), each breast; single duct) and 0047T (Catheter lavage of a mammary duct(s) for collection of cytology specimen(s), in high risk individuals (GAIL risk scoring or prior personal history of breast cancer), each breast; each additional duct) that were payable in ASCs in CY 2008 but were deleted effective for CY 2009. Some commenters requested the addition of unlisted CPT codes 55899 (Unlisted procedure, male genital system); 58999 (Unlisted procedure, female genital system (nonobstetrical)); and 64999 (Unlisted procedure, nervous system) because these codes may be used to report the procedures that were described by CPT codes 0027T (Endoscopic lysis of epidural adhesions with direct visualization using mechanical means (eg, spinal endoscopic catheter system) or solution injection (eg, normal saline) including radiologic localization and epidurography); 0031T (Speculoscopy); 0032T (Speculoscopy; with directed sampling); and 53853 (Transurethral destruction of prostate tissue; by water-induced thermotherapy) that were payable in ASCs in CY 2008 but were deleted effective for CY 2009.
Finally, the commenter who requested the addition of intravascular stent placement procedures, CPT codes 37205 (Transcatheter placement of an intravascular stent(s)(except coronary, carotid, and vertebral vessel), percutaneous; initial vessel) and 37206 (Transcatheter placement of an intravascular stent(s) (except coronary, carotid, and vertebral vessel), percutaneous; each additional vessel), claimed that the addition of these procedures to the ASC list of covered surgical procedures would improve access to care for patients with vascular access dysfunction, decrease costs to Medicare, and result in a higher quality of care for these patients. The commenters requested that if CMS is reluctant to add these procedures to the ASC list because CPT codes 37205 and 37206 are not restricted to the treatment of hemodialysis vascular access sites, CMS could allow reporting of the codes for ASC payment with a new and distinct modifier that would apply to hemodialysis vascular access procedures.
All of the procedures requested by commenters for addition to the ASC list of covered surgical procedures are displayed in Tables 60 and 61 below.
| CY 2010 CPT code | CY 2010 short descriptor |
|---|---|
| 27485 | Surgery to stop leg growth. |
| 29867 | Allgrft implnt, knee w/scope. |
| 29868 | Meniscal trnspl, knee w/scope. |
| 35470 | Repair arterial blockage. |
| 35474 | Repair arterial blockage. |
| 35493 | Atherectomy, percutaneous. |
| 35495 | Atherectomy, percutaneous. |
| 37205 | Transcath iv stent, percut. |
| 37206 | Transcath iv stent/perc addl. |
| 50593 | Perc cryo ablate renal tum. |
| 52649 | Prostate laser enucleation. |
| 57310 | Repair urethrovaginal lesion. |
| 60210 | Partial thyroid excision. |
| 60220 | Partial removal of thyroid. |
| Start Printed Page 60604 | |
| 63001 | Removal spinal lamina. |
| 63005 | Removal spinal lamina. |
| 63020 | Neck spine disk surgery. |
| 63030 | Low back disk surgery. |
| 63035 | Spinal disk surgery add-on. |
| 63040 | Laminotomy, single cervical. |
| 63042 | Laminotomy, single lumbar. |
| 63045 | Removal of spinal lamina. |
| 63047 | Removal of spinal lamina. |
| 63048 | Remove spinal lamina add-on. |
| CY 2010 CPT code | CY 2010 short descriptor |
|---|---|
| 17999 | Skin tissue procedure. |
| 19499 | Breast surgery procedure. |
| 23929 | Shoulder surgery procedure. |
| 27599 | Leg surgery procedure. |
| 27899 | Leg/ankle surgery procedure. |
| 28899 | Foot/toes surgery procedure. |
| 29999 | Arthroscopy of joint. |
| 31299 | Sinus surgery procedure. |
| 55899 | Genital surgery procedure. |
| 58999 | Genital surgery procedure. |
| 64999 | Nervous system surgery. |
| 66999 | Eye surgery procedure. |
| 67299 | Eye surgery procedure. |
| 67399 | Eye muscle surgery procedure. |
| 67999 | Revision of eyelid. |
| 68399 | Eyelid lining surgery. |
| 68899 | Tear duct system surgery. |
| 92499 | Eye service or procedure. |
Response: We reviewed all of the surgical procedures that commenters requested be added to the ASC list of covered surgical procedures. We did not review any of the procedures that may be reported by the CPT unlisted codes listed in Table 61 because those codes are not eligible for addition to the ASC list, consistent with our final policy which is discussed in detail in the August 2, 2007 final rule (72 FR 42484 through 42486). We do not agree that any of the procedures recommended by the commenters are appropriate for provision to Medicare beneficiaries in ASCs. Although the commenters asserted that some of the procedures they were requesting for addition to the list are less complex than procedures already on the list and that all of the requested procedures are as safe as procedures on the list, our review did not support those assertions.
We exclude from ASC payment any procedure for which standard medical practice dictates that the beneficiary who undergoes the procedure would typically be expected to require active medical monitoring and care at midnight following the procedure (overnight stay) as well as all surgical procedures that our medical advisors determine may be expected to pose a significant safety risk to Medicare beneficiaries. The criteria used under the revised ASC payment system to identify procedures that would be expected to pose a significant safety risk when performed in an ASC include, but are not limited to, those procedures that: generally result in extensive blood loss; require major or prolonged invasion of body cavities; directly involve major blood vessels; are emergent or life-threatening in nature; or commonly require systemic thrombolytic therapy (see § 416.166).
In our review of the procedures listed in Table 60, we found that all of the procedures either may be expected to pose a threat to beneficiary safety or require active medical monitoring at midnight following the procedure. Specifically, we found that prevailing medical practice called for inpatient hospital stays for beneficiaries undergoing many of the procedures and that some of the procedures directly involve major blood vessels and/or may result in extensive blood loss.
After consideration of the public comments we received, we are not adding any of the procedures requested by the commenters to the list of ASC covered surgical procedures. We also are not removing any of the procedures from the list as requested by commenters. We are finalizing, without modification, our proposal to add 28 procedures to the CY 2010 ASC list and to delete two Level II HCPCS codes. The procedures, their short descriptors, and payment indicators are displayed in Tables 62 and 63 below.
| CY 2010 CPT Code | CY 2010 short descriptor | Final CY 2010 ASC payment indicator |
|---|---|---|
| 26037 | Decompress fingers/hand | G2 |
| 27475 | Surgery to stop leg growth | G2 |
| 27479 | Surgery to stop leg growth | G2 |
| 27720 | Repair of tibia | G2 |
| 35460 | Repair venous blockage | G2 |
| 35475 | Repair arterial blockage | G2 |
| 41512 | Tongue suspension | G2 |
| 42225 | Reconstruct cleft palate | G2 |
| 42227 | Lengthening of palate | G2 |
| 43130 | Removal of esophagus pouch | G2 |
| 43752 | Nasal/orogastric w/stent | G2 |
| 45541 | Correct rectal prolapsed | G2 |
| 49435 | Insert subq exten to ip cath | G2 |
| 49436 | Embedded ip cath exit-site | G2 |
| 49442 | Place cecostomy tube perc | G2 |
| 50080 | Removal of kidney stone | G2 |
| 50081 | Removal of kidney stone | G2 |
| 50727 | Revise ureter | G2 |
| 51535 | Repair of ureter lesion | G2 |
| 57295 | Revise vag graft via vagina | G2 |
| 60210 | Partial thyroid excision | G2 |
| 60212 | Partial thyroid excision | G2 |
| 60220 | Partial removal of thyroid | G2 |
| 60225 | Partial removal of thyroid | G2 |
| 61770 | Incise skull for treatment | G2 |
| 0193T | Rf bladder neck microremodel | G2 |
| 0200T * | Perq sacral augmt unilat inj | G2 |
| 0201T * | Perq sacral augmt bilat inj | G2 |
| * Indicates codes are new, effective July 2009. | ||
| CY 2009 HCPCS Code | CY 2009 short descriptor | CY 2009 ASC payment indicator |
|---|---|---|
| G0392 | AV fistula or graft arterial | A2 |
| Start Printed Page 60605 | ||
| G0393 | AV fistula or graft venous | A2 |
b. Covered Surgical Procedures Designated as Office-Based
(1) Background
In the August 2, 2007 final rule, we finalized our policy to designate as “office-based” those procedures that are added to the ASC list of covered surgical procedures in CY 2008 or later years that we determine are performed predominantly (more than 50 percent of the time) in physicians' offices based on consideration of the most recent available volume and utilization data for each individual procedure code and/or, if appropriate, the clinical characteristics, utilization, and volume of related codes. In that rule, we also finalized our policy to exempt all procedures on the CY 2007 ASC list from application of the office-based classification (72 FR 42512). The procedures that were added to the ASC list of covered surgical procedures beginning in CY 2008 that we determined were office-based were identified in Addendum AA to that rule by payment indicator “P2” (Office-based surgical procedure added to ASC list in CY 2008 or later with MPFS nonfacility PE RVUs; payment based on OPPS relative payment weight); “P3” (Office-based surgical procedure added to ASC list in CY 2008 or later with MPFS nonfacility PE RVUs; payment based on MPFS nonfacility PE RVUs); or “R2” (Office-based surgical procedure added to ASC list in CY 2008 or later without MPFS nonfacility PE RVUs; payment based on OPPS relative payment weight), depending on whether we estimated it would be paid according to the standard ASC payment methodology based on its OPPS relative payment weight or at the MPFS nonfacility PE RVU amount.
Consistent with our final policy to annually review and update the list of surgical procedures eligible for payment in ASCs, each year we identify surgical procedures as either temporarily or permanently office-based after taking into account updated volume and utilization data.
(2) Changes to Covered Surgical Procedures Designated as Office-Based for CY 2010
In developing the CY 2010 OPPS/ASC proposed rule (74 FR 35381), we followed our policy to annually review and update the surgical procedures for which ASC payment is made and to identify new procedures that may be appropriate for ASC payment, including their potential designation as office-based. We reviewed CY 2008 volume and utilization data and the clinical characteristics for all surgical procedures that are assigned payment indicator “G2” in CY 2009, as well as for those procedures assigned one of the temporary office-based payment indicators, specifically “P2*,” “P3*,” or “R2*” in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68730 through 68733). As a result of that review, we proposed to newly designate 6 procedures as office-based for CY 2010 (74 FR 35381). We also proposed to make permanent the office-based designations of 4 surgical procedures that have temporary office-based designations in CY 2009.
The 6 procedures we proposed to permanently designate as office-based are: CPT code 15852 (Dressing change (for other than burns) under anesthesia (other than local)); CPT code 19105 (Ablation, cryosurgical, of fibroadenoma, including ultrasound guidance, each fibroadenoma); CPT code 20555 (Placement of needles or catheters into muscle and/or soft tissue for subsequent interstitial radioelement application (at the time of or subsequent to the procedure)); CPT code 36420 (Venipuncture, cutdown; younger than age 1 year); CPT code 50386 (Removal (via snare/capture) of internally dwelling ureteral stent via transurethral approach, without use of cystoscopy, including radiological supervision and interpretation); and CPT code 57022 (Incision and drainage of vaginal hematoma; obstetrical/postpartum). These procedures and their HCPCS code short descriptors and proposed CY 2010 payment indicators were displayed in Table 43 of the CY 2010 OPPS/ASC proposed rule (74 FR 35381).
Comment: Many commenters expressed their continued disagreement with the policy to make payment at the lower of the ASC rate or MPFS nonfacility PE RVU payment amount for procedures we identify as office-based and requested that CMS not finalize any of the proposed office-based designations. They believed that, due to the payment limits required by CMS' payment policy for providing these procedures in ASCs, beneficiaries who require the level of care provided in ASCs instead have to receive treatment in the more costly HOPD setting and that the policy makes the ASC payment rates subject to the fluctuations of the MPFS nonfacility PE RVUs and other problems of the MPFS.
The commenters recommended that CMS revise its policies in at least three ways. First, they recommended that CMS establish a minimum volume threshold before designating a procedure office-based. They asserted that it is unnecessary and inappropriate to designate as office-based procedures with extremely low volume because the utilization data for those procedures are variable and, thus, not reliable. Second, they recommended that CMS raise the utilization threshold above 50 percent for designating a procedure office-based and use multiple years of data for making the designation because of the variability in the utilization data across years. Third, the commenters recommended that CMS recognize the OPPS median costs for procedures as the best proxy for relative ASC costs and limit the reduction in payment to ASCs for the office-based procedures. The commenters reasoned that, because the ASC payment system was to be based on the relative payment weights under the OPPS, which are updated annually based on hospital claims and cost reports, OPPS median costs are a better source of relative payment weights than the MPFS, which is not based on facility costs estimated from claims and cost reports like the OPPS. In addition, they expressed concern that as more procedures are designated as office-based for ASC payment, the linkage between the OPPS and ASC ratesetting methodologies would be eroded and the ASC payment system would be increasingly affected by the unpredictable inflation updates under the MPFS.
Response: We continue to believe that our policy of identifying low complexity procedures that are usually provided in physicians' offices and limiting their payment in ASCs to the physician's office payment amount is necessary and valid. We believe this is the most appropriate approach to preventing the creation of payment incentives for services to move from physicians' offices to ASCs for the many newly-covered low complexity procedures on the ASC list. Moreover, we are confident that the CY 2008 claims data, the most recent full year of volume and utilization data, are an appropriate source to inform our decisions regarding the site-of-service for procedures. In our review process, when we believe that the available data are inadequate bases Start Printed Page 60606 upon which to make a determination that a procedure should be office-based, we either make no change to the procedure's payment status or make the change temporary and reevaluate our decision using data that become available for our next evaluation. We believe that it is appropriate to continue using our judgment regarding whether the volume of cases and the proportion of cases that are provided in the physicians' office setting indicate that the procedure is an office-based procedure in addition to our medical advisors' clinical judgments, utilization data for procedures that are closely related to the procedures being evaluated, and any other information that is available to us. Thus, we will not alter our review and decision processes with respect to establishing or changing volume or utilization thresholds as recommended by the commenters.
We continue to believe that it is appropriate that ASCs be paid no more for performing office-based procedures than those procedures would be paid when performed in physicians' offices, in order to deter inappropriate migration of these surgical procedures to ASCs based on financial considerations rather than clinical needs. Although our policy to pay for some services at the MPFS non-facility PE RVU amount does introduce payment for a number of procedures at rates not based on the ASC relative payment weights and, as such, may be viewed as an erosion of the linkage between the OPPS and ASC payment system, we do not believe that the alternative of making payments at the higher ASC rate is preferable. None of the office-based procedures was eligible for ASC payment prior to implementation of the revised payment system and we see no inherent unfairness in limiting ASC payment to the rate for the lower-intensity site of service (physician's office) that our data indicate is the care setting for most Medicare cases. In fact, the lower than expected CY 2008 ASC utilization for office-based procedures reported by commenters (discussed in section XV.I.2.of this final rule with comment period) may be an indication that our policy does not encourage inappropriate migration of these services to ASCs, as was our intention. While we acknowledge the potential volatility of the office-based payments under the MPFS, we continue to believe it is appropriate to treat office-based procedures similarly in the office and ASC settings for purposes of Medicare payment. Therefore, we also will not adopt the commenters' recommendation that ASC payment rates should be based only on OPPS median costs and will continue to update the office-based list of ASC covered surgical procedures annually, to account for changes in medical practice and new surgical procedures that may result in additional surgical procedures that are predominantly performed in physicians' offices.
The utilization data for the procedures listed in Table 43 of the proposed rule and restated in Table 64, below, did not change between the proposed rule and this final rule with comment period. We did not receive any public comments that specifically addressed our proposals to designate the 6 procedures listed in Table 64 as office-based for CY 2010. Therefore, we are finalizing our CY 2010 proposals, without modification, to designate the procedures displayed in Table 64 below as office-based for CY 2010.
| CY 2010 HCPCS code | CY 2010 short descriptor | CY 2009 ASC payment indicator | Proposed CY 2010 ASC payment indicator | Final CY 2010 ASC payment indicator * |
|---|---|---|---|---|
| 15852 | Dressing change not for burn | G2 | R2 | R2 |
| 19105 | Cryosurg ablate fa, each | G2 | P3 | P2 |
| 20555 | Place ndl musc/tis for rt | G2 | R2 | R2 |
| 36420 | Vein access cutdown < 1 yr | G2 | R2 | R2 |
| 50386 | Remove stent via transureth | G2 | P2 | P2 |
| 57022 | I & d vaginal hematoma, pp | G2 | R2 | R2 |
| * Payment indicators are based on a comparison of the rates according to the ASC standard ratesetting methodology and the MPFS final CY 2010 rates. Under current law, the MPFS payment rates have a negative update for CY 2010. For a discussion of those rates, we refer readers to the CY 2010 MPFS final rule with comment period. | ||||
In the CY 2010 OPPS/ASC proposed rule (74 FR 35381), we also reviewed CY 2008 volume and utilization data and other information for the 10 procedures with temporary office-based designations for CY 2009. Among these 10 procedures, there were no claims data for the 3 procedures with CPT codes that were new in CY 2009. Those 3 new procedure codes are: CPT code 46930 (Destruction of internal hemorrhoid(s) by thermal energy (eg, infrared coagulation, cautery, radiofrequency)); CPT code 64455 (Injection(s), anesthetic agent and/or steroid, plantar common digital nerve(s) (eg, Morton's neuroma)); and CPT code 64632 (Destruction by neurolytic agent; plantar common digital nerve). Consequently, in the CY 2010 OPPS/ASC proposed rule (74 FR 35381), we proposed to maintain their temporary office-based designations for CY 2010.
As a result of our review of the remaining 7 procedures that have temporary office-based designations for CY 2009, we proposed to make permanent the office-based designations for 4 procedures for CY 2010. The 4 surgical procedure codes are: CPT code 0084T (Insertion of a temporary prostatic urethral stent); CPT code 21073 (Manipulation of temporomandibular joint(s) (TMJ), therapeutic, requiring an anesthesia service (ie, general or monitored anesthesia care)); CPT code 55876 (Placement of interstitial device(s) for radiation therapy guidance (eg, fiducial markers, dosimeter), prostate (via needle, any approach), single or multiple); and HCPCS code C9728 (Placement of interstitial device(s) for radiation therapy/surgery guidance (eg, fiducial markers, dosimeter), other than prostate (any approach), single or multiple). Although we have no Medicare volume and utilization data in physicians' offices for HCPCS code C9728 because this code is not recognized for payment under the MPFS, we noted in the CY 2009 OPPS/ASC proposed rule (73 FR 41528) that because HCPCS code C9728 is Start Printed Page 60607 analogous to CPT code 55876, we believe they should be paid according to the same ASC payment methodology under the ASC payment system. In the CY 2010 OPPS/ASC proposed rule, we indicated our belief that the volume and utilization data for CPT codes 0084T, 21073, and 55876 are sufficient to support our determination that these procedures are most commonly provided in physicians' offices. Therefore, we proposed to make permanent the office-based designations for the four procedures (including HCPCS code C9728) for CY 2010.
We did not propose to make permanent the office-based designations for the 3 other procedures for which the CY 2009 office-based designations are temporary because we did not believe that the currently available volume and utilization data provided an adequate basis for proposing permanent office-based designations. Rather, available data supported our determination that maintaining the temporary office-based designation was appropriate for CY 2010 for CPT code 0099T (Implantation of intrastromal corneal ring segments); CPT code 0124T (Conjunctival incision with posterior extrascleral placement of pharmacological agent (does not include supply of medication)); and CPT code 67229 (Treatment of extensive or progressive retinopathy, 1 or more sessions; preterm infant (less than 37 weeks gestation at birth), performed from birth up to 1 year of age (eg, retinopathy of prematurity), photocoagulation or cryotherapy). Thus, we proposed to maintain the temporary office-based designation for those procedures for CY 2010.
The procedures that we proposed to permanently designate as office-based for CY 2010 that were temporarily designated as office-based procedures in CY 2009 were displayed in Table 44 in the CY 2010 OPPS/ASC proposed rule (74 FR 35382). The procedures that we proposed to continue to temporarily designate as office-based for CY 2010 were displayed in Table 45 in the CY 2010 OPPS/ASC proposed rule (74 FR 35382). The procedures for which the proposed office-based designation for CY 2010 is temporary also were indicated by an asterisk in Addendum AA to the proposed rule.
We did not receive any public comments that specifically addressed our proposals to designate the 4 procedures listed in Table 44 of the proposed rule and restated in Table 65, below, as permanently office-based for CY 2010. Therefore, we are adopting as final for CY 2010 permanent office-based payment indicators as proposed for the procedures reported by HCPCS codes 0084T, 21073, 55876, and C9728 that were designated temporarily office-based for CY 2009.
| CY 2009 HCPCS code | CY 2010 HCPCS code | CY 2010 short descriptor | CY 2009 ASC payment indicator | Final CY 2010 ASC payment indicator ** |
|---|---|---|---|---|
| 0084T | 53855 | Temp prostate urethral stent | R2 * | P2 |
| 21073 | 21073 | Mnpj of tmj w/anesth | P3 * | P3 |
| 55876 | 55876 | Place rt device/marker, pros | P3 * | P3 |
| C9728 | C9728 | Place device/marker, non pro | R2 * | R2 |
| * If designation is temporary. | ||||
| ** Payment indicators are based on a comparison of the rates according to the ASC standard ratesetting methodology and the MPFS final CY 2010 rates. Under current law, the MPFS payment rates have a negative update for CY 2010. For a discussion of those rates, we refer readers to the CY 2010 MPFS final rule with comment period. | ||||
Comment: One commenter supported the proposal to maintain CPT codes 64455 and 64632 as temporarily office-based pending the availability of actual claims data.
Response: We appreciate the commenter's support.
After consideration of the public comment we received, we are finalizing our CY 2010 proposal, without modification, to maintain the temporary office-based payment indicators as displayed in Table 66 for the 6 procedures reported by CPT codes 0099T, 0124T, 46930, 64455, 64632, and 67229 that were designated temporarily office-based for CY 2009.
| CY 2010 HCPCS Code | CY 2010 short descriptor | Final CY 2010 ASC payment indicator ** |
|---|---|---|
| 0099T | Implant corneal ring | R2 * |
| 0124T | Conjunctival drug placement | R2 * |
| 46930 | Destroy internal hemorrhoids | P3 * |
| 64455 | N block inj, plantar digit | P3 * |
| 64632 | N block inj, common digit | P3 * |
| 67229 | Tr retinal les preterm inf | R2* |
| * If designation is temporary. | ||
| ** Payment indicators are based on a comparison of the rates according to the ASC standard ratesetting methodology and the MPFS final CY 2010 rates. Under current law, the MPFS payment rates have a negative update for CY 2010. For a discussion of those rates, we refer readers to the CY 2010 MPFS final rule with comment period. | ||
Displayed in Table 67 below are new (or substantially revised) CY 2010 HCPCS codes to which we have assigned temporary office-based payment indicators. As explained in section XV.B.1. of this final rule with comment period, we reviewed all of the newly created HCPCS codes that became available after the issuance of the CY 2009 OPPS/ASC proposed rule that will be used to report surgical procedures in CY 2010 to evaluate their appropriateness for the ASC list of covered surgical procedures. Of the procedures reported by new or substantially revised CY 2010 HCPCS codes that we determined should not be excluded from the ASC list based on our clinical review, including assessment of Start Printed Page 60608 available utilization and volume data for any closely related procedures and consideration of other available information, we determined that 16 of the procedures would predominantly be performed in physicians' offices. However, because we had no utilization data for the procedures specifically described by these new HCPCS codes, we made the office-based designations temporary rather than permanent and will reevaluate the procedures when data become available. The temporary payment indicators for the 16 office-based procedures displayed in Table 67 are interim designations and are open to public comment during the 60-day comment period for this final rule with comment period. HCPCS codes that are new (or substantially revised) for CY 2010 are designated with an “NI” comment indicator in Addendum AA. We will respond to public comments on the interim designations in the CY 2011 OPPS/ASC final rule with comment period.
| CY 2010 HCPCS Code | CY 2010 short descriptor | Final CY 2010 ASC payment indicator ** |
|---|---|---|
| 21015 | Resection of facial tumor | R2 * |
| 21555 | Remove lesion, neck/chest | P3 * |
| 21930 | Remove lesion, back or flank | P3 * |
| 23075 | Removal of shoulder lesion | P3 * |
| 24075 | Remove arm/elbow lesion | P3 * |
| 25075 | Removal forearm lesion subcu | P3 * |
| 26115 | Removal hand lesion, subcut | P3 * |
| 27047 | Remove hip/pelvis lesion | P3 * |
| 27327 | Removal of thigh lesion | P3 * |
| 27618 | Remove lower leg lesion | P3 * |
| 28039 | Exc foot/toe tum sc > 1.5 cm | P3 * |
| 28041 | Exc foot/toe tum deep > 1.5 cm | R2 * |
| 28043 | Excision of foot lesion | P3 * |
| 28045 | Excision of foot lesion | P3 * |
| 28046 | Resection of tumor, foot | R2 * |
| 37761 | Ligate leg veins open | R2 * |
| * If designation is temporary. | ||
| ** Payment indicators are based on a comparison of the rates according to the ASC standard ratesetting methodology and the MPFS final CY 2010 rates. Under current law, the MPFS payment rates have a negative update for CY 2010. For a discussion of those rates, we refer readers to the CY 2010 MPFS final rule with comment period. | ||
c. ASC Covered Surgical Procedures Designated as Device-Intensive
(1) Background
As discussed in the August 2, 2007 final rule (72 FR 42503 through 42508), we adopted a modified payment methodology for calculating the ASC payment rates for covered surgical procedures that are assigned to the subset of OPPS device-dependent APCs with a device offset percentage greater than 50 percent of the APC cost under the OPPS, in order to ensure that payment for the procedure is adequate to provide packaged payment for the high-cost implantable devices used in those procedures. We assigned payment indicators “H8” (Device-intensive procedure on ASC list in CY 2007; paid at adjusted rate) and “J8” (Device-intensive procedure added to ASC list in CY 2008 or later; paid at adjusted rate) to identify the procedures that were eligible for ASC payment calculated according to the modified methodology, depending on whether the procedure was included on the ASC list of covered surgical procedures prior to CY 2008 and, therefore, subject to transitional payment as discussed in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68739 through 68742). The 52 device-intensive procedures for which the modified rate calculation methodology applies in CY 2009 were displayed in Table 47 and in Addendum AA to the CY 2009 OPPS/ASC final rule with comment period (73 FR 68736 through 68738 and 68840 through 68933).
(2) Changes to List of Covered Surgical Procedures Designated as Device-Intensive for CY 2010
In the CY 2010 OPPS/ASC proposed rule (74 FR 35382), we proposed to update the ASC list of covered surgical procedures that are eligible for payment according to the device-intensive procedure payment methodology for CY 2010, consistent with the proposed OPPS device-dependent APC update, reflecting the proposed APC assignments of procedures, designation of APCs as device-dependent, and APC device offset percentages based on the CY 2008 OPPS claims and cost report data available for the proposed rule. The ASC covered surgical procedures that we proposed to designate as device-intensive and that would be subject to the device-intensive procedure payment methodology for CY 2010 were listed in Table 46 in the CY 2010 OPPS/ASC proposed rule (74 FR 35383 through 35384).
Comment: Some commenters expressed general concerns regarding the sufficiency of ASC payment for device-related services and recommended modifications to the ASC device-intensive payment methodology. First, the commenters argued that CMS should not apply the ASC conversion factor to the device-related portion of the payment for all procedures for which CMS can establish a median device cost, regardless of whether they are designated as device-intensive under the established methodology. The commenters stated that, unlike ASCs' general abilities to achieve greater operational efficiencies than HOPDs, ASCs are unable to extract greater discounts on devices and expensive operative supplies than their hospital counterparts. Second, the commenters argued that CMS should not adjust the device portion of the ASC payment for device-intensive procedures by the wage index. According to the commenters, the acquisition of devices occurs on a national market, and ASCs in rural areas pay approximately the same for medical devices and equipment as facilities in more expensive labor markets. The commenters stated that CMS is underpaying device costs in markets where the wage index is low, and overpaying in markets where the wage index is high.
One commenter specifically remarked that the procedures described by CPT code 19296 (Placement of radiotherapy afterloading balloon catheter into the breast for interstitial radioelement application following partial mastectomy, includes imaging guidance; on date separate from partial Start Printed Page 60609 mastectomy) and CPT code 19297 (Placement of radiotherapy afterloading balloon catheter into the breast for interstitial radioelement application following partial mastectomy, includes imaging guidance; concurrent with partial mastectomy), which map to OPPS device-dependent APC 0648 (Level IV Breast Surgery), require the use of a device that has a list price that exceeds 50 percent of the median costs calculated for those CPT codes and, therefore, concluded that these procedures should be added to the ASC list of device-intensive procedures. Another commenter requested that CMS add the procedure described by CPT code 66180 (Aqueous shunt to extraocular reservoir (eg, Molteno, Schocket, Denver-Krupin)) to the ASC list of device-intensive procedures, arguing that the list prices of devices involved in performing this procedure are greater than 50 percent of the proposed ASC payment rate for this procedure for CY 2010.
Response: In the August 2, 2007 final rule (72 FR 42508), we established that the modified payment methodology for calculating ASC payment rates for device-intensive procedures shall apply to ASC covered surgical procedures that are assigned to device-dependent APCs under the OPPS for the same calendar year, where those APCs have a device cost of greater than 50 percent of the APC cost (that is, the device offset percentage is greater than 50). We continue to believe these criteria ensure that ASC payment rates are adequate to provide packaged payment for high cost implantable devices and ensure Medicare beneficiaries have access to these procedures in all appropriate settings of care. We do not agree that we should change our criteria and treat as device-intensive ASC services that are assigned to APCs for which the device offset percentage is less than 50 percent (such as the procedures described by CPT codes 19296 and 19297) or ASC services that are not assigned to OPPS device-dependent APCs (such as the procedure described by CPT code 66180). Under the modified payment methodology for ASC covered surgical procedures designated as device-intensive, we separately determine both the device payment and service payment portions of the ASC payment rate, and apply the ASC conversion factor only to the specifically calculated OPPS relative payment weight for the service portion, while providing the same packaged payment for the device portion as would be made under the OPPS. The 50-percent device offset threshold is established to ensure that the ASC conversion factor is not applied to the costs of high cost implantable devices, which likely do not vary between ASCs and HOPDs in the same manner service costs have been shown to vary. As we have stated in the past (73 FR 68734), we believe that when device costs comprise less than 50 percent of total procedure costs, those costs are less likely to be as predictable across sites-of-service. Accordingly, we believe that it is possible for ASCs to achieve efficiencies relative to HOPDs when providing those procedures, and that the application of the ASC conversion factor to the entire ASC payment weight is appropriate.
We also do not believe it is appropriate to vary the percentage of the national payment that is wage adjusted for different services. Under the revised ASC payment system, we utilize 50 percent as the labor-related share to adjust national ASC payment rates for geographic wage differences. We apply to ASC payments the IPPS pre-floor, pre-reclassification wage index values associated with the June 2003 OMB geographic localities, as recognized under the IPPS and OPPS, in order to adjust the labor-related portion of the national ASC payment rates for geographic wage differences. Consistent with the OPPS, we apply the ASC geographic wage adjustment to the entire ASC payment rate for device-intensive procedures. As we have noted in the past (73 FR 68735), MedPAC has indicated its intent to evaluate CMS' method for adjusting payments for variations in labor costs in light of differences in labor-related costs for device-implantation services. We look forward to reviewing the results of its evaluation, as well as any recommendations it may provide, regarding the OPPS or ASC wage adjustment policy.
Comment: Some commenters argued that CMS should not subject procedures that were on the ASC list of covered surgical procedures in CY 2007 but were rarely performed in ASCs prior to CY 2008 to the transitional adjustment. One commenter provided a data analysis demonstrating that CPT code 55873 (Cryosurgical ablation of the prostate (includes ultrasonic guidance and monitoring)) was present on three ASC claims in CY 2007, on one claim in CY 2006, and was not billed at all by ASCs in CY 2005. According to the commenter, the transitional payment for CPT code 55873 is inadequate to cover ASCs' costs of providing the procedure and will prevent Medicare beneficiaries from accessing this procedure in the ASC setting.
Response: As established in regulation at § 416.171(c), the transitional adjustment applies to all services on the CY 2007 ASC list of covered services. We cannot make an exception for procedures, such as the one described by CPT code 55873, that were on the CY 2007 list of covered services but were rarely performed in ASCs according to the commenter. Furthermore, as we stated in the August 2, 2007 final rule (72 FR 42520), the transition to the fully implemented revised ASC system payment system should not be asymmetrical, meaning that procedures with decreasing payments under the revised payment system should not be transitioned differently from those with increasing payments.
Comment: One commenter requested that CMS adjust the OPPS device offset percentages for ASC device-intensive payment purposes to account for the effects of charge compression. The commenter suggested that CMS “decompress” the supply median costs to minimize any artificial reductions that charge compression causes in the estimate of the OPPS device offset percentages.
Response: Charge compression is the practice of applying a lower charge markup to higher-cost services and a higher charge markup to lower-cost services. As a result of charge compression, the cost-based OPPS weights incorporate aggregation bias, undervaluing high cost items and overvaluing low cost items when an estimate of average markup, embodied in a single CCR, is applied to items of widely varying costs in the same cost center. As discussed in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68524), we did not adopt any short-term statistical regression-based adjustments under the OPPS that would serve to “decompress” the median costs for procedures involving devices, or for any other procedures. Rather, we chose to focus on long-term changes to Medicare cost reporting to address the effects of charge compression, including the creation of two new cost centers, “Medical Supplies Charged to Patients” and “Implantable Devices Charged to Patients,” as discussed in more detail in section II.A.1.c.(2) of this final rule with comment period. We believe that this change to how hospitals report costs for devices and supplies will improve our future estimates of costs related to high cost implantable devices, including the device offset percentages upon which we base the device portions of ASC payment rates for device-intensive procedures. Start Printed Page 60610
Comment: Several commenters remarked on the adequacy of the proposed payment rates calculated according to the ASC device-intensive payment methodology for procedures involving cochlear implants, described by CPT code 69930 (Cochlear device implantation, with or without mastoidectomy). According to the commenters, the proposed increase to the ASC payment rate for CPT code 69930 is a necessary improvement to ensure beneficiary access to cochlear implants. Several commenters also supported the proposed payment rate increases for procedures involving auditory osseointegrated devices, described by CPT codes 69714 (Implantation, osseointegrated implant, temporal bone, with percutaneous attachment to external speech processor/cochlear stimulator; without mastoidectomy); 69715 (Implantation, osseointegrated implant, temporal bone, with percutaneous attachment to external speech processor/cochlear stimulator; with mastoidectomy); 69717 (Replacement (including removal of existing device), osseointegrated implant, temporal bone, with percutaneous attachment to external speech processor/cochlear stimulator; without mastoidectomy); and 69718 (Replacement (including removal of existing device), osseointegrated implant, temporal bone, with percutaneous attachment to external speech processor/cochlear stimulator; with mastoidectomy). Other commenters encouraged CMS to finalize a somewhat higher payment rate for these procedures.
Response: We appreciate the commenters' support of the proposed payment rates for procedures involving cochlear implants and auditory osseointegrated devices. We believe that the final CY 2010 ASC payment rates for these procedures, calculated according to the ASC device-intensive ratesetting methodology, are appropriate and adequate to ensure beneficiaries have access to these procedures in the ASC setting.
After consideration of the public comments we received, we are designating the ASC covered surgical procedures displayed in Table 68 below as device-intensive for CY 2010. The HCPCS code, the HCPCS code short descriptor, the CY 2010 ASC payment indicator, the CY 2010 OPPS APC assignment, the OPPS APC Title, and the CY 2010 OPPS APC device offset percentage are listed in Table 68. Each device-intensive procedure is assigned payment indicator “H8” or “J8,” depending on whether it is subject to transitional payment. All of these procedures are included in Addendum AA to this final rule with comment period. The OPPS device-dependent APCs are discussed further in section II.A.2.d.(1) of this final rule with comment period.
| CY 2010 CPT code | CY 2010 short descriptor | Final CY 2010 ASC payment indicator | Final CY 2010 OPPS APC | OPPS APC title | Final CY 2010 device- dependent APC offset percentage |
|---|---|---|---|---|---|
| 24361 | Reconstruct elbow joint | H8 | 0425 | Level II Arthroplasty or Implantation with Prosthesis | 58 |
| 24363 | Replace elbow joint | H8 | 0425 | Level II Arthroplasty or Implantation with Prosthesis | 58 |
| 24366 | Reconstruct head of radius | H8 | 0425 | Level II Arthroplasty or Implantation with Prosthesis | 58 |
| 25441 | Reconstruct wrist joint | H8 | 0425 | Level II Arthroplasty or Implantation with Prosthesis | 58 |
| 25442 | Reconstruct wrist joint | H8 | 0425 | Level II Arthroplasty or Implantation with Prosthesis | 58 |
| 25446 | Wrist replacement | H8 | 0425 | Level II Arthroplasty or Implantation with Prosthesis | 58 |
| 27446 | Revision of knee joint | J8 | 0425 | Level II Arthroplasty or Implantation with Prosthesis | 58 |
| 33206 | Insertion of heart pacemaker | J8 | 0089 | Insertion/Replacement of Permanent Pacemaker and Electrodes | 72 |
| 33207 | Insertion of heart pacemaker | J8 | 0089 | Insertion/Replacement of Permanent Pacemaker and Electrodes | 72 |
| 33208 | Insertion of heart pacemaker | J8 | 0655 | Insertion/Replacement/Conversion of a permanent dual chamber pacemaker | 75 |
| 33212 | Insertion of pulse generator | H8 | 0090 | Insertion/Replacement of Pacemaker Pulse Generator | 74 |
| 33213 | Insertion of pulse generator | H8 | 0654 | Insertion/Replacement of a permanent dual chamber pacemaker | 75 |
| 33214 | Upgrade of pacemaker system | J8 | 0655 | Insertion/Replacement/Conversion of a permanent dual chamber pacemaker | 75 |
| 33224 | Insert pacing lead & connect | J8 | 0418 | Insertion of Left Ventricular Pacing Elect. | 81 |
| 33225 | Lventric pacing lead add-on | J8 | 0418 | Insertion of Left Ventricular Pacing Elect. | 81 |
| 33240 | Insert pulse generator | J8 | 0107 | Insertion of Cardioverter-Defibrillator | 89 |
| 33249 | Eltrd/insert pace-defib | J8 | 0108 | Insertion/Replacement/Repair of Cardioverter-Defibrillator Leads | 88 |
| 33282 | Implant pat-active ht record | J8 | 0680 | Insertion of Patient Activated Event Recorders | 73 |
| 53440 | Male sling procedure | H8 | 0385 | Level I Prosthetic Urological Procedures | 59 |
| 53444 | Insert tandem cuff | H8 | 0385 | Level I Prosthetic Urological Procedures | 59 |
| 53445 | Insert uro/ves nck sphincter | H8 | 0386 | Level II Prosthetic Urological Procedures | 71 |
| 53447 | Remove/replace ur sphincter | H8 | 0386 | Level II Prosthetic Urological Procedures | 71 |
| 54400 | Insert semi-rigid prosthesis | H8 | 0385 | Level I Prosthetic Urological Procedures | 59 |
| 54401 | Insert self-contd prosthesis | H8 | 0386 | Level II Prosthetic Urological Procedures | 71 |
| 54405 | Insert multi-comp penis pros | H8 | 0386 | Level II Prosthetic Urological Procedures | 71 |
| 54410 | Remove/replace penis prosth | H8 | 0386 | Level II Prosthetic Urological Procedures | 71 |
| 54416 | Remv/repl penis contain pros | H8 | 0386 | Level II Prosthetic Urological Procedures | 71 |
| Start Printed Page 60611 | |||||
| 55873 | Cryoablate prostate | H8 | 0674 | Prostate Cryoablation | 56 |
| 61885 | Insrt/redo neurostim 1 array | H8 | 0039 | Level I Implantation of Neurostimulator Generator | 85 |
| 61886 | Implant neurostim arrays | H8 | 0315 | Level II Implantation of Neurostimulator Generator | 88 |
| 62361 | Implant spine infusion pump | H8 | 0227 | Implantation of Drug Infusion Device | 83 |
| 62362 | Implant spine infusion pump | H8 | 0227 | Implantation of Drug Infusion Device | 83 |
| 63650 | Implant neuroelectrodes | H8 | 0040 | Percutaneous Implantation of Neurostimulator Electrodes | 58 |
| 63655 | Implant neuroelectrodes | J8 | 0061 | Laminectomy, Laparoscopy, or Incision for Implantation of Neurostimulator Electr | 64 |
| 63685 | Insrt/redo spine n generator | H8 | 0039 | Level I Implantation of Neurostimulator Generator | 85 |
| 64553 | Implant neuroelectrodes | H8 | 0040 | Percutaneous Implantation of Neurostimulator Electrodes | 58 |
| 64555 | Implant neuroelectrodes | J8 | 0040 | Percutaneous Implantation of Neurostimulator Electrodes | 58 |
| 64560 | Implant neuroelectrodes | J8 | 0040 | Percutaneous Implantation of Neurostimulator Electrodes | 58 |
| 64561 | Implant neuroelectrodes | H8 | 0040 | Percutaneous Implantation of Neurostimulator Electrodes | 58 |
| 64565 | Implant neuroelectrodes | J8 | 0040 | Percutaneous Implantation of Neurostimulator Electrodes | 58 |
| 64573 | Implant neuroelectrodes | H8 | 0225 | Implantation of Neurostimulator Electrodes, Cranial Nerve | 73 |
| 64575 | Implant neuroelectrodes | H8 | 0061 | Laminectomy, Laparoscopy, or Incision for Implantation of Neurostimulator Electr | 64 |
| 64577 | Implant neuroelectrodes | H8 | 0061 | Laminectomy, Laparoscopy, or Incision for Implantation of Neurostimulator Electr | 64 |
| 64580 | Implant neuroelectrodes | H8 | 0061 | Laminectomy, Laparoscopy, or Incision for Implantation of Neurostimulator Electr | 64 |
| 64581 | Implant neuroelectrodes | H8 | 0061 | Laminectomy, Laparoscopy, or Incision for Implantation of Neurostimulator Electr | 64 |
| 64590 | Insrt/redo pn/gastr stimul | H8 | 0039 | Level I Implantation of Neurostimulator Generator | 85 |
| 65770 | Revise cornea with implant | H8 | 0293 | Level V Anterior Segment Eye Procedures | 62 |
| 69714 | Implant temple bone w/stimul | H8 | 0425 | Level II Arthroplasty or Implantation with Prosthesis | 58 |
| 69715 | Temple bne implnt w/stimulat | H8 | 0425 | Level II Arthroplasty or Implantation with Prosthesis | 58 |
| 69717 | Temple bone implant revision | H8 | 0425 | Level II Arthroplasty or Implantation with Prosthesis | 58 |
| 69718 | Revise temple bone implant | H8 | 0425 | Level II Arthroplasty or Implantation with Prosthesis | 58 |
| 69930 | Implant cochlear device | H8 | 0259 | Level VII ENT Procedures | 85 |
d. ASC Treatment of Surgical Procedures Removed From the OPPS Inpatient List for CY 2010
As we discussed in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68724), we adopted a policy to include in our annual evaluation procedures proposed for removal from the OPPS inpatient list for possible inclusion on the ASC list of covered surgical procedures. The final list of procedures removed from the inpatient list for CY 2009 may be found in section XI.B. of that final rule with comment period.
We evaluated each of the 3 procedures we proposed to remove from the OPPS inpatient list for CY 2010 according to the criteria for exclusion from the list of covered ASC surgical procedures. As we stated in the CY 2010 OPPS/ASC proposed rule (74 FR 35384), we believe that all of these procedures should continue to be excluded from the ASC list of covered surgical procedures for CY 2010 because they would be expected to pose a significant risk to beneficiary safety or to require an overnight stay in ASCs. A full discussion about the APC Panel's recommendations regarding the procedures we proposed to remove from the OPPS inpatient list for CY 2010 may be found in section XI.B. of this final rule with comment period. The HCPCS codes for these 3 procedures and their long descriptors were listed in Table 47 in the CY 2010 OPPS/ASC proposed rule (74 FR 35384).
We did not receive any public comments specifically about our proposal to continue to exclude from the ASC list the 3 procedures reported by CPT codes 21256 (Reconstruction of orbit with osteotomies (extracranial) and with bone grafts (includes obtaining autografts) (eg, micro-ophthalmia); 27179 (Open treatment of slipped femoral epiphysis; osteoplasty of femoral neck (Heyman type procedure); and 51060 (Transvesical Start Printed Page 60612 ureterolithotomy). However, we did receive public comments requesting that we remove additional procedures from the OPPS inpatient list. In response to those comments, we removed 5 additional procedures from the OPPS inpatient list for CY 2010. The comments requesting that we remove additional procedures from the inpatient list and our responses may be found in section XI.B. of this final rule with comment period. Our review of the 5 procedures removed from the OPPS inpatient list in response to comments convinced us that none of them was appropriate for performance in the ASC setting. Our medical advisors determined that the procedures were expected to pose significant risks to beneficiary safety or to require an overnight stay when provided in ASCs.
The final list of procedures that have been removed from the CY 2010 OPPS inpatient list but that continue to be excluded from the ASC list of covered surgical procedures is displayed in Table 69 below.
| CY 2010 HCPCS code | CY 2010 long descriptor |
|---|---|
| 21256 | Reconstruction of orbit with osteotomies (extracranial) and with bone grafts (includes obtaining autografts) (eg, micro-ophthalmia). |
| 27179 | Open treatment of slipped femoral epiphysis; osteoplasty of femoral neck (Heyman type procedure). |
| 28805 | Amputation, foot; transmetatarsal. |
| 37215 | Transcatheter placement of intravascular stent(s), cervical carotid artery, percutaneous; with distal embolic protection. |
| 44950 | Appendectomy. |
| 44955 | Appendectomy; when done for indicated purpose at time of other major procedure (not as separate procedure) (List separately in addition to code for primary procedure). |
| 51060 | Transvesical ureterolithotomy. |
| 63076 | Discectomy, anterior, with decompression of spinal cord and/or nerve root(s), including osteophytectomy; cervical, each additional interspace (List separately in addition to code for primary procedure). |
2. Covered Ancillary Services
In the CY 2010 OPPS/ASC proposed rule (74 FR 35384), consistent with the established ASC payment system policy, we proposed to update the ASC list of covered ancillary services to reflect the proposed payment status for the services under the CY 2010 OPPS. Maintaining consistency with the OPPS resulted in proposed changes to ASC payment indicators for some covered ancillary items and services because of changes that were proposed under the OPPS for CY 2010. Comment indicator “CH,” discussed in section XV.F. of the CY 2010 OPPS/ASC proposed rule (74 FR 35390), was used in Addendum BB to that proposed rule to indicate covered ancillary services for which we proposed a change in the ASC payment indicator to maintain consistency with a proposed change in the OPPS treatment of the service for CY 2010.
Except for the Level II HCPCS codes listed in Table 40 of the proposed rule (74 FR 35379), all ASC covered ancillary services and their proposed payment indicators for CY 2010 were included in Addendum BB to the proposed rule.
We did not receive any public comments on our proposal to update the ASC list of covered ancillary services to reflect the payment status for the services under the OPPS. Therefore, we are finalizing, without modification, our proposed updates to the ASC list of covered ancillary services as proposed. All CY 2010 ASC covered ancillary services and their final payment indicators are included in Addendum BB to this final rule with comment period.
D. ASC Payment for Covered Surgical Procedures and Covered Ancillary Services
1. Payment for Covered Surgical Procedures
a. Background
Our ASC payment policies for covered surgical procedures under the revised ASC payment system are fully described in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66828 through 66831). Under our established policy for the revised ASC payment system, the ASC standard ratesetting methodology of multiplying the ASC relative payment weight for the procedure by the ASC conversion factor for that same year is used to calculate the national unadjusted payment rates for procedures with payment indicator “G2.” For procedures assigned payment indicator “A2,” our final policy established blended rates to be used during the transitional period and, beginning in CY 2011, ASC rates calculated according to the ASC standard ratesetting methodology. The rate calculation established for device-intensive procedures (payment indicators “H8” and “J8”) is structured so that the packaged device payment amount is the same as under the OPPS, and only the service portion of the rate is subject to the ASC standard ratesetting methodology. In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68722 through 68759), we updated the CY 2008 ASC payment rates for ASC covered surgical procedures with payment indicators of “A2,” “G2,” “H8,”and “J8” using CY 2007 data, consistent with the CY 2009 OPPS update. Payment rates for device-intensive procedures also were updated to incorporate the CY 2009 OPPS device offset percentages.
Payment rates for office-based procedures (payment indicators “P2,” “P3,” and “R2”) are the lower of the MPFS nonfacility PE RVU amount (we refer readers to the CY 2010 MPFS final rule with comment period) or the amount calculated using the ASC standard ratesetting methodology for the procedure. In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68722 through 68759), we updated the payment amounts for office-based procedures (payment indicators “P2,” “P3,” and “R2”) using the most recent available MPFS and OPPS data. We compared the estimated CY 2009 rate for each of the office-based procedures, calculated according to the ASC standard ratesetting methodology, to the MPFS nonfacility PE RVU amount to determine which was lower and, therefore, would be the CY 2009 payment rate for the procedure according to the final policy of the revised ASC payment system (see § 416.171(d)).
b. Update to ASC Covered Surgical Procedure Payment Rates for CY 2010
In the CY 2010 OPPS/ASC proposed rule (74 FR 35385), we proposed to update ASC payment rates for CY 2010 Start Printed Page 60613 using the established rate calculation methodologies under § 416.171. Thus, we proposed to calculate CY 2010 payments for procedures subject to the transitional payment methodology (payment indicators “A2” and “H8”) using a blend of 75 percent of the proposed CY 2010 ASC rate calculated according to the ASC standard ratesetting methodology and 25 percent of the CY 2007 ASC payment rate, incorporating the device-intensive procedure methodology, as appropriate, for procedures assigned ASC payment indicator “H8.” We proposed to use the amount calculated under the ASC standard ratesetting methodology for procedures assigned payment indicator “G2” because these procedures are not subject to the transitional payment methodology.
We proposed payment rates for office-based procedures (payment indicators “P2,” “P3,” and “R2”) and device-intensive procedures not subject to transitional payment (payment indicator “J8”) calculated according to our established policies. Thus, we proposed to update the payment amounts for device-intensive procedures based on the CY 2010 OPPS proposal that reflects updated OPPS device offset percentages, and to make payment for office-based procedures at the lesser of the CY 2010 proposed MPFS nonfacility PE RVU amount or the proposed CY 2010 ASC payment amount calculated according to the ASC standard ratesetting methodology.
Comment: Many commenters requested that CMS modify its packaging policy to provide separate payment for procedures that were eligible for separate ASC payment prior to becoming packaged into separately payable services under the OPPS that are not reported by any of the codes within the CPT surgical code range. The commenters stated that these HCPCS codes into which minor procedure payments are packaged are not on the list of ASC covered surgical procedures. The commenters believed that, as an unintended result of CMS' OPPS packaging policies, procedural services that meet the criteria for performance in ASCs are being excluded from coverage. They recommended that CMS adopt a policy under which packaging policy changes under the OPPS would not result in surgical procedures that were on the ASC list of covered surgical procedures in CY 2007 or CY 2008 becoming nonpayable.
Response: We did not propose to make any change in our policy to adopt the packaging decisions made under the OPPS for the ASC payment system. Further, we do not know which procedures the commenters were referring to in their comments and, therefore, are unable to fully address their other concerns.
Comment: One commenter requested that CMS correct the ASC payment rates for the procedures reported by CPT codes 64626 (Destruction by neurolytic agent, paravertebral facet joint nerve; cervical or thoracic, single level); 64627 (Destruction by neurolytic agent, paravertebral facet joint nerve; cervical or thoracic, each additional level); and 64680 (Destruction by neurolytic agent, with or without radiologic monitoring; celiac plexus). The commenter stated that the rates in Addendum AA to the proposed rule for these procedures were incorrectly listed as $299.12, $158.13, and $312.90. respectively.
Response: We reviewed the proposed payment rates for these three procedures and found that they are all correct. We believe that the commenter failed to notice that we proposed to assign two of the procedures, CPT codes 64626 and 64680, to different APCs under the OPPS for CY 2010. The proposed changes in their OPPS APC assignments resulted in lower OPPS relative payment weights and, therefore, lower proposed ASC payment rates for CY 2010. The proposed payment rate for the third procedure, CPT code 64627, is correct as displayed in Addendum AA to the CY 2010 OPPS/ASC proposed rule. There was no proposed change to the APC assignment for that procedure under the OPPS for CY 2010. Therefore, the proposed ASC payment rate change for CY 2010 is due to the recalibration of the OPPS APC relative payment weight, which was subsequently incorporated into the ASC payment system, and also due to the progression to the third year of the transition to ASC rates calculated entirely based on the standard ratesetting methodology.
After consideration of the public comments we received, we are finalizing our CY 2010 proposal, without modification, to calculate the CY 2010 final ASC payment rates according to our established methodologies.
c. Adjustment to ASC Payments for No Cost/Full Credit and Partial Credit Devices
Our ASC policy with regard to payment for costly devices implanted in ASCs at no cost or with full or partial credit as set forth in § 416.179 is consistent with the OPPS policy. The CY 2010 OPPS APCs and devices subject to the adjustment policy are discussed in section IV.B.2. of this final rule with comment period. The established ASC policy includes adoption of the OPPS policy for reduced payment to providers when a specified device is furnished without cost or with full or partial credit for the cost of the device for those ASC covered surgical procedures that are assigned to APCs under the OPPS to which this policy applies. We refer readers to the CY 2009 OPPS/ASC final rule with comment period for a full discussion of the ASC payment adjustment policy for no cost/full credit and partial credit devices (73 FR 68742 through 68745).
In the CY 2010 OPPS/ASC proposed rule (74 FR 35385), consistent with the OPPS, we proposed to update the list of ASC covered device-intensive procedures and devices that would be subject to the no cost/full credit and partial credit device adjustment policy for CY 2010. Table 48 in the CY 2010 OPPS/ASC proposed rule (74 FR 35386 through 35387) displayed the ASC covered device-intensive procedures that we proposed would be subject to the no cost/full credit and partial credit device adjustment policy for CY 2010. Specifically, when a procedure that is listed in Table 48 is performed to implant a device that is listed in Table 49 of the proposed rule (74 FR 35387), where that device is furnished at no cost or with full credit from the manufacturer, the ASC would append the HCPCS “FB” modifier on the line with the procedure to implant the device. The contractor would reduce payment to the ASC by the device offset amount that we estimate represents the cost of the device when the necessary device is furnished without cost to the ASC or with full credit. We would provide the same amount of payment reduction based on the device offset amount in ASCs that would apply under the OPPS under the same circumstances. We stated in the CY 2010 OPPS/ASC proposed rule (74 FR 35385) that we continue to believe that the reduction of ASC payment in these circumstances is necessary to pay appropriately for the covered surgical procedure being furnished by the ASC.
We also proposed to reduce the payment for implantation procedures listed in Table 48 of the proposed rule by one-half of the device offset amount that would be applied if a device was provided at no cost or with full credit, if the credit to the ASC is 50 percent or more of the cost of the new device. The ASC would append the HCPCS “FC” modifier to the HCPCS code for a surgical procedure listed in Table 48 in the proposed rule when the facility receives a partial credit of 50 percent or more of the cost of a device listed in Table 49. In order to report that they received a partial credit of 50 percent or Start Printed Page 60614 more of the cost of a new device, ASCs would have the option of either: (1) Submitting the claim for the device replacement procedure to their Medicare contractor after the procedure's performance but prior to manufacturer acknowledgment of credit for the device, and subsequently contacting the contractor regarding a claim adjustment once the credit determination is made; or (2) holding the claim for the device implantation procedure until a determination is made by the manufacturer on the partial credit and submitting the claim with the “FC” modifier appended to the implantation procedure HCPCS code if the partial credit is 50 percent or more of the cost of the replacement device. Beneficiary coinsurance would continue to be based on the reduced payment amount.
We did not receive any comments on our CY 2010 proposal to continue the no cost/full credit and partial credit device adjustment policy for ASCs. For CY 2010, as we proposed, we will reduce the payment for the device implantation procedures listed in Table 70, below, by the full device offset amount for no cost/full credit cases. ASCs must append the modifier “FB” to the HCPCS procedure code when the device furnished without cost or with full credit is listed in Table 71, below, and the associated implantation procedure code is listed in Table 70. In addition, for CY 2010, we will reduce the payment for implantation procedures listed in Table 70 by one half of the device offset amount that would be applied if a device were provided at no cost or with full credit, if the credit to the ASC is 50 percent or more of the device cost. If the ASC receives a partial credit of 50 percent or more of the cost of a device listed in Table 71, the ASC must append the modifier “FC” to the associated implantation procedure code if the procedure is listed in Table 70. We are adding device HCPCS code L8680 (Implantable neurostimulator electrode, each) to the list of devices in Table 71 because we are recognizing this code as packaged under the OPPS for CY 2010, as described in section IV.B.2. of this final rule with comment period.
Start Printed Page 60615 Start Printed Page 60616 Start Printed Page 60617| CY 2010 device HCPCS code | CY 2010 short descriptor |
|---|---|
| C1721 | AICD, dual chamber. |
| C1722 | AICD, single chamber. |
| C1764 | Event recorder, cardiac. |
| C1767 | Generator, neurostim, imp. |
| C1771 | Rep dev, urinary, w/sling. |
| C1772 | Infusion pump, programmable. |
| C1776 | Joint device (implantable). |
| C1778 | Lead, neurostimulator. |
| C1779 | Lead, pmkr, transvenous VDD. |
| C1785 | Pmkr, dual, rate-resp. |
| C1786 | Pmkr, single, rate-resp. |
| C1813 | Prosthesis, penile, inflatab. |
| C1815 | Pros, urinary sph, imp. |
| C1820 | Generator, neuro rechg bat sys. |
| C1881 | Dialysis access system. |
| C1882 | AICD, other than sing/dual. |
| C1891 | Infusion pump, non-prog, perm. |
| C1897 | Lead, neurostim, test kit. |
| C1898 | Lead, pmkr, other than trans. |
| C1900 | Lead coronary venous. |
| C2619 | Pmkr, dual, non rate-resp. |
| C2620 | Pmkr, single, non rate-resp. |
| C2621 | Pmkr, other than sing/dual. |
| Start Printed Page 60618 | |
| C2622 | Prosthesis, penile, non-inf. |
| C2626 | Infusion pump, non-prog, temp. |
| C2631 | Rep dev, urinary, w/o sling. |
| L8614 | Cochlear device/system. |
| L8680 | Implt neurostim elctr each. |
| L8685 | Implt nrostm pls gen sng rec. |
| L8686 | Implt nrostm pls gen sng non. |
| L8687 | Implt nrostm pls gen dua rec. |
| L8688 | Implt nrostm pls gen dua non. |
| L8690 | Aud osseo dev, int/ext comp. |
2. Payment for Covered Ancillary Services
a. Background
Our final payment policies under the revised ASC payment system for covered ancillary services vary according to the particular type of service and its payment policy under the OPPS. Our overall policy provides separate ASC payment for certain ancillary items and services integrally related to the provision of ASC covered surgical procedures that are paid separately under the OPPS and provides packaged ASC payment for other ancillary items and services that are packaged under the OPPS. Thus, we established a final policy to align ASC payment bundles with those under the OPPS (72 FR 42495).
Our ASC payment policies provide separate payment for drugs and biologicals that are separately paid under the OPPS at the OPPS rates, while we pay for separately payable radiology services at the lower of the MPFS nonfacility PE RVU (or technical component) amount or the rate calculated according to the ASC standard ratesetting methodology (72 FR 42497). In all cases, ancillary items and services must be provided integral to the performance of ASC covered surgical procedures for which the ASC bills Medicare, in order for those ancillary services also to be paid.
ASC payment policy for brachytherapy sources generally mirrors the payment policy under the OPPS. We finalized our policy in the CY 2008 OPPS/ASC final rule with comment period (72 FR 42499) to pay for brachytherapy sources applied in ASCs at the same prospective rates that were adopted under the OPPS or, if OPPS rates were unavailable, at contractor-priced rates. Subsequent to publication of that rule, section 106 of the Medicare, Medicaid, and SCHIP Extension Act of 2007 (Pub. L. 110–173) mandated that, for the period January 1, 2008 through June 30, 2008, brachytherapy sources be paid under the OPPS at charges adjusted to cost. Therefore, consistent with our final overall ASC payment policy, we paid ASCs at contractor-priced rates for brachytherapy sources provided in ASCs during that period of time. Beginning July 1, 2008, brachytherapy sources applied in ASCs were to be paid at the same prospectively set rates that were finalized in the CY 2008 OPPS/ASC final rule with comment period (72 FR 67165 through 67188). Immediately prior to the publication of the CY 2009 OPPS/ASC proposed rule, section 142 of the Medicare Improvements for Patients and Providers Act of 2008 (Pub. L. 110–275) amended section 1833(t)(16)(C) of the Act (as amended by section 106 of the Medicare, Medicaid, and SCHIP Extension Act of 2007) to extend the requirement that brachytherapy sources be paid under the OPPS at charges adjusted to cost through December 31, 2009. Therefore, consistent with final ASC payment policy, ASCs continued to be paid at contractor-priced rates for brachytherapy sources provided integral to ASC covered surgical procedures during that period of time.
Other separately paid covered ancillary services in ASCs, specifically corneal tissue acquisition and device categories with OPPS pass-through status, do not have prospectively established ASC payment rates according to the final policies of the revised ASC payment system (72 FR 42502 and 42509). Under the revised ASC payment system, corneal tissue acquisition is paid based on the invoiced costs for acquiring the corneal tissue for transplantation. As discussed in section IV.A.1. of this final rule with comment period, new pass-through device categories may be established on a quarterly basis, but currently there are no OPPS device pass-through categories that would continue for OPPS pass-through payment (and, correspondingly, separate ASC payment) in CY 2010.
b. Payment for Covered Ancillary Services for CY 2010
In the CY 2010 OPPS/ASC proposed rule (74 FR 35388), we proposed to update the ASC payment rates and make changes to ASC payment indicators as necessary to maintain consistency between the OPPS and ASC payment system regarding the packaged or separately payable status of services and the proposed CY 2010 OPPS and ASC payment rates. The proposed CY 2010 OPPS payment methodologies for separately payable drugs and biologicals and brachytherapy sources were discussed in sections V. and VII. of the CY 2010 OPPS/ASC proposed rule (74 FR 35324 through 35333 and 74 FR 35340 through 35343), respectively, and we proposed to set the CY 2010 ASC payment rates for those services equal to the proposed CY 2010 OPPS rates.
Consistent with established ASC payment policy (72 FR 42497), the proposed CY 2010 payment for separately payable covered radiology services was based on a comparison of the CY 2010 proposed MPFS nonfacility PE RVU amounts (74 FR 33687 through 33800) and the proposed CY 2010 ASC payment rates calculated according to the ASC standard ratesetting methodology and then set at the lower of the two amounts. Alternatively, payment for a radiology service may be packaged into the payment for the ASC covered surgical procedure if the radiology service is packaged under the OPPS. The payment indicators in Addendum BB to the CY 2010 OPPS/ASC proposed rule indicated whether the proposed payment rates for radiology services are based on the MPFS nonfacility PE RVU amount or the ASC standard ratesetting methodology, or whether payment for a radiology service is packaged into the payment for the covered surgical procedure (payment indicator “N1”). Radiology services that we proposed to pay based on the ASC standard ratesetting methodology are assigned payment indicator “Z2” (Radiology service paid separately when provided integral to a surgical procedure on ASC list; payment based on OPPS relative payment weight) and those for which the proposed payment is based on the MPFS nonfacility PE RVU amount are assigned payment indicator “Z3” (Radiology service paid separately when provided integral to a surgical procedure on ASC list; payment based on MPFS nonfacility PE RVUs).
All covered ancillary services and their proposed payment indicators were listed in Addendum BB to the CY 2010 OPPS/ASC proposed rule.
Comment: One commenter expressed continued disagreement with the ASC packaging policy related to discography services. According to the policy, the injection procedures reported by CPT codes 62290 (Injection procedure for Start Printed Page 60619 discography, each level; lumbar) and 62291 (Injection procedure for discography, each level; cervical or thoracic) are packaged into the services reported by CPT codes 72285 (Discography, cervical or thoracic, radiological supervision and interpretation) and 72295 (Discography, lumbar, radiological supervision and interpretation) and, therefore, separate payment is made to an ASC only when the radiology service is provided integral to a covered surgical procedure. The commenter asserted that the injection procedures reported by CPT codes 62290 and 62291 are the major procedures of the discography because they require more time and resources than the radiological services and, as such, should not be packaged into the lesser radiological services.
The commenter believed that discography has many similarities to vertebroplasty, for which separate payment is made under the ASC payment system. The commenter stated that both procedures require sedation, insertion of a needle into the spine (one into the disc and the other into the bone), and image guidance, and that material (contrast agent or bone cement, respectively) is injected into the spine in both procedures. Based on discography's similarities to the separately payable vertebroplasty procedures, the commenter requested that CMS implement separate payments for discography and radiological supervision and interpretation, recognizing that the injection procedures are the major procedures in discography.
Response: As we explained fully in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68747), we continue to believe that our packaging policy for discography services is appropriate and we do not agree that packaging policies under the ASC payment system should vary from those under the OPPS. Also, we continue to believe that discography is a radiology service, even though a component of it may be defined as surgical, and that radiology services are not appropriate for performance and separate payment in ASCs unless they are integral to covered surgical procedures.
Comment: Several commenters requested that CMS pay for low dose rate (LDR) prostate brachytherapy services under the ASC payment system based on the composite APC methodology used under the OPPS rather than making two separate payments for the services reported by CPT codes 55875 (Transperineal placement of needles or catheters into prostate for interstitial radioelement application, with or without cystoscopy) and 77778 (Interstitial radiation source application; complex). The composite APCs were developed for procedures like LDR prostate brachytherapy in which two procedures are frequently performed in a single hospital visit. The commenters asserted that basing ASC payments for the services on the composite APC methodology in which one payment is made for the combination of the two services, would result in a more accurate payment than is currently being made to ASCs because ASC payment is based on the median costs from single-service claims that CMS has acknowledged are mostly incorrectly coded claims.
Response: Although we have tried to align the ASC and OPPS packaging polices to the fullest extent, in the case of the LDR prostate brachytherapy composite APC and other composite APCs, the differences in the payment policies across the two payment systems pose some obstacles to making payment to ASCs using the composite packages of services. In the case of the two services included in the LDR brachytherapy composite APC, the surgical procedure was on the ASC list in CY 2007 and, therefore, is subject to the transitional payment methodology in CY 2010. The other service in the LDR brachytherapy composite APC is a covered ancillary service for which the ASC payment is made at the lesser of the ASC rate calculated according to the ASC standard ratesetting methodology or the MPFS nonfacility PE RVU amount for that year. We do not see a method by which to calculate an ASC rate for the package of the two procedures that is consistent with the established ASC payment policies. Further, we did not propose to implement composite payment policies under the ASC payment system.
After consideration of the public comments we received, we are providing CY 2010 payment for covered ancillary services in accordance with the final policies of the revised ASC payment system as described in the CY 2008 OPPS/ASC final rule with comment period (72 FR 42493 through 42501). Covered ancillary services and their final CY 2010 payment indicators are listed in Addendum BB to this final rule with comment period.
E. New Technology Intraocular Lenses (NTIOLs)
1. Background
In the CY 2007 OPPS/ASC final rule with comment period (71 FR 68176), we finalized our current process for reviewing applications to establish new active classes of new technology intraocular lenses (NTIOLs) and for recognizing new candidate intraocular lenses (IOLs) inserted during or subsequent to cataract extraction as belonging to a NTIOL class that is qualified for a payment adjustment. Specifically, we established the following process:
• We announce annually in the Federal Register a document that proposes the update of ASC payment rates for the following calendar year, a list of all requests to establish new NTIOL classes accepted for review during the calendar year in which the proposal is published and the deadline for submission of public comments regarding those requests. Pursuant to Section 141(b)(3) of Public Law 103–432 and our regulations at § 416.185(b), the deadline for receipt of public comments is 30 days following publication of the list of requests.
• In the Federal Register document that finalizes the update of ASC payment rates for the following calendar year, we—
○ Provide a list of determinations made as a result of our review of all new class requests and public comments; and
○ Announce the deadline for submitting requests for review of an application for a new NTIOL class for the following calendar year.
In determining whether a lens belongs to a new class of NTIOLs and whether the ASC payment amount for insertion of that lens in conjunction with cataract surgery is appropriate, we expect that the insertion of the candidate IOL would result in significantly improved clinical outcomes compared to currently available IOLs. In addition, to establish a new NTIOL class, the candidate lens must be distinguishable from lenses already approved as members of active or expired classes of NTIOLs that share a predominant characteristic associated with improved clinical outcomes that was identified for each class. Furthermore, in the CY 2007 OPPS/ASC final rule with comment period (71 FR 68227), we finalized our proposal to base our determinations on consideration of the following factors set out at § 416.195:
- The IOL must have been approved by the FDA and claims of specific clinical benefits and/or lens characteristics with established clinical relevance in comparison with currently available IOLs must have been approved by the FDA for use in labeling and advertising;
• The IOL is not described by an active or expired NTIOL class; that is, it does not share the predominant, class- Start Printed Page 60620 defining characteristic associated with improved clinical outcomes with designated members of an active or expired NTIOL class; and
- Evidence demonstrates that use of the IOL results in measurable, clinically meaningful, improved outcomes in comparison with use of currently available IOLs. According to the statute, and consistent with previous examples provided by CMS, superior outcomes that we consider include the following:
○ Reduced risk of intraoperative or postoperative complication or trauma;
○ Accelerated postoperative recovery;
○ Reduced induced astigmatism;
○ Improved postoperative visual acuity;
○ More stable postoperative vision; and/or
○ Other comparable clinical advantages, such as—
■ Reduced dependence on other eyewear (for example, spectacles, contact lenses, and reading glasses);
■ Decreased rate of subsequent diagnostic or therapeutic interventions, such as the need for YAG laser treatment;
■ Decreased incidence of subsequent IOL exchange; and
■ Decreased blurred vision, glare, other quantifiable symptom or vision deficiency.
For a request to be considered complete, we require submission of the information that is found in the guidance document entitled “Application Process and Information Requirements for Requests for a New Class of New Technology Intraocular Lens (NTIOL)” posted on the CMS Web site at: http://www.cms.hhs.gov/ASCPayment/08_NTIOLs.asp#TopOfPage.
As we stated in the CY 2007 OPPS/ASC final rule with comment period (71 FR 68180), there are three possible outcomes from our review of a request for establishment of a new NTIOL class. As appropriate, for each completed request for consideration of a candidate IOL into a new class that is received by the established deadline, one of the following determinations is announced annually in the final rule updating the ASC payment rates for the next calendar year:
- The request for a payment adjustment is approved for the candidate IOL for 5 full years as a member of a new NTIOL class described by a new HCPCS code;
- The request for a payment adjustment is approved for the candidate IOL for the balance of time remaining as a member of an active NTIOL class; or
- The request for a payment adjustment is not approved.
We also discussed our plan to summarize briefly in the final rule with comment period the evidence that we reviewed, the public comments, and the basis for our determinations in consideration of applications for establishment of a new NTIOL class. We established that when a new NTIOL class is created, we identify the predominant characteristic of NTIOLs in that class that sets them apart from other IOLs (including those previously approved as members of other expired or active NTIOL classes) and that is associated with improved clinical outcomes. The date of implementation of a payment adjustment in the case of approval of an IOL as a member of a new NTIOL class would be set prospectively as of 30 days after publication of the ASC payment update final rule, consistent with the statutory requirement.
2. NTIOL Application Process for Payment Adjustment
In CY 2007, we posted an updated guidance document to the CMS Web site to provide process and information requirements for applications requesting a review of the appropriateness of the payment amount for insertion of an IOL to ensure that the ASC payment for covered surgical procedures includes payment that is reasonable and related to the cost of acquiring a lens that is approved as belonging to a new class of NTIOLs. This guidance document can be accessed on the CMS Web site at: http://www.cms.hhs.gov/ASCPayment/downloads/NTIOLprocess.pdf.
We note that we have also issued a guidance document entitled “Revised Process for Recognizing Intraocular Lenses Furnished by Ambulatory Surgery Centers (ASCs) as Belonging to an Active Subset of New Technology Intraocular Lenses (NTIOLs).” This guidance document can be accessed on the CMS Web site at: http://www.cms.hhs.gov/ASCPayment/Downloads/Request_for_inclusion_in_current_NTIOL_subset.pdf.
This second guidance document provides specific details regarding requests for recognition of IOLs as belonging to an existing, active NTIOL class, the review process, and information required for a request to review. Currently, there is one active NTIOL class whose defining characteristic is the reduction of spherical aberration. CMS accepts requests throughout the year to review the appropriateness of recognizing an IOL as a member of an active class of NTIOLs. That is, review of candidate lenses for membership in an existing, active NTIOL class is ongoing and not limited to the annual review process that applies to the establishment of new NTIOL classes. We ordinarily complete the review of such a request within 90 days of receipt of all information that we consider pertinent to our review, and upon completion of our review, we notify the requestor of our determination and post on the CMS Web site notification of a lens newly approved for a payment adjustment as an NTIOL belonging to an active NTIOL class when furnished in an ASC.
3. Classes of NTIOLs Approved and New Requests for Payment Adjustment
a. Background
Since implementation of the process for adjustment of payment amounts for NTIOLs that was established in the June 16, 1999 Federal Register , we have approved three classes of NTIOLs, as shown in the following table, with the associated qualifying IOLs to date:
| NTIOL class | HCPCS code | $50 approved for services furnished on or after | NTIOL characteristic | IOLs eligible for adjustment |
|---|---|---|---|---|
| 1 | Q1001 | May 18, 2000, through May 18, 2005 | Multifocal | Allergan AMO Array Multifocal lens, model SA40N. |
| 2 | Q1002 | May 18, 2000, through May 18, 2005 | Reduction in Preexisting Astigmatism | STAAR Surgical Elastic Ultraviolet-Absorbing Silicone Posterior Chamber IOL with Toric Optic, models AA4203T, AA4203TF, and AA4203TL. |
| Start Printed Page 60621 | ||||
| 3 | Q1003 | February 27, 2006, through February 26, 2011 | Reduced Spherical Aberration | Advanced Medical Optics (AMO) Tecnis® IOL models Z9000, Z9001, Z9002, ZA9003, and AR40xEM and Tecnis® 1–Piece model ZCB00; Alcon Acrysof® IQ Model SN60WF, Acrysert Delivery System model SN60WS and Acrysof ® IQ Toric model SN6ATT; Bausch & Lomb Sofport AO models LI61AO and LI61AOV and Akreos AO models AO60 and MI60; STAAR Affinity Collamer model CQ2015A and CC4204A and Elastimide model AQ2015A; Hoya model FY–60AD, FC–60AD, PY–60AD, and PC–60AD. |
b. Request To Establish New NTIOL Class for CY 2010 and Deadline for Public Comment
As explained in the guidance document on the CMS Web site, the deadline for each year's requests for review of the appropriateness of the ASC payment amount for insertion of a candidate IOL as a member of a new class of NTIOLs is announced in the final rule updating the ASC and OPPS payment rates for that calendar year. Therefore, a request for review for a new class of NTIOLs for CY 2010 must have been submitted to CMS by March 2, 2009, the due date published in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68752). We did not receive any requests for review to establish a new NTIOL class for CY 2010 by the March 2, 2009 due date.
4. Payment Adjustment
The current payment adjustment for a 5-year period from the implementation date of a new NTIOL class is $50. In the CY 2007 OPPS/ASC final rule with comment period, we revised § 416.200(a) through (c) to clarify how the IOL payment adjustment is made and how an NTIOL is paid after expiration of the payment adjustment, and made minor editorial changes to § 416.200(d). For CY 2008 and CY 2009, we did not revise the payment adjustment amount, and, in the CY 2010 OPPS/ASC proposed rule (74 FR 35390), we did not propose to revise the payment adjustment amount for CY 2010 in light of our limited experience with the revised ASC payment system, implemented initially on January 1, 2008. Therefore, the final ASC payment adjustment amount for NTIOLS in CY 2010 is $50.
5. ASC Payment for Insertion of IOLs
In accordance with the final policies of the revised ASC payment system, for CY 2010, payment for IOL insertion procedures is established according to the standard payment methodology of the revised payment system, which multiplies the ASC conversion factor by the ASC payment weight for the surgical procedure to implant the IOL. CY 2010 ASC payment for the cost of a conventional lens is packaged into the payment for the associated covered surgical procedures performed by the ASC. The HCPCS codes for IOL insertion procedures were included in Table 50 in the CY 2010 OPPS/ASC proposed rule (74 FR 35390), and their proposed CY 2010 payment rates were included in Addendum AA to that proposed rule.
We did not receive any public comments concerning the proposed CY 2010 payment rates for the insertion of IOL procedures. Therefore, we are finalizing the payment rates for the insertion of IOL procedures, calculated according to the standard methodology of the revised ASC payment system. The HCPCS codes for IOL insertion procedures are displayed in Table 72 below, and their final CY 2010 payment rates may be found in Addendum AA to this final rule with comment period.
| CY 2009 HCPCS code | CY 2009 long descriptor |
|---|---|
| 66983 | Intracapsular cataract extraction with insertion of intraocular lens prosthesis (one stage procedure). |
| 66984 | Extracapsular cataract removal with insertion of intraocular lens prosthesis (one stage procedure), manual or mechanical technique (eg, irrigation and aspiration or phacoemulsification). |
| 66985 | Insertion of intraocular lens prosthesis (secondary implant), not associated with concurrent cataract removal. |
| 66986 | Exchange of intraocular lens. |
6. Announcement of CY 2010 Deadline for Submitting Requests for CMS Review of Appropriateness of ASC Payment for Insertion of an NTIOL Following Cataract Surgery
In accordance with § 416.185(a) of our regulations as revised by the CY 2007 OPPS/ASC final rule with comment period, CMS announces that in order to be considered for payment effective January 1, 2011, requests for review of applications for a new class of new technology IOLs must be received at CMS by 5 p.m. EST, on March 8, 2010. Send requests to ASC/NTIOL, Division of Outpatient Care, Mailstop C4–05–17, Centers for Medicare and Medicaid, 7500 Security Boulevard, Baltimore, MD 21244–1850.
To be considered, requests for NTIOL reviews must include the information on the CMS Web site at: http://www.cms.hhs.gov/ASCPayment/downloads/NTIOLprocess.pdf.
F. ASC Payment and Comment Indicators
1. Background
In addition to the payment indicators that we introduced in the August 2, 2007 final rule, we also created final comment indicators for the ASC payment system in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66855). We created Addendum DD1 to define ASC payment indicators that we use in Addenda AA and BB to provide payment information regarding covered surgical procedures and covered ancillary services, respectively, under the revised ASC payment system. The ASC payment indicators in Addendum DD1 are intended to capture policy-relevant characteristics of HCPCS codes that may receive packaged or separate payment in ASCs, including: their ASC payment status prior to CY 2008; their designation as device-intensive or office-based and the corresponding ASC payment methodology; and their classification as separately payable radiology services, Start Printed Page 60622 brachytherapy sources, OPPS pass-through devices, corneal tissue acquisition services, drugs or biologicals, or NTIOLs.
We also created Addendum DD2 that lists the ASC comment indicators. The ASC comment indicators used in Addenda AA and BB to the proposed rules and final rules with comment period serve to identify, for the revised ASC payment system, the status of a specific HCPCS code and its payment indicator with respect to the timeframe when comments will be accepted. The comment indicator “NI” is used in the OPPS/ASC final rule with comment period to indicate new HCPCS codes for the next calendar year or existing codes with substantial revisions to their descriptors such that we consider them to be describing new services or procedures for which their ASC payment indicators may change. All HCPCS codes to which the interim payment indicator is assigned are subject to comment.
The “CH” comment indicator was used in Addenda AA and BB to the CY 2010 OPPS/ASC proposed rule to indicate that a new payment indicator (in comparison with the indicator for the CY 2009 ASC April quarterly update) was proposed for assignment to an active HCPCS code for the next calendar year; an active HCPCS code was proposed for addition to the list of procedures or services payable in ASCs; or an active HCPCS code was proposed for deletion at the end of the current calendar year. The “CH” comment indicators that are published in the final rule with comment period are provided to alert readers that a change has been made from one calendar year to the next, but do not indicate that the change is subject to comment. The full definitions of the payment indicators and comment indicators are provided in Addenda DD1 and DD2 to this final rule with comment period.
2. ASC Payment and Comment Indicators
In the CY 2010 OPPS/ASC proposed rule (74 FR 35390 through 35391), we did not propose any changes to the definitions of the ASC payment and comment indicators for CY 2010 and we did not receive any public comments on the payment and comment indicators. Therefore, we are finalizing our proposed CY 2010 payment and comment indicators in Addenda DD1 and DD2 to this final rule with comment period, with modification to the meaning of comment indicator “NI” as follows. We want to clarify our policy regarding the use of comment indicator “NI” in this CY 2010 OPPS/ASC final rule with comment period to describe a new code. There are numerous instances in which the descriptor of an existing Category I CPT code is substantially revised for CY 2010 so that it describes a new service or procedure that could have been assigned a new code number by the CPT Editorial Panel and that new code number would then have been assigned the “NI” comment indicator. Because, for CY 2010, not all new services or procedures will be assigned a new CPT code number, but instead will be described by an existing CPT code number with a substantially revised code descriptor, we are assigning the comment indicator “NI” to these codes in order to allow for comment on these substantially revised codes. Like all codes labeled with comment indicator “NI,” we will respond to public comments and finalize their ASC treatment in the CY 2011 OPPS/ASC final rule with comment period. In accordance with our usual practice, CPT and Level II HCPCS code numbers that are new for CY 2010 and are ASC covered surgical procedures or covered ancillary items and services are also labeled with comment indicator “NI” in Addenda AA and BB to this final rule with comment period.
G. ASC Policy and Payment Recommendations
MedPAC was established under section 1805 of the Act to advise the U.S. Congress on issues affecting the Medicare program. Sections 1805(b)(1)(B) and 1805(b)(1)(C) of the Act require MedPAC to submit reports to Congress not later than March 1 and June 15 of each year that present its Medicare payment policy reviews and recommendations. The following section describes a recent MedPAC recommendation that is relevant to the ASC payment system.
The March 2009 MedPAC “Report to the Congress: Medicare Payment Policy” included the following recommendation relating specifically to the ASC payment system for CY 2010:
Recommendation 2B–4: The Congress should increase payments for ambulatory surgery center (ASC) services in calendar year 2010 by 0.6 percent. In addition, the Congress should require ASCs to submit to the Secretary cost data and quality data that will allow for an effective evaluation of the adequacy of ASC payment rates.
CMS Response: As we proposed in the CY 2010 proposed rule (74 FR 35391), in this final rule with comment period we are increasing the payment amounts for the ASC payment system according to our established policy as stated in the August 2, 2007 final rule (72 FR 42518 through 42519). Section 1833(i)(2)(C) of the Act requires that, if the Secretary has not updated the ASC payment amounts in a calendar year, the payment amounts shall be increased by the percentage increase in the Consumer Price Index for All Urban Consumers (CPI–U) as estimated by the Secretary for the 12-month period ending with the midpoint of the year involved. We indicated that we planned to implement the annual updates through an adjustment to the conversion factor under the ASC payment system beginning in CY 2010 when the statutory requirement for a zero update no longer applies. Further, we noted that we were proposing to update the conversion factor for the CY 2010 ASC payment system by the percentage increase in the CPI–U, consistent with our policy as codified under § 416.171(a)(2).
We also did not propose to require ASCs to submit cost data to the Secretary for CY 2010 and, therefore, will not require cost reporting in this final rule with comment period. We explained that the 2006 GAO report, “Medicare: Payment for Ambulatory Surgical Centers Should Be Based on the Hospital Outpatient Payment System” (GAO–07–86), concluded that the APC groups in the OPPS reflect the relative costs of surgical procedures performed in ASCs in the same way they reflect the relative costs of the same procedures when they are performed in HOPDs. Consistent with the GAO findings, CMS is using the OPPS as the basis for the ASC payment system, which provides for an annual revision of the ASC payment rates under the budget neutral ASC payment system. In addition, we noted that under the methodology of the revised ASC payment system, we do not utilize ASC cost information to set and revise the payment rates for ASCs but, instead, rely on the relativity of hospital outpatient costs developed for the OPPS, consistent with the recommendation of the GAO. Furthermore, we explained that we have never required ASCs to routinely submit cost data and expressed our concern that a new Medicare requirement for ASCs to do so could be administratively burdensome for ASCs. However, in light of the MedPAC recommendation, in the CY 2010 OPPS/ASC proposed rule (74 FR 35391), we solicited public comment on the feasibility of ASCs submitting cost information to CMS, including whether costs should be collected from a sample or the universe of ASCs, the administrative burden associated with Start Printed Page 60623 such an activity, the form that such a submission could take considering existing Medicare requirements for other types of facilities and the scope of ASC services, the expected accuracy of such cost information, and any other issues or concerns of interest to the public on this topic.
Finally, we noted that section 109(b) of the MIEA–TRHCA (Pub. L. 109–432) gives the Secretary the authority to implement ASC quality measure reporting and to reduce the payment update for ASCs that fail to report those required measures. We restated our belief that promoting high quality care in the ASC setting through quality reporting is highly desirable and fully in line with our efforts under other payment systems. For the reasons discussed in section XVI.G. of this final rule with comment period, we did not require ASC quality data reporting for CY 2010, but our intention is to implement ASC quality reporting in a future rulemaking.
Comment: Commenters' expressed varied opinions regarding the feasibility of requiring ASCs to submit cost data to the Secretary. MedPAC's comments on CMS' solicitation in the CY 2010 OPPS/ASC proposed rule (74 FR 35391) stated that, although ASCs are generally small facilities that may have limited resources for collecting cost data, other small providers submit cost reports to CMS and, therefore, MedPAC did not believe that the resources involved in submitting cost data would be an insurmountable obstacle for ASCs. Further, MedPAC suggested that the scale of cost reporting for ASCs would be limited to the information that analysts would need to assess the adequacy of Medicare payments and evaluate the ASC update and may be satisfied by implementing either a streamlined cost report or a random survey. If a survey method is used, MedPAC recommended that CMS require ASCs to respond in order to ensure adequate data.
Other commenters, mostly those representing hospitals, believed that in light of the MedPAC recommendation that ASCs be required to submit cost data, ASCs should be required to do so even though ASC cost data are not used to set or revise the payment rates. They suggested that collection of ASC cost data could be accomplished through implementing an ASC cost reporting system or through the periodic collection of ASC cost data at the procedure level. On the other hand, some commenters (predominantly commenters who represented ASCs) opposed a requirement that ASCs submit cost data to CMS. The commenters believed that a requirement to submit cost data would be both unnecessary and administratively burdensome for ASCs. Further, some commenters stated that requiring ASCs to submit cost data that would not be used to update or set payment rates would very likely result in submissions of data that would not be reliable.
In its comment on the statement in the proposed rule (74 FR 35391) that CMS has the authority under section 109(b) of the MIEA–TRHCA (Pub. L. 109–432) to implement ASC quality measure reporting, MedPAC noted that CMS was not required to implement that reporting as MedPAC recommended in its March 2009 Report to Congress. MedPAC expressed concern about CMS' proposal to delay implementation of ASC quality measurement reporting and argued it should be technically feasible for ASCs to report in CY 2010 on at least the five quality measures that were developed by the ASC industry-sponsored ASC Quality Collaboration and endorsed by the National Quality Forum. Briefly, these five facility-level measures are: patient being burned; patient fall in the ASC; errors related to wrong surgery, wrong patient, wrong side, wrong site or wrong implant; timing of prophylactic intravenous antibiotic; and hospital transfer/admission upon discharge from the ASC. MedPAC believed that ASCs could report these five measures without undue administrative burden and that CMS should require ASCs to report these measures without further delay.
Many other commenters also urged CMS to implement ASC quality reporting as soon as possible and reported that ASCs are anxious to begin the process. The commenters believed that CMS should ensure the availability of fair and accurate quality data from ASCs in order to promote transparency and allow beneficiaries to make meaningful comparisons across outpatient surgical settings. Some commenters believed that ASCs should be required to report quality data because ASCs should be held to the same accountability standards as hospitals with respect to the quality of care they deliver and that the ASC quality measures should be consistent, and where possible, identical to the measures reported by HOPDs.
Response: We thank all of the commenters for their thoughts regarding the feasibility and value of requiring ASCs to submit cost data that could be used to evaluate the adequacy of the Medicare ASC payment rates. We will keep the commenters' perspectives about collecting cost information from ASCs in mind as we further consider the adequacy of the Medicare ASC payment rates.
We also appreciate the commenters' thoughtful remarks and suggestions regarding ASC quality reporting. We will be mindful of the opinions and information shared in the public comments as we move toward implementation of ASC quality reporting.
H. Revision to Terms of Agreements for Hospital-Operated ASCs
1. Background
The August 5, 1982 ASC final rule (47 FR 34082) established the initial Medicare ASC payment system and implementing Federal regulations under 42 CFR part 416. Under § 416.26 of our regulations, ASCs operated by hospitals, like other ASCs, must meet the applicable conditions for coverage and enter into an agreement with CMS in which CMS accepts the ASC as qualified to furnish ambulatory surgical services. Sections 416.30(a) through (g) of our regulations specify terms of agreement for ASCs. Section 416.30(f) specifies the following additional terms of agreement for an ASC operated by a hospital—
- The agreement is made effective on the first day of the next Medicare cost reporting period of the hospital that operates the ASC;
- The ASC participates and is paid only as an ASC, without the option of converting to or being paid as a hospital outpatient department, unless CMS determines there is good cause to do otherwise; and
- Costs incurred by the ASC are treated as a nonreimbursable cost center on the hospital's Medicare cost report.
In addition, § 416.35 provides guidance regarding the termination of ASC agreements with CMS. Voluntary terminations are those initiated by an ASC and as specified in § 416.35, an ASC may terminate its agreement either by sending written notice to CMS or by ceasing to furnish services to the community.
Although some sections of part 416 of the regulations governing ASCs have been revised since they were established in 1982, most recently for CY 2008 with the adoption of the revised ASC payment system, §§ 416.30(a) through 416.30(g) have not been changed or updated. At the time §§ 416.30 and 416.35 were promulgated, Medicare paid for hospital outpatient services on a reasonable cost basis. In contrast, Medicare initially paid ASCs for a small number of surgical procedures at one of only four prospective rates that were Start Printed Page 60624 developed for the ASC payment system using cost data obtained from surveys of ASCs. Since then, Medicare has adopted a prospective payment system for HOPDs (the OPPS), the ASC list of covered surgical procedures and payment rates have been updated a number of times, and, beginning in CY 2008, the revised ASC payment system was introduced.
Under the revised ASC payment system, Medicare greatly increased the number and types of surgical procedures that are eligible for payment in ASCs. As a result, many more of the same surgical procedures may be paid under the OPPS and ASC payment system, with the specific payment determined by whether the service is provided by a hospital or an ASC. Further, under the current, revised payment methodology, ASC payment rates have a direct relationship to the relative payment weights under the OPPS for the same services. Today, hospital outpatient and ASC surgical procedures are paid based on the relative weights adopted for the OPPS, and the difference between payments under the two systems is largely a reflection of the differences in capital and operating costs attributable to being an ASC or being an HOPD.
Another change that has taken place since the establishment of the Medicare ASC payment system and the implementing regulations at § 416.30 has been our effort to simplify the Medicare regulations to reduce the burden on providers and suppliers. As discussed in the August 1, 2002 IPPS final rule (67 FR 50084 through 50090), as part of that effort, we revised the provider-based status regulations at § 413.65 that outline the requirements for a determination that a facility or an organization has provider-based status as a department or entity of a hospital (main provider). The provider-based status rules generally apply to situations where there is a financial incentive for a facility or organization to claim affiliation with a main provider. The provider-based status rules establish criteria for a facility or organization to demonstrate that it is integrated with the main provider for payment purposes. We do not make provider-based status determinations for certain facilities, listed under § 413.65(a)(1)(ii) of the regulations, because the outcome of the determination (that is, whether a facility, unit, or department is found to be freestanding or provider-based) would not affect the methodology used to make Medicare or Medicaid payment, the scope of benefits available to a Medicare beneficiary in or at the facility, or the deductible or coinsurance liability of a Medicare beneficiary in or at the facility. According to § 413.65(a)(1)(ii), we do not make provider-based determinations for ASCs or other suppliers that have active supplier agreements with Medicare because services provided in such entities are paid under other fee schedules, specifically in the case of ASCs regardless of whether the ASC is operated by a hospital.
In the August 1, 2002 IPPS final rule (67 FR 50084 through 50090), we revised the provider-based status rules where the main providers were no longer required to submit an attestation to CMS to demonstrate that their provider-based departments or entities met the provider-based status rules. However, the provider-based department or entity of a main provider must still meet the provider-based status rules in § 413.65 in order for the main provider to bill for services performed in the provider-based department or entity.
2. Change to the Terms of Agreements for ASCs Operated by Hospitals
In the CY 2010 OPPS/ASC proposed rule (74 FR 35392), in order to further streamline our regulations to reduce the administrative burden on providers and suppliers, we proposed to revise existing § 416.30(f)(2) to remove the language requiring a hospital-operated ASC to satisfy CMS that there is good cause for its request to become a provider-based department of a hospital prior to being recognized as such. Specifically, we proposed to remove the language, “without the option of converting to or being paid as a hospital outpatient department, unless CMS determines there is good cause to do otherwise.” We believe that this proposed revision to the requirements that apply to hospital-operated ASCs is consistent with our earlier regulation simplification activities related to the provider-based status rules under § 413.65. We believe that we would reduce the administrative burden on hospitals and ASCs that terminate their supplier agreements with Medicare and bring the requirements into closer alignment with the provider-based status rules for other facilities or organizations that wish to be integrated with the main provider for payment purposes. While an ASC participating in Medicare would continue to be paid only as an ASC, an ASC would also continue to be able to voluntarily terminate its agreements in accordance with § 416.35. Thus, if an ASC chooses to voluntarily terminate its agreement as an ASC and a main provider wants to consider the surgical facility a provider-based department of that main provider, the facility must meet the provider-based status rules under § 413.65.
We did not receive any public comments on our proposal to revise § 416.30(f)(2) to remove the language, “without the option of converting to or being paid as a hospital outpatient department, unless CMS determines there is good cause to do otherwise.” Therefore, we are adopting as final our proposed revision of § 416.30(f)(2), without modification.
I. Calculation of the ASC Conversion Factor and ASC Payment Rates
1. Background
In the August 2, 2007 final rule (72 FR 42493), we established our policy to base ASC relative payment weights and payment rates under the revised ASC payment system on APC groups and relative payment weights. Consistent with that policy and the requirement at section 1833(i)(2)(D)(ii) of the Act that the revised payment system be implemented so that it would be budget neutral, the initial ASC conversion factor (CY 2008) was calculated so that estimated total Medicare payments under the revised ASC payment system in the first year would be budget neutral to estimated total Medicare payments under the prior (CY 2007) ASC payment system. That is, application of the ASC conversion factor was designed to result in aggregate Medicare expenditures under the revised ASC payment system in CY 2008 equal to aggregate Medicare expenditures that would have occurred in CY 2008 in the absence of the revised system, taking into consideration the cap on ASC payments in CY 2007 as required under section 1833(i)(2)(E) of the Act (72 FR 42521 through 42522).
We note that we consider the term “expenditures” in the context of the budget neutrality requirement under section 1833(i)(2)(D)(ii) of the Act to mean expenditures from the Medicare Part B Trust Fund. We do not consider expenditures to include beneficiary coinsurance and copayments. This distinction was important for the CY 2008 ASC budget neutrality model that considered payments across hospital outpatient, ASC, and MPFS payment systems. However, because coinsurance is almost always 20 percent for ASC services, this interpretation of expenditures has minimal impact for subsequent budget neutrality adjustments calculated within the revised ASC payment system.
In the CY 2008 OPPS/ASC final rule with comment period (72 FR 66857 through 66858), we set out a step-by-step illustration of the final budget Start Printed Page 60625 neutrality adjustment calculation based on the methodology finalized in the August 2, 2007 final rule (72 FR 42521 through 42531) and as applied to updated data available for the CY 2008 OPPS/ASC final rule with comment period. The application of that methodology to the data available for the CY 2008 OPPS/ASC final rule with comment period resulted in a budget neutrality adjustment of 0.65.
For CY 2008, we adopted the OPPS relative payment weights as the ASC relative payment weights for most services and, consistent with the final policy, we calculated the CY 2008 ASC payment rates by multiplying the ASC relative payment weights by the final CY 2008 ASC conversion factor of $41.401. For covered office-based surgical procedures and covered ancillary radiology services, the established policy is to set the relative payment weights so that the national unadjusted ASC payment rate does not exceed the MPFS unadjusted nonfacility PE RVU amount. Further, as discussed in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66841 through 66847), we also adopted alternative ratesetting methodologies for specific types of services (for example, device-intensive procedures).
As discussed in the August 2, 2007 final rule (72 FR 42518) and as codified under § 416.172(c) of the regulations, the revised ASC payment system accounts for geographic wage variation when calculating individual ASC payments by applying the pre-floor and pre-reclassified hospital wage indices to the labor-related share, which is 50 percent of the ASC payment amount. Beginning in CY 2008, CMS accounted for geographic wage variation in labor cost when calculating individual ASC payments by applying the pre-floor and pre-reclassified hospital wage index values that CMS calculates for payment, using updated Core Based Statistical Areas (CBSAs) issued by the Office of Management and Budget in June 2003. The reclassification provision provided at section 1886(d)(10) of the Act is specific to hospitals. We believe the use of the most recent available raw pre-floor and pre-reclassified hospital wage indices results in the most appropriate adjustment to the labor portion of ASC costs. In addition, use of the unadjusted hospital wage data avoids further reductions in certain rural statewide wage index values that result from reclassification. We continue to believe that the unadjusted hospital wage indices, which are updated yearly and are used by many other Medicare payment systems, appropriately account for geographic variation in labor costs for ASCs.
Comment: Several commenters recommended that CMS adopt for the ASC payment system the same wage index values used for hospital payment under the OPPS. They believed that applying different wage indices in the ASC payment system than are used in the OPPS is inequitable because, in many market areas, ASCs compete directly with hospitals for employees with skills and functions that are applicable in both settings. The commenters believed that, in all but a few instances, the adjusted wage index values used in the OPPS would be higher than the current wage index values used in the ASC payment system. Specifically, the commenters believed the adjustments that are applied to the wage indices used in the OPPS system also should be applied to the ASC wage indices. The adjustments that commenters requested be applied to the wage index values used in the ASC payment system are: an imputed statewide rural wage index for States with no counties outside of an urban area; a mechanism to prevent urban areas from having indices below the statewide rural wage index; a mechanism to prevent the wage index of urban areas that cross state lines from falling below the State-specific rural floor; and an adjustment for counties where a significant proportion of residents commute to other counties for work.
Response: We continue to believe that the unadjusted hospital wage indices, which are updated yearly and are used by almost all Medicare payment systems, appropriately account for geographic variance in labor costs for ASCs. The post-reclassification wage indices for 1886(d) hospitals include many statutory adjustments specific to 1886(d) hospitals and some regulatory adjustments for 1886(d) hospitals including, but not limited to, the areas requested by commenters: an imputed statewide rural wage index for States with no counties outside of an urban area; a “rural floor” mechanism to prevent urban areas from having indices below the statewide rural wage index; a mechanism to prevent the wage index of urban areas that cross state lines from falling below the State-specific rural floor; and an adjustment for counties where a significant proportion of residents commute to other counties. Because many of these adjustments are specified in statute for 1886(d) hospitals, we believe it is appropriate to apply these adjustments to 1886(d) hospitals. The OPPS adopts the post-reclassification wage indices (adjusted hospital wage indices) because the majority of participating hospitals are section 1886(d) hospitals and, in these hospitals, the exact same personnel staff the ancillary departments of the hospital that simultaneously treat both inpatients and outpatients. For payments systems for other providers and suppliers for which there is no specific statutory provision for adjustments to the wage index values, CMS calculates and employs unadjusted hospital wage indices that reflect the reported cost of hospital labor in each area. Specifically, CMS uses some form of the unadjusted hospital wage indices to pay long-term care, psychiatric, and inpatient rehabilitation hospitals for inpatient care, as well as skilled nursing facilities, hospice programs, home health agencies, and ESRD facilities. CMS historically has only applied the adjusted, post-reclassification hospital wage indices to pay section 1886(d) hospitals for both inpatient and outpatient services for the reasons noted above. It is our policy to treat ASCs as we do all other providers and suppliers using hospital wage index values.
Further, adopting the post-reclassification hospital wage indices with rural floor and associated statewide budget neutrality adjustment would not increase overall ASC payment because we apply a budget neutrality adjustment for changes in the wage indices to the conversion factor. Therefore, any anticipated increases in aggregate ASC payment created by adopting the post-reclassification wage indices would lead to a comparable downward adjustment to the conversion factor to ensure that the only increase in payments to ASCs are those allowed by the update factor. We discuss our budget neutrality adjustment for changes to the wage indices below in section XV.I.2.b. of this final rule with comment period.
2. Calculation of the ASC Payment Rates
a. Updating the ASC Relative Payment Weights for CY 2010 and Future Years
We update the ASC relative payment weights each year using the national OPPS relative payment weights (and MPFS nonfacility PE RVU amounts, as applicable) for that same calendar year and uniformly scale the ASC relative payment weights for each update year to make them budget neutral (72 FR 42531 through 42532). In the CY 2010 OPPS/ASC proposed rule (74 FR 35393), consistent with our established policy, we proposed to scale the CY 2010 relative payment weights for ASCs according to the following method. Holding ASC utilization and the mix of Start Printed Page 60626 services constant from CY 2008, for CY 2010, we proposed to compare the total payment weight using the CY 2009 ASC relative payment weights under the 50/50 blend (of the CY 2007 payment rate and the ASC payment rate calculated under the ASC standard ratesetting methodology) with the total payment weight using the CY 2010 ASC relative payment weights under the 25/75 blend (of the CY 2007 ASC payment rate and the ASC payment rate calculated under the ASC standard ratesetting methodology) to take into account the changes in the OPPS relative payment weights between CY 2009 and CY 2010. We proposed to use the ratio of CY 2009 to CY 2010 total payment weight (the weight scaler) to scale the ASC relative payment weights for CY 2010. The proposed CY 2010 ASC scaler was 0.9514 and scaling would apply to the ASC relative payment weights of the covered surgical procedures and covered ancillary radiology services for which the ASC payment rates are based on OPPS relative payment weights.
Scaling would not apply in the case of ASC payment for separately payable covered ancillary services that have a predetermined national payment amount (that is, their national ASC payment amounts are not based on OPPS relative payment weights), such as drugs and biologicals that are separately paid or services that are contractor-priced or paid at reasonable cost in ASCs. Any service with a predetermined national payment amount would be included in the ASC budget neutrality comparison, but scaling of the ASC relative payment weights would not apply to those services. The ASC payment weights for those services without predetermined national payment amounts (that is, those services with national payment amounts that would be based on OPPS relative payment weights if a payment limitation did not apply) would be scaled to eliminate any difference in the total payment weight between the current year and the update year.
The proposed weight scaler used to model CY 2010 ASC fully implemented payment rates in order to reflect our estimated of rates if there was no transition was equal to 0.9329. We applied this scaler to the payment weights subject to scaling, in order to estimate the ASC payment rates for CY 2010 without the transition, for purposes of the ASC impact analysis discussed in section XXI.C. of the CY 2010 OPPS/ASC proposed rule (74 FR 35418).
For any given year's ratesetting, we typically use the most recent full calendar year of claims data to model budget neutrality adjustments. When we developed the CY 2010 OPPS/ASC proposed rule, we had available 98 percent of CY 2008 ASC claims data. In the CY 2010 OPPS/ASC proposed rule (74 FR 35393), we reported that we had 95 percent of the CY 2008 ASC claims data available to model proposed revisions to the CY 2010 ASC payment system, but we have since confirmed that we had a slightly higher percentage available at that time. For this final rule with comment period, we have close to 100 percent of all claims for CY 2008. CY 2010 is the first year that the claims data used for ratesetting include new covered surgical procedures and covered ancillary services under the revised ASC payment system. Because we had almost all of the CY 2008 claims data available when we calculated the conversion factor and budget neutrality adjustments for our proposed rule, for the final CY 2010 budget neutrality adjustments, we did not expect there would be significant changes in our calculated budget neutrality adjustments (the weight scaler or wage adjustment) that could be attributable to more utilization available from additional claims data for this CY 2010 OPPS/ASC final rule with comment period.
To create an analytic file to support calculation of the weight scaler and budget neutrality adjustment for the wage index (discussed below), we summarized available CY 2008 ASC claims by provider and by HCPCS code. We created a unique supplier identifier solely for the purpose of identifying unique ASCs within the CY 2008 claims data. We used the supplier zip code reported on the claim to associate State, county, and CBSA with each ASC. This file, available to the public as a supporting data file for the CY 2010 OPPS/ASC proposed rule, is posted on the CMS Web site at: http://www.cms.hhs.gov/ASCPayment/01_Overview.asp#TopOfPage.
Comment: Many commenters again expressed their opposition to scaling the ASC relative payment weights. Many of the commenters on the CY 2010 proposed rule offered the same views as the public commenters on the CY 2009 OPPS/ASC final rule with comment period, the year when CMS first applied the scaling policy that was finalized in the August 2, 2007 final rule. The commenters expressed many concerns, including that scaling is contrary to the intent of using the cost-based OPPS relative payment weights as the bases for determining the relative payments for the same services in ASCs and that scaling would continue to erode the payment relationship between the OPPS and ASC payment system. Further, the commenters stated that increasing the difference between ASC and OPPS payments is in direct conflict with the goal of ensuring that patients have continued access to surgical care in the lowest priced setting appropriate to their clinical needs. They asserted that, although scaling is intended to maintain budget neutrality within the ASC payment system, it is instead creating increasingly large payment differentials between the ASC and OPPS payments for the same services, without evidence of growing differences in capital and operating costs between the two settings.
The commenters argued that CMS is not required to scale the ASC relative weights and that it should use its authority to suspend the application of scaling the ASC relative weights for CY 2010. They noted that CMS established at § 416.171(e)(2) of the regulations a process by which it may (emphasis added) make annual adjustment to the relative payment weights, as needed (emphasis added).
The commenters also expressed their continuing disagreement with aspects of the budget neutrality adjustment methodology used by CMS to establish the conversion factor. They provided the results of their comparison of actual volume and payment for services that were new to the ASC list in CY 2008. Based upon the results of their analyses of CY 2008 claims data, the commenters concluded that the migration estimates used by CMS to establish budget neutrality in CY 2008 were several times higher than the actual ASC spending for newly covered procedures and, therefore, that the resulting CY 2008 conversion factor was too low. They believed that these findings provide a further basis for CMS not to scale the ASC relative payment weights for CY 2010 after the weights are scaled under the OPPS.
In addition, many of the commenters reasoned that because the ASC payment system is based on the OPPS relative weights, the weights should be equal in both settings and because the weights are scaled to ensure budget neutrality under the OPPS, the weights should not be scaled again to ensure budget neutrality under the ASC system. The commenters believed that the CY 2010 OPPS relative payment weights reflected real growth in the relative costs of surgical services provided in HOPDs and that the ASC scaler should not reclaim dollars from the ASC payment system because there also has been real cost growth for the surgical services provided in ASCs. However, they acknowledge that suspending Start Printed Page 60627 application of the scaler for CY 2010 would result in an aggregate increase in ASC spending in that year.
The commenters expressed concern that other payment adjustments are depressing the ASC payments for many procedures, including the freeze on the ASC payment update through CY 2009 and the transition policy and that scaling further reduces rates to inappropriately low levels. Further, the commenters argued that scaling is forcing procedures for which the OPPS median cost increased from CY 2009 to CY 2010 to finance the transitional payment policies, and that the procedures the transition was intended to aid are the procedures financing the bulk of the scaler.
Response: Many of these comments are similar to public comments on the proposal for the revised ASC payment system that we responded to in the August 2, 2007 final rule (72 FR 42531 through 42533). For example, with regard to scaling, we addressed these same concerns raised by commenters “that annual rescaling would cause divergence of the relative weights between the OPPS and the revised ASC payment system for individual procedures” in the August 2, 2007 final rule (72 FR 42532). We refer the commenters to that discussion for our detailed response in promulgating the scaling policy that was initially applied in CY 2009 (72 FR 42531 through 42533). Below, we address new issues raised by the commenters and provide a general summary of some of the relevant responses from the August 2, 2007 final rule and the CY 2009 OPPS/ASC final rule with comment period (73 FR 68754 through 68755).
The ASC weight scaling methodology is entirely consistent with the OPPS methodology for scaling the relative payment weights and, for the most part, the increasing payment differentials between the ASC and OPPS payments for the same services are not attributable to scaling ASC relative payment weights. Considerations of differences between the capital and operating costs of ASCs and HOPDs are not part of the ASC standard ratesetting methodology, which relies only on maintaining the same relativity of payments for services under the two payment systems, as well as budget neutrality within each payment system. Furthermore, unlike HOPDs, we do not have information about the costs of ASC services in order to assess differences in capital and operating costs over time between the two settings. In order to maintain budget neutrality of the ASC payment system, we need to adjust for the effects of changes in relative weights. The ASC payment system adopts the OPPS relative weights as the mechanism for apportioning total payments, after application of the update factor, among all of the services covered by the ASC payment system. The OPPS relative weights serve the same purpose in the OPPS. The OPPS relative weights do not represent an estimate of absolute cost of any given procedure; rather, they reflect our estimate of the cost of the procedure within the context of our cost estimation methodology for the OPPS. With the exception of services with a predetermined national payment amount, the use of a uniform scaling factor for changes in total weight between years in the ASC payment system does not alter the relativity of the OPPS payment weights as used in the ASC payment system. Differences in the relativity between the ASC relative payment weights and the OPPS relative payment weights are not driven by the application of the uniform scaling factor. The ASC weight scaling methodology is entirely consistent with the OPPS weight scaling methodology and the weights serve the same purpose in both systems, to apportion total budget neutral payment allowed under the update.
We do not believe that the application of the scaler will lead to beneficiary access problems. We believe that the fully implemented relative weights will be representative of relative costs across all ASC services and that payments will support the continued provision of high quality surgical procedures to Medicare beneficiaries in the most appropriate settings. We also expect that, over time, ASCs will provide an increased breadth of services. Appropriate beneficiary access to services in appropriate care settings is always an important concern and we will continue to monitor access under the revised ASC payment system.
As stated in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68754), with respect to the use of “as needed” in the text of § 416.171(e)(2) that commenters have interpreted to mean that CMS has the authority to suspend scaling the relative payment weights if it determines there is not a need to do so, the phrase does not mean that CMS will determine whether or not to adjust for budget neutrality. Rather, it means that CMS adjusts the relative payment weights as needed to ensure budget neutrality and, as acknowledged by the commenters on the CY 2010 OPPS/ASC proposed rule, if we were not to scale the ASC relative payment weights, we estimate that the CY 2010 revisions would not be budget neutral.
We agree that there are differences between the service volume estimates CMS used to establish budget neutrality based on CY 2006 claims data and those reflected in the CY 2008 claims data. In the final regulations implementing the revised ASC payment system, we made our best actuarial estimate to ensure budget neutrality. We did not intend to revisit the actuarial budget neutrality regardless of whether or not it could be determined that there was a difference between actual experience and our underlying data assumptions and regardless of whether or not any difference that could be determined resulted in increased or decreased expenditures under the revised ASC payment system.
Establishing budget neutrality under the OPPS does not result in budget neutrality under the revised ASC payment system; it is only to maintain budget neutrality under the OPPS. Scaling the ASC relative payment weights is an integral and separate process for maintaining budget neutrality under the ASC prospective payment system. Scaling is the budget neutrality adjustment that ensures that changes in the relative weights do not, in and of themselves, change aggregate payment to ASCs. It ensures a specific amount of payment for ASCs in any given year. Without scaling, total ASC payment could increase or decrease relative to changes in hospital outpatient payment.
Although the commenters believed that scaling prevents increases in ASC spending that may be appropriate because ASC costs have increased over time, increases in cost in a prospective payment system are handled by the update factor. In a budget neutral system, we remove the independent effects of increases or decreases in payments as a result of changes in the relative payment weights or the wage indices and constrain increases to the allowed update factor. Therefore, changes in aggregate ASC expenditures related to payment rates should be determined by the update to the ASC conversion factor, the CPI–U.
Regarding commenters' concern that other payment adjustments, including the freeze on ASC payment updates and the transitional payment policy, are depressing the ASC payments for many procedures and that scaling has a disproportionate effect on some covered surgical procedures, we note that the statute set a zero percent update for CY 2008 and CY 2009. We implemented the 4-year transitional payment policy in response to public comments that persuaded us that ASCs would benefit from more gradual implementation of the revised ASC payment rates, especially for historically high volume Start Printed Page 60628 procedures because the prior rates for those procedures were disproportionately high compared to the prior rates for other ASC procedures. As explained in the August 2, 2007 final rule (72 FR 42542), a major effect of the revised ASC payment system is redistribution of payments across all ASC procedures. Historically, the highest volume ASC procedures had payment rates that were close to the payments in HOPDs and, as such, accounted for most of the total Medicare payments to ASCs. As a result, payments for many of those high volume services are the most adversely affected under the revised payment system as the relative weights across all ASC procedures become more closely aligned with those of the OPPS. We appreciate the commenters' concern that scaling is forcing procedures for which the OPPS median cost increased from CY 2009 to CY 2010 to finance the transitional payment policies, and that the procedures the transition was intended to aid are the procedures financing the bulk of the scaler. However, as already noted, the ASC payment system adopts the relativity of the OPPS weights, not the actual median costs or payments for OPPS services. It is fully consistent that a budget neutrality adjustment for differences in aggregate payment weight, specifically scaling, would change the amount of payment under the ASC payment system relative to the OPPS median cost and to the previous year's payment under the ASC payment system for the same service. It is critical that the amount of payment allowed under the ASC payment system, after application of the update factor, distributes the appropriate proportional payment amount to each service. The same statement is true for commenters' concerns that scaling is reducing payment for services explicitly designated as receiving a transition payment. Scaling ensures that the changes in the relative weights do not, in and of themselves, change aggregate payment to ASCs. The calculation of the transition weight over a fully implemented weight for any procedures paid in CY 2007 under the previous ASC payment system changes the relativity of the weight of those services relative to other services newly covered by the revised ASC payment system. This clearly changes the proportional resources distributed to services subject to the transition compared to what would be distributed under a fully implemented system. However, entitlement to a transition weight under a budget neutral system does not guarantee a specific amount of payment in absolute dollar terms. A service that experienced an increase in the OPPS relative weight may very well experience a decline in payment relative to the previous year's actual payment rate because the scaling necessary to maintain equal weight in the system is greater than the proportional increase in the OPPS relative weight portion of the transition weight. Again, this outcome is fully consistent with implementation of a budget neutral prospective payment system with a specific update factor.
For this final rule with comment period, we used our proposed methodology described above to calculate the scaler adjustment using updated ASC claims data. The final CY 2010 scaler adjustment for the third year of the transition is 0.9567. This scaler adjustment is necessary to budget neutralize the difference in aggregate ASC payments calculated using the CY 2009 ASC transitional (50/50 blend) relative payment weights and the CY 2010 ASC transitional (75/25 blend) relative payment weights. We calculated the difference in aggregate payments due to the change in relative payment weights (including drugs and biologicals) holding constant the ASC conversion factor, the most recent CY 2008 ASC utilization from our claims data, and the CY 2009 wage index values. For this final CY 2010 calculation, we used the CY 2009 ASC conversion factor updated by the CY 2010 CPI–U, which is 1.2 percent.
After consideration of the public comments we received, we are finalizing our CY 2010 ASC relative payment weight scaling methodology, without modification. The final CY 2010 ASC payment weight scaler is 0.9567.
b. Updating the ASC Conversion Factor
Under the OPPS, we typically apply a budget neutrality adjustment for provider-level changes, most notably a change in the wage index values for the upcoming year, to the conversion factor. In the CY 2010 OPPS/ASC proposed rule (74 FR 35393), consistent with our final ASC payment policy, for the CY 2010 ASC payment system, we proposed to calculate and apply the pre-floor and pre-reclassified hospital wage indices that are used for ASC payment adjustment to the ASC conversion factor, just as the OPPS wage index adjustment is calculated and applied to the OPPS conversion factor (73 FR 41539). For CY 2010, we calculated this proposed adjustment for the ASC payment system by using the most recent CY 2008 claims data available and estimating the difference in total payment that would be created by introducing the CY 2010 pre-floor and pre-reclassified hospital wage indices. Specifically, holding CY 2008 ASC utilization and service-mix and CY 2010 national payment rates after application of the weight scaler constant, we calculated the total adjusted payment using the CY 2009 pre-floor and pre-reclassified hospital wage indices and the total adjusted payment using the proposed CY 2010 pre-floor and pre-reclassified hospital wage indices. We used the 50-percent labor-related share for both total adjusted payment calculations. We then compared the total adjusted payment calculated with the CY 2009 pre-floor and pre-reclassified hospital wage indices to the total adjusted payment calculated with the proposed CY 2010 pre-floor and pre-reclassified hospital wage indices and applied the resulting ratio of 0.9996 (the proposed CY 2010 ASC wage index budget neutrality adjustment) to the CY 2009 ASC conversion factor to calculate the proposed CY 2010 ASC conversion factor.
Section 1833(i)(2)(C) of the Act requires that, if the Secretary has not updated the ASC payment amounts in a calendar year, the payment amounts shall be increased by the percentage increase in the CPI–U as estimated by the Secretary for the 12-month period ending with the midpoint of the year involved. However, section 1833(i)(2)(C)(iv) of the Act required that the increase of ASC payment amounts for CYs 2008 and 2009 equal zero percent. As discussed in the August 2, 2007 final rule, we adopted a final policy to update the ASC conversion factor using the CPI–U in order to adjust ASC payment rates for CY 2010 and subsequent years (72 FR 42518 through 42519 and § 416.171(a)(2)). In the CY 2010 OPPS/ASC proposed rule (74 FR 35394), we proposed to implement the annual updates through an adjustment to the ASC conversion factor beginning in CY 2010 when the statutory requirement for a zero update no longer applies.
For our proposed rule, for the 12-month period ending with the midpoint of CY 2010, the Secretary estimated that the CPI–U is 0.6 percent. Therefore, we proposed to apply to the ASC conversion factor a 0.6 percent increase for CY 2010.
Thus, for CY 2010, we proposed to adjust the CY 2009 ASC conversion factor ($41.393) by the wage adjustment for budget neutrality of 0.9996 and the update of 0.6 percent, which resulted in a proposed CY 2010 ASC conversion factor of $41.625.
Comment: Many commenters requested that CMS adopt the hospital Start Printed Page 60629 market basket to update the ASC payment system. They explained that not only is the CPI–U lower than the hospital market basket but it is not appropriate for updating health care providers because, unlike the hospital market basket which analyzes hospital spending, the CPI–U is designed to capture household spending. The commenters stated that in the most recent years, the CPI–U has been dominated by energy and housing costs rather than healthcare provider spending. Further, the commenters stated that CMS' use of the midyear CPI–U percent change is problematic because other federal agencies, such as the Bureau of Labor Statistics and the Congressional Budget Office, use an end-of-year timeframe. They believed that a negative consequence of the midyear timing for CMS' forecasted CPI–U percent change is that the CPI–U used to update the ASC payment system cannot be validated directly with an independent source.
The commenters argued that the difference between the ASC and OPPS conversion factors is not due to real differences in the growth of costs of goods and services furnished by ASCs and HOPDs and should not be perpetuated. The commenters asserted that CMS clearly has the authority to use an alternative update mechanism, and believed CMS should adopt a more appropriate update for the ASC payment system to prevent further increases in differential between the ASC and OPPS conversion factors.
Response: We understand the commenters' concerns regarding the update to the conversion factor for CY 2010, but note that we did not propose to change the conversion factor update methodology. We refer readers to the discussion in the August 2, 2007 final rule on this issue (72 FR 42518 through 42519).
After consideration of the public comments we received, we are applying our established methodology for determining the final CY 2010 ASC conversion factor. Using more complete CY 2008 data for this final rule with comment period than was available for the proposed rule, we calculated a wage index budget neutrality adjustment of 0.9996 and the updated CPI–U projected for the midpoint of CY 2010 is 1.2 percent. The final ASC conversion factor of $41.873 is the product of the CY 2009 conversion factor of $41.393 multiplied by 0.9996 and the 1.2 percent CPI–U.
3. Display of ASC Payment Rates
Addenda AA and BB to this CY 2010 OPPS/ASC final rule with comment period display the updated ASC payment rates for CY 2010 for covered surgical procedures and covered ancillary services, respectively. These addenda contain several types of information related to the CY 2010 payment rates. Specifically, in Addendum AA, a “Y” in the column titled “Subject to Multiple Procedure Discounting” indicates that the surgical procedure would be subject to the multiple procedure payment reduction policy. As discussed in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66829 through 66830), most covered surgical procedures are subject to a 50-percent reduction in the ASC payment for the lower-paying procedure when more than one procedure is performed in a single operative session. Display of the comment indicator “CH” in the column titled “Comment Indicator” indicates a final change in payment policy for the item or service, including identifying discontinued HCPCS codes, designating items or services newly payable under the ASC payment system, and identifying items or services with changes in the ASC payment indicator for CY 2010. Display of the commenter indicator “NI” in the column titled “Comment Indicator” indicates that the code is new (or substantially revised) and that the payment indicator assignment is an interim assignment that is open to comment on this final rule with comment period.
The values displayed in the column titled “CY 2010 Third Year Transition Payment Weight” are the relative payment weights for each of the listed services for CY 2010, the third year of the 4-year transition period. The CY 2010 ASC payment rates for the covered surgical procedures subject to transitional payment (payment indicators “A2” and “H8” in Addendum AA) are based on a blend of 25 percent of the CY 2007 ASC payment rate for the procedure and 75 percent of the CY 2010 ASC rate calculated under the ASC standard ratesetting methodology before scaling for budget neutrality. The payment weights for all covered surgical procedures and covered ancillary services whose ASC payment rates are based on OPPS relative payment weights are scaled for budget neutrality. Thus, scaling was not applied to the device portion of the device-intensive procedures, services that are paid at the MPFS nonfacility PE RVU amount, separately payable covered ancillary services that have a predetermined national payment amount, such as drugs and biologicals that are separately paid under the OPPS, or services that are contractor-priced or paid at reasonable cost in ASCs.
To derive the CY 2010 national unadjusted payment rate displayed in the “CY 2010 Third Year Transition Payment” column, each ASC payment weight in the “CY 2010 Third Year Transition Payment Weight” column is multiplied by the final CY 2010 ASC conversion factor of $41.873. The conversion factor includes a budget neutrality adjustment for changes in the wage index values and the CPI–U percentage increase.
In Addendum BB, there are no relative payment weights displayed in the “CY 2010 Third Year Transition Payment Weight” column for items and services with predetermined national payment amounts, such as separately payable drugs and biologicals. The “CY 2010 Third Year Transition Payment” column displays the final CY 2010 national unadjusted ASC payment rates for all items and services. The CY 2010 ASC payment rates listed in the Addendum AA for separately payable drugs and biologicals are based on ASP data used for payment in physicians' offices in October 2009.
For informational purposes only, we also have posted on the CMS Web site the fully transitioned ASC payment rates for CY 2010. These rates do not represent what the payment rates would be once the transition is over, only what the CY 2010 rates would be if there were no transition. The Web site address is: https://www.cms.hhs.gov/ASCPayment/.
We did not receive any public comments regarding the continuation of our policy to provide CY 2010 ASC payment information as detailed in Addenda AA and BB. Therefore, Addenda AA and BB to this final rule with comment period display the updated ASC payment rates for CY 2010 for covered surgical procedures and covered ancillary services, respectively, and provide additional information related to the CY 2010 rates.
XVI. Reporting Quality Data for Annual Payment Rate Updates
A. Background
1. Overview
CMS has implemented quality measure reporting programs for multiple settings of care. These programs promote higher quality, more efficient health care for Medicare beneficiaries. The quality data reporting program for hospital outpatient care, known as the Hospital Outpatient Quality Data Reporting Program (HOP QDRP), has been generally modeled after the program for hospital inpatient services, Start Printed Page 60630 the Reporting Hospital Quality Data for Annual Payment Update (RHQDAPU) program. Both of these quality reporting programs for hospital services, as well as the program for physicians and other eligible professionals, known as the Physician Quality Reporting Initiative (PQRI), have financial incentives for reporting of quality data to CMS. CMS has also implemented quality reporting programs for home health agencies and skilled nursing facilities that are based on conditions of participation, and an end-stage renal disease quality reporting program that is based on conditions for coverage.
2. Hospital Outpatient Quality Data Reporting Under Section 109(a) of Pub. L. 109–432
Section 109(a) of the MIEA–TRHCA (Pub. L. 109–432) amended section 1833(t) of the Act by adding a new subsection (17) that affects the payment rate update applicable to OPPS payments for services furnished by hospitals in outpatient settings on or after January 1, 2009. Section 1833(t)(17)(A) of the Act, which applies to hospitals as defined under section 1886(d)(1)(B) of the Act, states that subsection (d) hospitals that fail to report data required for the quality measures selected by the Secretary in the form and manner required by the Secretary under section 1833(t)(17)(B) of the Act will receive a 2.0 percentage point reduction to their annual payment update factor. Section 1833(t)(17)(B) of the Act requires that hospitals submit quality data in a form and manner, and at a time, that the Secretary specifies. Section 1833(t)(17)(C)(i) of the Act requires the Secretary to develop measures appropriate for the measurement of the quality of care (including medication errors) furnished by hospitals in outpatient settings, that these measures reflect consensus among affected parties and, to the extent feasible and practicable, that these measures include measures set forth by one or more national consensus building entities.
The National Quality Forum (NQF) is a voluntary consensus standard-setting organization that is composed of a diverse representation of consumer, purchaser, provider, academic, clinical, and other health care stakeholder organizations. NQF was established to standardize health care quality measurement and reporting through its consensus development process. We generally prefer to adopt NQF-endorsed measures for CMS quality reporting programs. However, we believe that consensus among affected parties also can be reflected by other means, including: consensus achieved during the measure development process; consensus shown through broad acceptance and use of measures; and consensus through public comment. We also note that section 1833(t)(17) of the Act does not require that each measure we adopt for the HOP QDRP be endorsed by a national consensus building entity, or by the NQF specifically.
Section 1833(t)(17)(C)(ii) of the Act authorizes the Secretary to select measures for the HOP QDRP that are the same as (or a subset of) the measures for which data are required to be submitted under section 1886(b)(3)(B)(viii) of the Act (the RHQDAPU program). Section 1833(t)(17)(D) of the Act gives the Secretary the authority to replace measures or indicators as appropriate, such as when all hospitals are effectively in compliance or when the measures or indicators have been subsequently shown not to represent the best clinical practice. Section 1833(t)(17)(E) of the Act requires the Secretary to establish procedures for making data submitted under the HOP QDRP available to the public. Such procedures must include giving hospitals the opportunity to review their data before these data are released to the public.
As we stated in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68758 through 68759), we continue to believe that it is most appropriate and desirable to adopt measures that specifically apply to the hospital outpatient setting for the HOP QDRP. In other words, we do not believe that we should simply, without further analysis, adopt the RHQDAPU program measures as the measures for the HOP QDRP. Nonetheless, we note that section 1833(t)(17)(C)(ii) of the Act allows the Secretary to “[select] measures that are the same as (or a subset of) the measures for which data are required to be submitted” under the RHQDAPU program.
3. Reporting ASC Quality Data for Annual Payment Update
Section 109(b) of the MIEA–TRHCA amended section 1833(i) of the Act by redesignating clause (iv) as clause (v) and adding new clause (iv) to paragraph (2)(D) and adding paragraph (7). These amendments may affect ASC payments for services furnished in ASC settings on or after January 1, 2009. Section 1833(i)(2)(D)(iv) of the Act authorizes the Secretary to implement the revised payment system for services furnished in ASCs (established under section 1833(i)(2)(D) of the Act), “so as to provide for a reduction in any annual update for failure to report on quality measures.”
Section 1833(i)(7)(A) of the Act states that the Secretary may provide that any ASC that fails to report data required for the quality measures selected by the Secretary in the form and manner required by the Secretary under section 1833(i)(7) of the Act will incur a reduction in any annual payment update of 2.0 percentage points. Section 1833(i)(7)(A) of the Act also specifies that a reduction for one year cannot be taken into account in computing the ASC update for a subsequent calendar year.
Section 1833(i)(7)(B) of the Act provides that, “[e]xcept as the Secretary may otherwise provide,” the hospital outpatient quality data provisions of sections 1833(t)(17)(B) through (E) of the Act, summarized above, shall apply to ASCs. We did not implement an ASC quality reporting program for CY 2008 (72 FR 66875) or for CY 2009 (73 FR 68779).
We refer readers to section XVI.H. of this final rule with comment period for a discussion of our decision to implement ASC quality data reporting in a later rulemaking.
4. HOP QDRP Quality Measures for the CY 2009 Payment Determination
For the CY 2009 annual payment update, we required HOP QDRP reporting using seven quality measures—five Emergency Department (ED) AMI measures and two Perioperative Care measures. These measures address care provided to a large number of adult patients in hospital outpatient settings, across a diverse set of conditions, and were selected for the initial set of HOP QDRP measures based on their relevance as a set to all HOPDs.
Specifically, in order for hospitals to receive the full OPPS payment update for services furnished in CY 2009, in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66860), we required that subsection (d) hospitals paid under the OPPS submit data on the following seven measures for hospital outpatient services furnished on or after April 1, 2008: (1) ED–AMI–1: Aspirin at Arrival; (2) ED–AMI–2: Median Time to Fibrinolysis; (3) ED–AMI–3: Fibrinolytic Therapy Received within 30 Minutes of Arrival; (4) ED–AMI–4: Median Time to Electrocardiogram (ECG); (5) ED–AMI–5: Median Time to Transfer for Primary PCI; (6) PQRI #20: Perioperative Care—Timing of Antibiotic Prophylaxis; and (7) PQRI #21: Perioperative Care—Selection of Perioperative Antibiotic. Start Printed Page 60631
5. HOP QDRP Quality Measures for the CY 2010 Payment Determination
a. Background
In the CY 2009 OPPS/ASC final rule with comment period, for the CY 2010 payment update, we required continued submission of data on the existing seven measures discussed above (73 FR 68761), and adopted four imaging measures (73 FR 68766). For CY 2010, we changed the measure designations for the existing seven measures, including a change to an “OP–X” format in order to maintain a consistent sequential designation system that we could expand as we add additional measures.
The four imaging measures that we adopted beginning with the CY 2010 payment determination (OP–8: MRI Lumbar Spine for Low Back Pain, OP–9: Mammography Follow-up Rates, OP–10: Abdomen CT—Use of Contrast Material, and OP–11: Thorax CT—Use of Contrast Material) are claims-based measures that CMS will calculate using Medicare Part B claims data without imposing upon hospitals the burden of additional chart abstraction. For purposes of the CY 2010 payment determination, we will calculate these measures using CY 2008 Medicare administrative claims data.
In the CY 2009 OPPS/ASC proposed rule, OP–10 had two submeasures listed: OP–10a: CT Abdomen—Use of contrast material excluding calculi of the kidneys, ureter, and/or urinary tract, and OP–10b: CT Abdomen—Use of contrast material for diagnosis of calculi in the kidneys, ureter, and or urinary tract. In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68766), we finalized OP–10: Abdomen CT—Use of Contrast Material. To clarify, we are calculating OP–10 excluding patients with renal disease. This exclusion is described in greater detail in the Specifications Manual for Hospital Outpatient Department Quality Measures (HOPD Specifications Manual) located at the QualityNet Web site ( http://www.QualityNet.org).
The complete set of measures to be used for the CY 2010 payment determination is set out below, and is shown with the CY 2010 measure designations as well as their ED–AMI and PQRI designations:
| HOP QDRP measurement set to be used for CY 2010 payment determination | CY 2009 designation |
|---|---|
| OP–1: Median Time to Fibrinolysis | ED–AMI–2. |
| OP–2: Fibrinolytic Therapy Received Within 30 Minutes | ED–AMI–3. |
| OP–3: Median Time to Transfer to Another Facility for Acute Coronary Intervention | ED–AMI–5. |
| OP–4: Aspirin at Arrival | ED–AMI–1. |
| OP–5: Median Time to ECG | ED–AMI–4. |
| OP–6: Timing of Antibiotic Prophylaxis | PQRI #20. |
| OP–7: Prophylactic Antibiotic Selection for Surgical Patients | PQRI #21. |
| OP–8: MRI Lumbar Spine for Low Back Pain | NA. |
| OP–9: Mammography Follow-up Rates | NA. |
| OP–10: Abdomen CT—Use of Contrast Material | NA. |
| OP–11: Thorax CT—Use of Contrast Material | NA. |
b. Maintenance of Technical Specifications for Quality Measures
Technical specifications for each HOP QDRP measure are listed in the HOPD Specifications Manual, which is posted on the CMS QualityNet Web site at http://www.QualityNet.org. We maintain the technical specifications for the measures by updating this HOPD Specifications Manual and include detailed instructions and calculation algorithms for hospitals to use when collecting and submitting data on required measures.
In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68766), we established a subregulatory process for updates to the technical specifications that we use to calculate HOP QDRP measures. This process is used when changes to the measure specifications are necessary due to changes in scientific evidence or in the measure as endorsed by the consensus entity. Changes of this nature may not coincide with the timing of our regulatory actions, but nevertheless require inclusion in the measure specifications so that the HOP QDRP measures are calculated based on the most up-to-date scientific and consensus standards. We indicated that notification of changes to the measure specifications on the QualityNet Web site, http://www.QualityNet.org, and in the HOPD Specifications Manual that occurred as a result of changes in scientific evidence or national consensus would occur no less than 3 months before any changes become effective for purposes of reporting under the HOP QDRP.
The HOPD Specifications Manual is released every 6 months and addenda are released as necessary, providing at least 3 months of advance notice for nonsubstantive changes such as changes to ICD–9, CPT, NUBC, and HCPCS codes, and at least 6 months notice for substantive changes to data elements that would require significant systems changes.
Comment: A few commenters indicated that they agreed with the maintenance of the outpatient measure technical specifications in a manner consistent with the inpatient measure technical specifications. They agreed that providing a 3-month notification period for code updates is sufficient. One commenter also agreed the OP–X designations along with short measure names are appropriate. Commenters indicated that CMS should ensure that the subregulatory process that it uses to update the technical specifications for HOP QDRP measures is regular and transparent.
Response: We thank the commenters for their support of the subregulatory manual update process and timeframes. We will continue to make such updates on a regular semi-annual basis with addenda as necessary, and to issue notifications of updates via the QualityNet Web site, http://www.QualityNet.org, in order to maintain the transparency of the process. The HOPD Specifications Manual will continue to be released regularly and addenda will continue to be issued as necessary, providing at least 3 months of advance notice for nonsubstantive changes such as changes to ICD–9, CPT, NUBC, and HCPCS codes, and at least 6 months notice for substantive changes to data elements that would require significant systems changes.
c. Publication of HOP QDRP Data
Section 1833(t)(17)(E) of the Act requires that the Secretary establish procedures to make data collected under the HOP QDRP program available to the public. CMS also requires hospitals to complete and submit a registration form (“participation form”) in order to participate in the HOP QDRP. With submission of this form, participating Start Printed Page 60632 hospitals agree that they will allow CMS to publicly report the quality measures, including those that CMS calculates using Medicare claims, as required by the Act and the HOP QDRP.
In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68778), we established that for CY 2010, hospitals sharing the same CMS Certification Number (CCN, previously known as the Medicare Provider Number (MPN)) must combine data collection and submission across their multiple campuses for the clinical measures for public reporting purposes. We finalized the policy that, under the HOP QDRP, we will publish quality data by the corresponding CCN. This approach is consistent with the approach taken under the RHQDAPU program. In that final rule with comment period, we also stated that we intend to indicate instances where data from two or more hospitals are combined to form the publicly reported measures on the Web site.
We discuss our CY 2010 policy regarding publication of HOP QDRP data in section XVI.F. of this final rule with comment period.
B. Quality Measures for the CY 2011 Payment Determination
1. Considerations in Expanding and Updating Quality Measures Under the HOP QDRP
In general, when selecting measures for the HOP QDRP program, we take into account several considerations and goals. These include: (a) Expanding the types of measures beyond process of care measures to include an increased number of outcome measures, efficiency measures, and patients' experience-of-care measures; (b) expanding the scope of hospital services to which the measures apply; (c) considering the burden on hospitals in collecting chart-abstracted data; (d) harmonizing the measures used in the HOP QDRP program with other CMS quality programs to align incentives and promote coordinated efforts to improve quality; (e) seeking to use measures based on alternative sources of data that do not require chart abstraction or that utilize data already being reported by many hospitals, such as data that hospitals report to clinical data registries, or all-payer claims data bases; and (f) weighing the relevance and utility of the measures compared to the burden on hospitals in submitting data under the HOP QDRP program. Specifically, we give priority to quality measures that assess performance on: (a) Conditions that result in the greatest mortality and morbidity in the Medicare population; (b) conditions that are high volume and high cost for the Medicare program; and (c) conditions for which wide cost and treatment variations have been reported, despite established clinical guidelines. We have used and continue to use these criteria to guide our decisions regarding what measures to add to the HOP QDRP measure set.
Comment: Many commenters indicated that, although CMS is not required to adopt only measures that are endorsed by NQF, CMS should continue to rely on NQF evaluations to guide selection of measures, and to seek NQF approval for measures considered and adopted for the HOP QDRP in order to maintain consistency in the selection processes for quality measures across physician and hospital services. Many commenters indicated that they prefer that measures adopted for HOP QDRP first go through the rigorous, consensus-based assessment processes of both the NQF and HQA, and that given the number of NQF-endorsed and HQA-adopted measures currently available for use, it is both feasible and practicable for CMS to choose only NQF-endorsed and HQA-adopted measures. Other commenters indicated that although a consensus-based process may have been employed by CMS or CMS contractors to develop measures, it does not equal the rigor or broad stakeholder input of NQF endorsement and HQA adoption.
Response: Section 1833(t)(17)(C)(i) of the Act requires the Secretary to “develop measures that the Secretary determines to be appropriate for the measurement of the quality of care (including medication errors) furnished by hospitals in outpatient settings and that reflect consensus among affected parties and, to the extent feasible and practicable, shall include measures set forth by one or more national consensus building entities.” This provision does not require that the measures we adopt for the HOP QDRP be endorsed by any particular entity, and we believe that consensus among affected parties can be reflected by means other than endorsement by a national consensus building entity, including consensus achieved during the measure development process, consensus shown through broad acceptance and use of measures, and consensus through public comment. Nevertheless, we have stated on numerous occasions that we prefer to adopt quality measures that have been endorsed by the NQF because the NQF uses a formal consensus development process and has been recognized as a voluntary consensus standards-setting organization as defined by the National Technology Transfer and Advancement Act of 1995 (NTTAA) and Office of Management and Budget Circular A 119 (see http://www.qualityforum.org/Measuring_Performance/Consensus_Development_Process.aspx). We are unaware of any other organizations that qualify as an NTTAA consensus organization for the endorsement of quality measures. However, when we propose and adopt quality measures, we take into consideration the measures adopted by the HQA as well as an array of input from the public. We appreciate HQA's integral efforts to improve hospital quality of care by supporting CMS' public reporting programs.
Comment: Some commenters expressed concern regarding the accuracy of measures that rely solely on administrative (that is, claims) data and requested that CMS not consider these types of measures in the future. Several commenters questioned the value of measures based solely on claims data/administrative data for public reporting and pay-for-performance in terms of their capacity to improve care delivered to Medicare beneficiaries.
Response: We do not agree with these commenters' statements. We believe that claims data/administrative data are an appropriate data source upon which quality measures selected by the Secretary may be based. We note that many NQF-endorsed evidence-based quality measures that have been found appropriate for public reporting and quality improvement rely upon claims and administrative data as a data source. Furthermore, the use of claims-based measures reduces reliance upon chart abstraction and its associated burden for quality measurement.
Comment: Commenters submitted the following suggested measure selection criteria for the HOP QDRP:
- Potential for quality improvement;
- Processes measured are related to improved patient outcomes;
- Processes measured occur closer in time to patient outcomes of interest;
- Outcome measures are related to modifiable processes that affect patient outcomes;
- Minimal unintended adverse consequences;
- Alignment with national priorities as described in the NQF NPP project;
- Amenable to collection via alternative mechanisms such as electronic health records (EHRs), registries, and claims;
- Harmonizes with measures used for reporting programs in similar settings;
- Attributable to the facility rather than a prescribing physician;
- Data collection should not increase hospital operational burden; and
• Fully tested in a variety of outpatient settings. Start Printed Page 60633
Response: We thank the commenters for these suggestions, and we note that these suggestions were not submitted in reference to specific measures. In section XVI.B.1. of this final rule with comment period, we have set out the criteria that we use to guide our decisions regarding what measures to add to the HOP QDRP measure set. We determine the suitability of potential measures using consensus development processes, including, when appropriate, relying upon the NQF's voluntary consensus standards in addition to our rulemaking in determining the suitability of quality measures.
In the CY 2009 OPPS/ASC final rule with comment period, we adopted four claims-based quality measures that do not require a hospital to submit chart-abstracted clinical data. This supports our goal of expanding the measures for the HOP QDRP while minimizing the burden upon hospitals and, in particular, without significantly increasing the chart abstraction burden. In addition to claims-based measures, we are considering registries [1] and EHRs as alternative ways to collect data from hospitals. Many hospitals submit data to and participate in existing registries. In addition, registries often capture outcome information and provide ongoing quality improvement feedback to registry participants. Instead of requiring hospitals to submit the same data to CMS that they are already submitting to registries, we could collect the data directly from the registries with the permission of the hospital, thereby enabling us to expand the HOP QDRP measure set without increasing the burden of data collection for those hospitals participating in the registries. The data that we would receive from registries would be used to calculate quality measures required under the HOP QDRP, and would be publicly reported like other HOP QDRP quality measures, encouraging improvements in the quality of care. In the CY 2010 OPPS/ASC proposed rule (74 FR 35397), we invited public comment on such an approach.
Comment: Many commenters expressed concern about the potential use of registries as a source of data for the HOP QDRP. Many commenters indicated that the fees imposed by registries would be prohibitive for smaller hospitals and rural hospitals. Regarding registry-based data submission for the HOP QDRP, CMS was urged to do the following:
- Develop and test alternatives for hospitals choosing to submit data directly to CMS in lieu of participating in a registry (that is, chart abstraction, CART tool);
- Determine and articulate a process for validating data submitted through registries for completeness and accuracy;
- Determine and articulate a process to transmit registry-based data to the national data warehouse in a secure fashion and without violating HIPAA or other rules;
- Explore and determine the willingness and ability of the ORYX vendors to submit data for those hospitals not participating in a registry; and
- Require standardized, externally verifiable sampling for the measures.
Response: We are interested in minimizing the burden associated with quality measurement. If hospitals are participating in registries and submit the same data to those registries that they would otherwise have to submit for measures that are part of the HOP QDRP, we believe that the registry-based data would be an efficient alternative source from which to collect the data, and that this would prevent the hospital from having to report the same data twice. Many hospitals are currently participating in a number of registries that collect data on quality measures that are topics of interest to us. However, we acknowledge the commenters' concerns regarding the cost associated with participation in certain registries that may make this alternative mechanism for data submission less feasible for some hospitals, and the need for standardized validation strategies for registry-based data. We will take these considerations into account when considering registry-based measure submission options for this and other reporting programs in the future.
Comment: Some commenters strongly supported the use of registries as an alternative source of data for the HOP QRDP. These commenters stated that registries provide a substantial advantage over chart-abstracted data because registries provide regular feedback reports to participating hospitals on their performance, further minimize the reporting burden for physicians and facilities because registry-based data could be used for more than one reporting program, and aggregate clinical data from a provider's entire patient population and enable these data to be analyzed and tracked over time for adherence to evidence-based medicine and health outcomes. Commenters encouraged CMS to continue to explore this mechanism and to develop the infrastructure standards needed to accurately capture such data as soon as practicable.
Response: We thank these commenters for their encouragement and will continue to investigate the feasibility of such an approach to the HOP QDRP and other quality data reporting programs.
Comment: One commenter recommended that CMS request legislative authority to base payments on pay-for-performance so that a portion of payments will depend on providers' performance on the selected quality measures, not simply on whether they report the specified data to CMS. This commenter also expressed support for CMS' efforts to collect data on measures of hospital quality as a valuable step toward pay-for-performance.
Response: We thank the commenter for sharing this suggestion for future program direction and for supporting current program operations.
In the CY 2009 OPPS/ASC final rule with comment period, we also stated our intention to explore mechanisms for data submission using EHRs (73 FR 68769). Establishing such a system will require interoperability between EHRs and CMS data collection systems, additional infrastructure development on the part of hospitals and CMS, and the adoption of standards for the capturing, formatting, and transmission of data elements that make up the measures. However, once these activities are accomplished, the adoption of measures that rely on data obtained directly from EHRs will enable us to expand the HOP QDRP measure set with less cost and burden to hospitals.
Comment: Some commenters strongly supported the use of EHRs and other health information technology (IT). These commenters believed that such technology has the ability to capture, store, and readily report the types of clinical data not available from medical claims data, such as diagnostic laboratory test results and prescription drug dispensing data. Commenters commended CMS for encouraging the development and adoption of uniform data content and information technology standards across the health care industry that will support automated data collection and reporting of clinical data from EHR systems. These commenters believed that such efforts would streamline hospital data submission procedures and enable providers to view real-time measurement results to initiate their own improvement interventions in a more timely and efficient manner. Start Printed Page 60634
Response: We appreciate these supportive comments regarding EHR-based data collection as an alternative data source for quality measures. We agree that EHR-based data submission may provide an alternative means of submitting quality data that would benefit hospitals by reducing their chart abstraction burden. We also agree that such systems may enable providers to implement more timely improvement efforts. Although we encourage adoption of EHRs, we also acknowledge the challenges that must be met both by hospitals and CMS to establish the infrastructure and interoperability necessary to collect data on quality measures via EHRs. We will continue to work collaboratively with health IT standard-setting and consensus development organizations to ensure that quality measures can be collected in a standardized manner.
Comment: Many commenters were concerned about the ability of EHRs to accurately capture the data required for meaningful and accurate quality measures for the HOP QDRP. Commenters indicated that, currently, all of the necessary information for measuring performance against essential metrics of quality (such as exclusion and inclusion criteria and contraindications) is not codified within EHRs, and that the need for such information will still require medical record review because the information cannot be adequately found in EHRs. Other commenters indicated that current products feature inconsistent communication standards and may pose privacy concerns. Several commenters indicated that small rural hospitals may not be able to enhance their health IT infrastructure to support EHR-based reporting. Several commenters supported one-way transmission of specific data elements from EHRs, but would not support providing access to the whole EHR to abstract clinical information for quality measures. Commenters encouraged CMS to consider postponing new measure implementation for CY 2012 until new measures can be verified to be structured for EHR data collection, especially given impending challenges of ICD–10 implementation.
Response: We do not agree with the commenters' belief that quality data produced from EHRs is not likely to accurately capture data elements needed for quality measurement. The data collected from the EHR would essentially be the same data that hospitals would otherwise have to manually abstract from a medical chart. These data are what we currently use for quality measure reporting. We acknowledge that additional programming work may be needed in order to enable current EHR systems to collect and submit quality measure data. We are currently working with the Healthcare Information Technology Standards Panel (HITSP), a public-private partnership working to establish health IT interoperability standards under contract to the HHS Office of the National Coordinator on Health IT (ONC), to standardize the specifications of data elements used in several measure sets so that they may be collected and reported via EHRs. Standardization of the specifications allows software to convert clinical data of different types into a form that can be analyzed for quality measurement. We encourage collaboration among standard-setting organizations and measure developers on the creation of standards for electronic collection of data elements for other quality measures as well, particularly those used in our quality data reporting programs.
With regard to the commenters' concern about having to provide access to the entire EHR, we would only require that the hospital provide access to those data elements in the EHRs that are needed to calculate the measures. We also acknowledge the burden faced by hospitals in implementing multiple technological changes, including the ICD–10 coding system. We will carefully consider any additional burden that may be imposed by adopting additional measures for the HOP QDRP and will continue to consider other feasible alternatives to data collection such as registries.
Comment: Several commenters encouraged the collection of all-payer or multiple-payer claims information in order to calculate measures for the HOP QDRP as it would provide a more complete picture of care to consumers. Commenters also encouraged CMS to ensure the validity of any third party data used in the development or calculation of measures for public reporting.
Response: We thank the commenters for their encouragement of the collection of all-payer claims data, and we agree that all-payer claims data would enable us to provide consumers with more comprehensive claims-based quality measures that provide a comprehensive picture of the quality of care provided by a hospital. We currently collect other all-payer data where feasible for the hospital quality data reporting programs, and currently this is feasible for chart-abstracted data elements. It has been our policy to collect all-payer chart-abstracted data since the inception of both inpatient (RHQDAPU program) and outpatient (HOP QDRP) quality data reporting. While we currently do not have the infrastructure in place to accept all-payer claims data, we intend to work with stakeholders to identify options, processes, and opportunities to collect all-payer claims data to supplement the Medicare claims data we currently use in many of our reporting programs.
Comment: Several commenters indicated that CMS should only concern itself with obtaining information and outcomes for Medicare beneficiaries, and should not collect information regarding patients for whom other payers are responsible.
Response: For the HOP QDRP, section 1833(t)(17)(C)(i) of the Act requires the Secretary to develop measures appropriate for the measurement of the quality of care (including medication errors) furnished by hospitals in outpatient settings. The collection and publication of quality measures based on all-payer data captures variations in the care delivered by a hospital to different populations and payers, and therefore allows us to obtain comprehensive information regarding the quality of care provided to its beneficiaries. Therefore, we are collecting all-payer data elements to calculate the chart-abstracted measures adopted into the HOP QDRP. We wish to eventually provide a similarly comprehensive picture of the quality of care provided by HOPDs with respect to the claims-based measures adopted into the HOP QDRP.
2. Retirement of HOP QDRP Quality Measures
In the FY 2010 IPPS/RY 2010 LTCH PPS proposed rule, we proposed a process for immediate retirement of RHQDAPU program measures based on evidence that the continued use of the measure as specified raises patient safety concerns (74 FR 24168). As we explained in that proposed rule, in situations such as the one prompting immediate retirement of the AMI–6 measure from the RHQDAPU program in December 2008, we do not believe that it would be appropriate to wait for the annual rulemaking cycle to retire a measure. This proposal was later finalized for the RHQDAPU program in the FY 2010 IPPS/RY 2010 LTCH PPS final rule (74 FR 43863). We proposed to adopt this same immediate retirement policy for the HOP QDRP (74 FR 35397). Specifically, in the CY 2010 OPPS/ASC proposed rule, we proposed that if we receive evidence that continued collection of a measure that has been adopted for the HOP QDRP raises patient safety concerns, we would Start Printed Page 60635 promptly retire the measure and notify hospitals and the public of the retirement of the measure and the reasons for its retirement through the usual means by which we communicate with hospitals, including but not limited to hospital e-mail blasts and the QualityNet Web site. We also proposed to confirm the retirement of the measure in the next OPPS rulemaking. In other circumstances, where we do not believe that continued use of a measure raises specific patient safety concerns, we stated that we intend to use the regular rulemaking process to retire a measure.
We invited public comment on this proposal allowing for immediate retirement of a HOP QDRP measure following evidence of a patient safety concern followed by confirmation in the next rulemaking cycle.
Comment: Several commenters applauded the proposal to immediately retire a HOP QDRP measure if CMS receives evidence that the continued collection of a measure raises patient safety concerns. They encouraged CMS to establish consistent and transparent processes that address changes in evidence-based guidelines more quickly and to establish channels to exchange this type of information between the agency and measure developers. The commenters also encouraged CMS to retire measures under the following conditions:
- A measure is no longer consistent with current clinical guidelines;
- Another indicator exists that better, or more accurately, assesses good quality care;
- Redundancy of measurement on a given topic or process; and
- The burden associated with data collection and reporting a measure outweighs the benefit of public reporting;
Response: We thank the commenters for their support for the proposed policy of prompt retirement when potential patient harm could result from the continued collection of a measure, and are finalizing our policy in this final rule with comment period. With respect to the suggestions we received, these criteria reflect examples of conditions that may warrant retirement via notice and comment rulemaking as opposed to prompt retirement because continued collection of the measure does not raise patient safety concerns. Another example of a nonurgent circumstance where we would use the rulemaking process to retire a measure would be when a measure is “topped out.” While we did not solicit public comments on criteria for retirement under circumstances other than potential patient harm, we will consider these suggestions as we consider whether to propose to retire measures in nonurgent circumstances.
After consideration of the public comments we received, we are finalizing our proposal to promptly retire measures under circumstances in which we receive evidence that continued collection of a measure that has been adopted for the HOP QDRP raises patient safety concerns, to notify hospitals and the public of the retirement of the measure and the reasons for its retirement through the usual means by which we communicate with hospitals, including, but not limited to, hospital e-mail blasts and the QualityNet Web site, and to confirm the retirement of measures retired in this manner in the next rulemaking cycle.
3. HOP QDRP Quality Measures for the CY 2011 Payment Determination
For the CY 2011 payment determination, in the CY 2010 OPPS/ASC proposed rule (74 FR 35397), we proposed to continue requiring that hospitals submit data on the existing 11 HOP QDRP measures. These measures continue to address areas of topical importance regarding the quality of care provided in HOPDs, and reflect consensus among affected parties. Seven of these 11 measures are chart-abstracted measures in two areas of importance that are also measured for the inpatient setting: AMI care and surgical care. The remaining four measures address imaging efficiency in HOPDs.
For the CY 2011 payment determination, we proposed not to add any new HOP QDRP measures. Although we considered adding a number of chart-abstracted measures, we are sensitive to the burden upon HOPDs associated with chart abstraction and believe that adopting such measures at this time would not be consistent with our stated goal to minimize the collection burden associated with quality measurement. We will continue to assess whether we can collect data on additional quality measures through mechanisms other than chart abstraction, such as from Medicare administrative claims data and EHRs.
We invited public comment on our proposal to retain the existing 11 HOP QDRP measures and to not adopt additional measures for the CY 2011 payment determination.
Comment: Most commenters were pleased that CMS recognizes the burden that data collection and reporting places on facilities and did not propose to add new measures to the HOP QDRP measurement set for the CY 2011 payment determination. In particular, some hospitals indicated that they have only one staff member performing chart abstraction for both the inpatient and outpatient quality data reporting programs, and that the burden of adding measures has a great impact under such circumstances.
Response: We thank the commenters for expressing their support for this proposal. We will continue to carefully weigh the burden associated with adding chart-abstracted measures to quality reporting programs such as the HOP QDRP against the benefit of adding such measures in the future.
We also received specific comments, discussed below, on some of the measures we proposed to retain.
- OP–3: Median Time To Transfer to Another Facility for Acute Coronary Intervention
Comment: One commenter recommended that CMS consider measuring the overall median time to percutaneous coronary intervention (PCI) in transferred patients because this captures the entire process of care and will encourage collaboration between transferring and receiving ST-segment elevation myocardial infarction (STEMI) centers.
Response: We thank the commenter for this suggestion. The current measure is meant to be one of accountability for the initial (transferring) facility rather than for both the transferring and receiving facility. Therefore, the outpatient measure that is currently in place (OP–3) focuses on the measurable time of arrival to time of physical departure from the first hospital, which is an important component of the total time to reperfusion. A modification to the measure as suggested would not currently be feasible to implement as it would require capturing information from medical records at two separate facilities.
• OP–4: Aspirin at Arrival & OP–5: Median Time to ECG
Comment: One commenter recommended that CMS consider excluding “Chest Pain NEC” from the list of eligible cases for these two measures because many of these cases are not “probable cardiac chest pain” as is the intent of the measures. This commenter also recommended only using the working diagnosis in the “final impression,” rather than working diagnoses used throughout the ED documentation forms, and recommended excluding patients in observation status, as patients believed to have “probable cardiac chest pain” or AMI will likely not be kept under observation status. The commenter believed implementing these Start Printed Page 60636 recommendations will eliminate many cases that these measures did not intend to capture. Another commenter noted that OP–4 has the potential to become “topped out” as the program matures.
Response: We will consider these suggestions as part of maintenance of the technical specifications for the measure. We also will evaluate the performance of OP–4 over time as we do with other measures that have been adopted for public reporting programs.
• Imaging Efficiency Measures Generally
Comment: Several commenters objected to CMS continuing to include the four imaging efficiency measures in the HOP QDRP. Many of these commenters objected because none of the four measures have been adopted by the HQA. Other commenters acknowledged that OP–8 and OP–11 are NQF-endorsed, and also acknowledged that NQF endorsement is not required, but recommended that CMS obtain endorsement for OP–9 and OP–10 in order to establish their credibility. Some commenters opined that the two non-NQF endorsed Imaging Efficiency measures, OP–9 and OP–10, are inappropriate for the HOP QDRP and could cause patient harm. One commenter cautioned that, because the protocols for reporting contrast media on claims have varied over the years, CMS should be aware that the use of contrast media may not be reliably documented in claims.
Response: Many of the concerns raised by the commenters about the imaging efficiency measures were also raised at the time the imaging measures were proposed. We responded to these concerns when we adopted the measures (74 FR 68762 through 68766). We stated that the measures meet the statutory definition of reflecting consensus among affected parties through their consensus-based development, and that the measures address important patient safety concerns related to exposure to unnecessary radiation and contrast materials. We also stated that the Secretary is not required to limit measures considered for selection to only those adopted by the HQA or to those that have been NQF-endorsed. We anticipate submitting OP–9 and OP–10 for NQF endorsement, along with national performance information and other supporting information, when an appropriate call for measures occurs. We note the cautionary advice regarding the varying requirements for reporting contrast media on claims. However, the OP–10 and OP–11 measures rely on procedure codes rather than on specific material codes to determine whether a with-contrast procedure or without-contrast procedure was performed. In other words, these measures only consider whether contrast media was appropriately used during diagnostic imaging procedures, regardless of type.
• OP–8: MRI Lumbar Spine for Low Back Pain
Comment: Some commenters indicated that complete details of a patient treatment plan and history such as conservative therapy for the previous 60 days would often be unavailable to an imaging provider or outpatient center. Other commenters indicated that this measure would be appropriate for the PQRI program.
Response: While a HOPD may not have complete information about a patient's treatment plan and history, such as conservative therapy, HOPDs are in a position to consult and directly communicate with ordering physicians and the radiologists employed by the HOPD. HOPDs can also educate hospital medical staff and community physicians on the appropriate use of MRI for low back pain. We thank the commenters for suggesting that OP–8 may be appropriate for the PQRI program, and will consider this suggestion. We agree that the basis for the measure may be appropriately applied at the ordering physician level. However, we note that the measure has been endorsed by the NQF as appropriate for facility-level measurement.
• OP–9: Mammography Follow-up Rates
Comment: Some commenters objected to this measure because they believed that there is a lack of consensus as to what the appropriate recall rate should be, and that there is no established link between providers' recall rates and patient outcomes. Many commenters expressed concern that the measure implies that high follow-up rates are undesirable, leading to decreased access to these tests, and an increase in undiagnosed early cases of cancer. Other commenters supported this measure, stating that mammography is a life saving tool that is currently underutilized. In addition, some commenters suggested revisions that they believed would improve the current measure. These suggestions include:
- Extending the call back period to 3 months to allow adequate time for a patient to return;
- Counting breast MRI within 3 months of a screening examination as a call back;
- Revising the measure to be a proportion of screening mammograms interpreted as positive, or where the radiologist has recommended further evaluation.
Response: We do not believe that HOPDs should refuse access to mammograms when appropriate follow-up study is needed. We also do not believe that the measure encourages HOPDs to do so. The measure allows identification of facilities with abnormally high rates of “call-backs” from indeterminate or inadequate screening studies. We will evaluate the commenters' suggestions for improvements to the measure specifications as part of the maintenance process for the measure.
• OP–10: Abdomen CT—Use of Contrast Material
Comment: Some commenters indicated that there is a lack of evidence in the literature to determine the appropriate use of contrast material for these patients, and, thus, there is no accepted best practice. In addition, some commenters asserted that, because the measure contains a number of patient exclusions, the applicable patient population is unclear. Other commenters approved of the decision to exclude renal disease patients from this measure.
Response: We have incorporated existing clinical guidelines for appropriate use of combined imaging studies (with and without contrast) into the imaging efficiency measures. Nevertheless, imaging efficiency measures are not intended to define absolutes and should not be interpreted to mean that combined studies would never be considered appropriate. We believe that the measures will promote more careful consideration in individual cases as to whether, in the particular circumstance, a combined study is necessary and thus enhance the efficient use of combined studies. We also anticipate that the variation that exists will lessen and approaches to the use of combined studies will become more standardized.
By implementing the denominator exclusions, we seek to more clearly define the applicable patient population for the quality measure. We thank the commenters that supported the exclusion of renal disease patients from the denominator of this measure.
• OP–11: Thorax CT—Use of Contrast Material
Comment: One commenter objected to the inclusion of this measure, stating that current guidelines indicate that it is acceptable to perform a with-contrast Start Printed Page 60637 study followed by a without-contrast study as clinically indicated.
Response: We agree that, if clinically indicated, such dual studies are appropriate. As with the OP–10 measure, the intent of the OP–11 measure is not to reduce the use of contrast studies or dual studies to zero, but to identify facilities utilizing dual study protocols in the majority of cases when not clinically appropriate.
After consideration of the public comments we received, we have decided to adopt as final our proposal to retain the existing 11 HOP QDRP measures without adding new measures to the measure set for the CY 2011 payment determination. The measure set that will be used for the CY 2011 payment determination is displayed below.
| OP–1: Median Time to Fibrinolysis |
| OP–2: Fibrinolytic Therapy Received Within 30 Minutes |
| OP–3: Median Time to Transfer to Another Facility for Acute Coronary Intervention |
| OP–4: Aspirin at Arrival |
| OP–5: Median Time to ECG |
| OP–6: Timing of Antibiotic Prophylaxis |
| OP–7: Prophylactic Antibiotic Selection for Surgical Patients |
| OP–8: MRI Lumbar Spine for Low Back Pain |
| OP–9: Mammography Follow-up Rates |
| OP–10: Abdomen CT—Use of Contrast Material |
| OP–11: Thorax CT—Use of Contrast Material |
C. Possible Quality Measures Under Consideration for CY 2012 and Subsequent Years
In previous years' rulemakings, we have provided lists of quality measures that are under consideration for future adoption into the HOP QRDP measurement set. In the CY 2010 OPPS/ASC proposed rule (74 FR 35398), we set out a list of measures under consideration for the CY 2012 payment determination and subsequent years. That list is displayed below.
| Topic | No. | Measure | Potential data sources |
|---|---|---|---|
| Cancer | 1 | Adjuvant Chemotherapy Is Considered or Administered Within 4 Months of Surgery to Patients Under Age 80 With AJCC III Colon Cancer The measure specifications are similar to PQRI # 72 found at the PQRI manual Web site: http://www.cms.hhs.gov/apps/ama/license.asp?file=/PQRI/downloads/2009PQRIQualityMeasureSpecificationsManualandReleaseNotes.zip | Registry. |
| 2 | Adjuvant Hormonal Therapy for Patients with Breast Cancer | Claims, Registry. | |
| The measure specifications are similar to PQRI # 71 found at the PQRI manual Web site: http://www.cms.hhs.gov/apps/ama/license.asp?file=/PQRI/downloads/2009PQRIQualityMeasureSpecificationsManualandReleaseNotes.zip | |||
| 3 | Needle Biopsy To Establish Diagnosis of Cancer Precedes Surgical Excision/Resection | Claims, Registry. | |
| The measure specifications can be found at: http://www.qualityforum.org/pdf/reports/Cancer_Nonmember_Report.pdf | |||
| ED Throughput | 4 | Median Time From ED Arrival to ED Departure for Discharged ED Patients | Chart, EHR. |
| The measure specifications can be found at http://www.qualitynet.org/ in Appendix P of the specifications manual under Hospital—Outpatient | |||
| Diabetes | 5 | Low Density Lipoprotein Control in Type 1 or 2 Diabetes Mellitus | Claims, EHR. |
| The measure specifications are similar to PQRI # 2 found at the PQRI manual Web site: http://www.cms.hhs.gov/apps/ama/license.asp?file=/PQRI/downloads/2009PQRIQualityMeasureSpecificationsManualandReleaseNotes.zip | |||
| 6 | Urine protein screening or medical attention for nephrology during at least one office visit within last year for patient with diabetes mellitus | Claims, EHR. | |
| The measure specifications are similar to PQRI # 119 found at the PQRI manual Web site: http://www.cms.hhs.gov/apps/ama/license.asp?file=/PQRI/downloads/2009PQRIQualityMeasureSpecificationsManualandReleaseNotes.zip | |||
| 7 | Eligible diabetes patients with documentation of an eye exam or referral for an eye exam within the last 24 months | Claims, EHR. | |
| Start Printed Page 60638 | |||
| The measure specifications are similar to PQRI # 117 found at the PQRI manual Web site: http://www.cms.hhs.gov/apps/ama/license.asp?file=/PQRI/downloads/2009PQRIQualityMeasureSpecificationsManualandReleaseNotes.zip | |||
| 8 | Patients who received at least one complete foot exam (visual inspection, sensory examination with monofilament and pulse exam within the last 12 months | Claims, EHR. | |
| The measure specifications are similar to PQRI # 126 found at the PQRI manual Web site: http://www.cms.hhs.gov/apps/ama/license.asp?file=/PQRI/downloads/2009PQRIQualityMeasureSpecificationsManualandReleaseNotes.zip | |||
| Medication Reconciliation | 9 | Medication Reconciliation The measure specifications are similar to PQRI # 46 found at the PQRI manual Web site: http://www.cms.hhs.gov/apps/ama/license.asp?file=/PQRI/downloads/2009PQRIQualityMeasureSpecificationsManualandReleaseNotes.zip | Claims, EHR. |
| Immunization | 10 | Pneumococcal Vaccination Status—Overall Rate | Chart, EHR. |
| The measure specifications are available at http://www.qualityforum.org/pdf/reports/Immunization/4%2029%20Immunizations_Nonmembers.pdf | |||
| 11 | Influenza Vaccination Status—Overall Rate | Chart, EHR. | |
| The measure specifications are available at http://www.qualityforum.org/pdf/reports/Immunization/4%2029%20Immunizations_Nonmembers.pdf | |||
| Imaging Efficiency | 12 | SPECT MPI and Stress Echocardiography for Preoperative Evaluation for Low-Risk Non-Cardiac Surgery Risk Assessment | Claims. |
| The measure specifications can be found at http://www.imagingmeasures.com/ | |||
| 13 | Use of Stress Echocardiography or SPECT MPI Post-Revascularization Coronary Artery Bypass Graft | Claims. | |
| The measure specifications can be found at http://www.imagingmeasures.com/ | |||
| 14 | Use of Computed Tomography in Emergency Department for Headache | Claims. | |
| The measure specifications can be found at http://www.imagingmeasures.com/ | |||
| 15 | Simultaneous Use of Brain Computed Tomography and Sinus Computed Tomography | Claims. | |
| The measure specifications can be found at http://www.imagingmeasures.com/ | |||
| Surgery | 16 | Appropriate surgical site hair removal | Chart, EHR. |
| The measure specifications are similar to Surgical Care Improvement Project Infection (SCIP)–6 which can be found at http://www.qualitynet.org/ under Hospital—Inpatient | |||
We invited public comment on these quality measures and topics that we may consider proposing to adopt beginning with the CY 2012 payment determination. We also sought suggestions and rationales to support the adoption of measures and topics for the HOP QDRP which do not appear in the table above.
• Cancer (Potential Measures 1, 2, and 3)
Comment: A number of commenters supported the cancer measures because: (1) They align with national priorities and CMS priority condition areas; (2) they provide insight into an area of care that is very relevant to the consumer; and (3) the measure set seems to address health care provided across settings. One commenter indicated that CMS should more clearly state whether CMS or the HOPD will collect information on chemotherapy within the 4-month timeframe stated in the measure, and how this information will be collected. Some commenters stated that, because some of the cancer measures are registry-based measures, the added costs of implementing measures that require paying a fee to a nongovernmental entity would hinder small rural hospitals from being able to report data. Other commenters indicated that a process for validating registry-based data should be proposed prior to implementing quality measures based on registry data.
Response: We agree with the commenters who supported the cancer measures. We acknowledge that receiving data from registries presents additional issues, but believe that in circumstances where substantial timeframes are involved, registries may Start Printed Page 60639 provide the best data collection mechanism. We will take these comments into consideration in deciding whether to propose these measures in the future for the HOP QDRP, and would specify the form and manner for data submission required should we, in the future, adopt these measures.
• Emergency Department Throughput (Potential Measure 4)
Comment: A few commenters expressed strong support for the ED throughput Measure 4 (Median Time from ED Arrival to ED Departure for Discharged ED Patients) and recommended its inclusion in the HOP QDRP. Some commenters stated that a measure assessing delays in patient care is important as providers experience a growth in demand for ED services. Commenters saw the measure as making significant contributions to reducing overcrowding, and in turn increasing the quality of care delivered, particularly when public reporting occurs.
Response: We thank these commenters for their supportive statements. We agree with the commenters that this measure addresses the issue of timely emergency department care and delays which have an adverse impact on quality of care due to overcrowding.
Comment: One commenter indicated that the ED throughput measure is overly burdensome for hospitals to collect as it will require an arrival time to be noted for each patient, whether the patient is on observation, and will require sampling over 300 records per quarter. Other commenters indicated that, as currently structured, the measure includes the time spent receiving care in the ED in addition to the time spent waiting in the ED. These commenters indicated that the time spent receiving care in the ED should not be counted against the hospital, as it does not represent a delay in care. The commenters stated that, for patients discharged back into the community, and not admitted or transferred to another facility, there is no wait time in the ED after the patient has received the appropriate care. The commenters noted that, for these patients, any time spent waiting in the ED occurs before they see a provider. The commenters suggested that CMS modify the measure so that it reflects only the time spent waiting in the ED to see a provider. One commenter questioned whether the measure actually measures quality because fast care is not necessarily better care. Another commenter indicated that it could not locate the specifications for this measure.
Response: We do not agree that the measure, as currently specified, would be overly burdensome to collect, because hospitals routinely collect the key information needed to calculate the median time (ED arrival date and time and ED departure date and time) for each emergency department patient. The current measure is an NQF-endorsed measure of quality, and feasibility of collection was among the considerations for its endorsement. Revising the measure in the manner suggested by the commenters to exclude active treatment times would be impractical, as it would impose a severe burden for hospitals to accurately track and collect the time spent in the ED not receiving care. We do not agree with the comment that prolonged ED throughput is solely due to time elapsed between arrival and first contact with a provider. The measure specifications are currently available in Appendix P of the HOPD Specifications Manual (versions 2.1b and 3.0) which is posted on the QualityNet Web site ( http://www.qualitynet.org/).
• Diabetes (Potential Measures 5, 6, 7, and 8)
Comment: A few commenters indicated that they believed the three diabetes measures would be better suited to measure the quality of care in physician offices or physician-based clinics rather than in HOPDs. Other commenters indicated that, if finalized, CMS should consider HOPD participation in a disease management registry, or recognition and/or certification in disease management, as substitutes for the requirement of submitting diabetes-related quality measures to the CMS for the HOP QDRP. One commenter indicated that, for the LDL Control measure, CMS should account for the fact that some patients may not reach the goal but their risk may be mitigated by high HDL. One commenter indicated that the timeframe for the eye examination should be 24 months if there is no retinopathy and 12 months if retinopathy is known to be present.
Response: We agree on the suitability of such measures for the physician office setting and note that these measures are currently part of the PQRI program. We would anticipate that these measures would be appropriate for reporting by those HOPDs that function as a primary care provider. We would not view participation in a registry or disease management program certification/recognition as a substitute for reporting quality measures for the HOP QDRP because it would not allow us to achieve the goal of providing comparative quality information on HOPDs to Medicare beneficiaries. These measures are currently being specified for the HOPD setting, and we will consider the suggestions for enhancements submitted by commenters.
• Medication Reconciliation (Potential Measure 9)
Comment: A few commenters supported the medication reconciliation measure but urged CMS to clarify its expectation of medication reconciliation in the ED. Some commenters indicated that, although medication reconciliation measures were recently NQF-endorsed, implementing a quality measure in multiple outpatient settings may result in more medication errors, and recommended that the measure be implemented in primary care settings.
Response: We are interested in medication reconciliation in all settings of care because medication errors may result in serious avoidable complications, and receiving the appropriate medications throughout the continuum of care may prevent the onset or worsening of serious medical conditions. Thus, the reduction of medication errors would contribute to overall improvements in patient outcomes and quality of life and would reduce mortality and hospital readmissions. We would expect that, prior to administration of or prescription of drugs in an ED setting, a patient's current medications, drug allergies, current acute condition, and chronic conditions would be assessed to the extent possible in order to prevent adverse drug-drug interactions and drug-disease interactions. We will take these comments into consideration in determining whether to propose this measure for the HOP QDRP in the future.
• Immunization (Potential Measures 10 and 11)
Comment: One commenter stated that the influenza and pneumococcal vaccination measures will contribute to ED overcrowding, and that the measures are not appropriate for the HOPD setting as administering influenza and pneumococcal vaccinations are not part of routine emergency care protocols like administering a tetanus vaccine would be for wound care. The commenter believed that the measures will work against the ED throughput measures and would be more appropriate for physician offices and community public health departments. Start Printed Page 60640
Response: These measures are currently being specified for HOPDs and are not intended for EDs. These measures are intended to apply to the facility under circumstances where the HOPD serves as a primary care provider. We will consider these comments in deciding whether to propose this measure for the HOP QDRP.
• Imaging Efficiency—SPECT MPI and Stress Echocardiography (Potential Measures 12 and 13)
Comment: Some commenters indicated that the two measures on SPECT MPI and Stress Echocardiography should not be considered for the following reasons:
- Lack of benchmarks;
- Preoperative or postoperative period difficult for provider of test to track;
- Lack of medical history makes it difficult for a provider to determine if a test is appropriate for a patient;
- Not clear how the purpose of test (preoperative evaluation) is captured in Medicare claims; and
- Medicare claims provide an incomplete picture of facility performance.
In addition, for Measure 13 (Use of Stress Echocardiography or SPECT MPI Post-Revascularization Coronary Artery Bypass Graft (CABG)), the commenters indicated that the measure's long time span of a 5-year period post-CABG hinders its usability as the information will be unavailable for a number of years and will be irrelevant by the time it becomes available.
Response: These measures are currently under development, and we will take these comments into consideration as the measures are developed further.
Comment: Some commenters applauded CMS' effort to obtain consensus among affected parties as evidenced by hosting of a public comment period during the measure development process, and supported Measure 12 (SPECT MPI and Stress Echocardiography for Preoperative Evaluation of Low Risk Non-Cardiac Surgery) and Measure 13. However, these commenters also recommended stratification of the measures by imaging procedure.
Response: We appreciate the supportive comments regarding our consensus-based measure development process. We will consider these suggestions for these measures as we continue measure development.
• Imaging Efficiency—Computed Tomography (Potential Measures 14 and 15)
Comment: For both measures, several commenters indicated that the measures target important areas where overuse of diagnostic imaging may be detrimental to patient care, and the measures appear valid and usable. However, the commenters believed that because the measures are based on Medicare claims, they would provide an incomplete picture of facility performance. One commenter suggested excluding “sign of meningeal irritation (stiff neck)” from Measure 14 (Use of Computed Tomography in Emergency Department for Headache).
Response: Both of these measures are under development, and both address overutilization of CT scans in the outpatient setting which have implications for patient safety due to radiation exposure. The goal of these measures is not to reduce outpatient diagnostic CT imaging in these circumstances to zero, but to encourage its use only in circumstances where it is clinically indicated. Though all-payer claims are not currently included in these measures, due to the high volume of these services in the Medicare population relative to other populations, we believe that calculation of these measures based on Medicare claims only will target performance improvement where it is most needed: in the population that is at high risk for inappropriate imaging studies. We appreciate the supportive comments, and will consider these suggestions in the continuing development of these measures.
• Surgery (Potential Measure 16)
Comment: Some commenters indicated that Measure 16 (Appropriate surgical site hair removal) is an unnecessary measure, as performance on the measure in the inpatient setting is already in the high 90 percent range for the Nation. One commenter also indicated that SCIP officials may retire the measure because it is “topped out” and no longer distinguishes between high performers and low performers. Other commenters suggested that CMS not use this measure for quality reporting or, at the very least, exclude cases for which there is no supporting evidence that the use of razors results in lesser quality of care, and cases in which razors would prevent wound bandages from falling off, thus decreasing the chance of infection.
Response: While hospitals may perform highly on this measure in the inpatient setting, we currently do not know if this is the case for the outpatient setting. We will take these comments into consideration in determining whether to propose this measure for the HOP QDRP in the future.
• Other Suggested Measures or Measurement Areas
Comment: Commenters suggested several measures or measurement areas for CMS to consider for future development and adoption. The suggestions include:
- Heart Failure: ACE or ARB for LVSD (NQF #0137);
- Pneumonia: Empiric antibiotic for CAP (NQF #0096);
- Diabetes: Hemoglobin A1c poor control in type 1 or 2 diabetes mellitus (NQF #0059);
- Outcome-based measures;
- Radiation therapy administered within 1 year of diagnosis for women under 70 receiving breast conserving surgery for breast cancer;
- Patient centeredness;
- Total lipid treatment;
- ED throughput;
- Orthopedic procedures;
- Diagnostic Mammography Positive Predictive Value;
- Screening Mammography Positive Predictive Value;
- Cancer Detection Rate (CDR);
- Abnormal Interpretation Rate;
- Emergency Department AMI mortality;
- Emergency Department-related nonmortality outcome measures (that is, NQF Sepsis measures);
- Overall cardiac care;
- Use and overuse of Cardiac CT;
- Percutaneous Cardiac Interventions (“PCI”);
- Care transitions/care coordination;
- AMI–2: Aspirin prescribed at discharge;
- AMI–5: Beta Blocker prescribed at discharge;
- HF–1: Discharge instructions;
- PN–3b: Blood culture performed before first antibiotic received in hospital;
- COPD management;
- NQF-endorsed ASC quality measures;
- Rate of surgical infections in outpatient surgery centers; and
- Rate of infection outbreaks related to contaminated scopes, syringes, and other medical equipment.
Response: We thank the commenters for these suggestions for quality measures and measurement areas for the HOP QDRP, and we will consider them for the future. Some of the topics are reflected in the current list of measures and topics for future consideration. Start Printed Page 60641 Some of the specific measures suggested were considered in the past for the HOP QDRP but, upon evaluation, were either found not to be appropriate measures for HOPD services or were found to be overly burdensome. Other measures and measure topics on this list are currently under consideration as future areas of measurement for inpatient quality measure reporting, and we will examine the appropriateness of these measures for the HOP QDRP as well.
D. Payment Reduction for Hospitals That Fail To Meet the HOP QDRP Requirements for the CY 2010 Payment Update
1. Background
Section 1833(t)(17)(A) of the Act, which applies to hospitals as defined under section 1886(d)(1)(B) of the Act, requires that hospitals that fail to report data required for the quality measures selected by the Secretary, in the form and manner required by the Secretary under section 1833(t)(17)(B) of the Act, incur a 2.0 percentage point reduction to their OPD fee schedule increase factor, that is, the annual payment update factor. Section 1833(t)(17)(A)(ii) of the Act specifies that any reduction would apply only to the payment year involved and would not be taken into account in computing the applicable OPD fee schedule increase factor for a subsequent payment year.
In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68769 through 68772), we discussed how the payment reduction for failure to meet the administrative, data collection, and data submission requirements of the HOP QDRP affected the CY 2009 payment update applicable to OPPS payments for HOPD services furnished by the hospitals defined under section 1886(d)(1)(B) of the Act to which the program applies. The application of a reduced OPD fee schedule increase factor results in reduced national unadjusted payment rates that apply to certain outpatient items and services provided by hospitals that are required to report outpatient quality data and that fail to meet the HOP QDRP requirements. All other hospitals paid under the OPPS receive the full OPPS payment update without the reduction.
The national unadjusted payment rates for many services paid under the OPPS equal the product of the OPPS conversion factor and the scaled relative weight for the APC to which the service is assigned. The OPPS conversion factor, which is updated annually by the OPD fee schedule increase factor, is used to calculate the OPPS payment rate for services with the following status indicators (listed in Addendum B to this final rule with comment period): “P,” “Q1,” “Q2,” “Q3,” “R,” “S,” “T,” “V,” “U,” or “X.” In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68770), we adopted a policy that payment for all services assigned these status indicators would be subject to the reduction of the national unadjusted payment rates for applicable hospitals, with the exception of services assigned to New Technology APCs, assigned status indicator “S” or “T,” and brachytherapy sources, assigned status indicator “U,” which were paid at charges adjusted to cost in CY 2009. We excluded services assigned to New Technology APCs from the list of services subject to the reduced national unadjusted payment rates because the OPD fee schedule increase factor is not used to update the payment rates for these APCs.
In addition, section 1833(t)(16)(C) of the Act, as amended by section 142 of Public Law 110–275, specifically required that brachytherapy sources be paid during CY 2009 on the basis of charges adjusted to cost, rather than under the standard OPPS methodology. Therefore, the reduced conversion factor also was not applicable to CY 2009 payment for brachytherapy sources because payment would not be based on the OPPS conversion factor and, consequently, the payment rates for these services were not updated by the OPD fee schedule increase factor. However, in accordance with section 1833(t)(16)(C) of the Act, as amended by section 142 of Public Law 110–275, payment for brachytherapy sources at charges adjusted to cost is set to expire on January 1, 2010. For CY 2010, in the CY 2010 OPPS/ASC proposed rule (74 FR 35399), we proposed to pay prospectively for brachytherapy sources. Therefore, we proposed that the CY 2010 payment for brachytherapy sources would be based on the conversion factor and the quality reporting reduction policy would be applicable to brachytherapy sources, which are assigned status indicator “U.”
We did not receive any public comments on our proposal to apply the reporting reduction to payment for brachytherapy sources, effective for services furnished on and after January 1, 2010. Therefore, we are finalizing our CY 2010 proposal, without modification, to apply the reduction to payment for brachytherapy sources to hospitals that fail to meet the quality data reporting requirements of the HOP QDRP for the CY 2010 OPD fee schedule increase factor.
The OPD fee schedule increase factor, or market basket update, is an input into the OPPS conversion factor, which is used to calculate OPPS payment rates. To implement the requirement to reduce the market basket update for hospitals that fail to meet reporting requirements, in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68770 through 68771), we calculated two conversion factors: a full market basket conversion factor (that is, the full conversion factor), and a reduced market basket conversion factor (that is, the reduced conversion factor). We then calculated a reduction ratio by dividing the reduced conversion factor by the full conversion factor. We refer to this reduction ratio as the “reporting ratio” to indicate that it applies to payment for hospitals that fail to meet their reporting requirements. Applying this reporting ratio to the OPPS payment amounts results in reduced national unadjusted payment rates that are mathematically equivalent to the reduced national unadjusted payment rates that would result if we multiplied the scaled OPPS relative weights by the reduced conversion factor. To determine the reduced national unadjusted payment rates that applied to hospitals that failed to meet their quality reporting requirements for the CY 2009 OPPS, we multiplied the final full national unadjusted payment rate in Addendum B to the CY 2009 OPPS/ASC final rule with comment period by the CY 2009 OPPS final reporting ratio of 0.981 (73 FR 68771).
In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68771 through 68772), we established a policy that the Medicare beneficiary's minimum unadjusted copayment and national unadjusted copayment for a service to which a reduced national unadjusted payment rate applies would each equal the product of the reporting ratio and the national unadjusted copayment or the minimum unadjusted copayment, as applicable, for the service. We applied the reporting ratio to both the minimum unadjusted copayment and national unadjusted copayment for those hospitals that received the payment reduction for failure to meet the HOP QDRP reporting requirements. This application of the reporting ratio to the national unadjusted and minimum unadjusted copayments was calculated according to § 419.41 of the regulations, prior to any adjustment for hospitals' failure to meet the quality reporting standards according to § 419.43(h). Beneficiaries and secondary payers thereby share in the reduction of payments to these hospitals.
In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68772), we Start Printed Page 60642 established the policy that all other applicable adjustments to the OPPS national unadjusted payment rates apply in those cases when the OPD fee schedule increase factor is reduced for hospitals that fail to meet the requirements of the HOP QDRP. For example, the following standard adjustments apply to the reduced national unadjusted payment rates: the wage index adjustment; the multiple procedure adjustment; the interrupted procedure adjustment; the rural sole community hospital adjustment; and the adjustment for devices furnished with full or partial credit or without cost. We believe that these adjustments continue to be equally applicable to payments for hospitals that do not meet the HOP QDRP requirements. Similarly, outlier payments will continue to be made when the criteria are met. For hospitals that fail to meet the quality data reporting requirements, the hospitals' costs are compared to the reduced payments for purposes of outlier eligibility and payment calculation. This policy conforms to current practice under the IPPS. For a complete discussion of the OPPS outlier calculation and eligibility criteria, we refer readers to section II.F. of this CY 2010 OPPS/ASC final rule with comment period.
2. Reporting Ratio Application and Associated Adjustment Policy for CY 2010
In the CY 2010 OPPS/ASC proposed rule (74 FR 35400), we proposed to continue our established policy of applying the reduction of the OPD fee schedule increase factor through the use of a reporting ratio for those hospitals that fail to meet the HOP QDRP requirements for the full CY 2010 annual payment update factor. For the CY 2010 OPPS, the proposed reporting ratio was 0.980, calculated by dividing the reduced conversion factor of $66.118 by the full conversion factor of $67.439. The final CY 2010 OPPS reporting ratio is 0.980, calculated by dividing the reduced conversion factor of $66.086 by the full conversion factor of $67.406. We proposed to continue to apply the reporting ratio to all services calculated using the OPPS conversion factor. For the CY 2010 OPPS, we proposed to apply the reporting ratio, when applicable, to all HCPCS codes to which we have assigned status indicators “P,” “Q1,” “Q2,” “Q3,” “R,” “S,” “T,” “V,” or “X” and, effective for services furnished on or after January 1, 2010, to also apply it to the HCPCS codes for brachytherapy sources, to which we have assigned status indicator “U.” Under our established policy, we would continue to exclude services paid under New Technology APCs. We proposed to continue to apply the reporting ratio to the national unadjusted payment rates and the minimum unadjusted and national unadjusted copayment rates of all applicable services for those hospitals that fail to meet the HOP QDRP reporting requirements. We also proposed to continue to apply all other applicable standard adjustments to the OPPS national unadjusted payment rates for hospitals that fail to meet the requirements of the HOP QDRP. Similarly, we proposed to continue to calculate OPPS outlier eligibility and outlier payment based on the reduced payment rates for those hospitals that fail to meet the reporting requirements.
We did not receive any public comments on our CY 2010 proposal to apply the HOP QDRP reduction in the manner described in the paragraph above and, therefore, are finalizing our proposal, without modification. For the CY 2010 OPPS, we are applying a reporting ratio of 0.980 to the national unadjusted payments, minimum unadjusted copayments, and national unadjusted copayments for all applicable services reported by those hospitals failing to meet the HOP QDRP reporting requirements. This reporting ratio applies to lines with HCPCS codes assigned status indicators “P,” “Q1,” “Q2,” “Q3,” “R,” “S,” “T,” “U,” “V,” or “X,” excluding services paid under New Technology APCs. All other applicable standard adjustments to the OPPS national unadjusted payment rates will continue to apply. This includes the OPPS outlier eligibility and payment calculations, which are determined using the reduced payment rates.
E. Requirements for HOPD Quality Data Reporting for CY 2011 and Subsequent Years
In order to participate in the HOP QDRP, hospitals must meet administrative, data collection and submission, and data validation requirements (if applicable). Hospitals that do not meet the requirements of the HOP QDRP, as well as hospitals not participating in the program and hospitals that withdraw from the program, will not receive the full OPPS payment rate update. Instead, in accordance with section 1833(t)(17)(A) of the Act, those hospitals will receive a reduction of 2.0 percentage points in their updates for the applicable payment year.
For payment determinations affecting the CY 2011 payment update, in the CY 2010 OPPS/ASC proposed rule (74 FR 35400), we proposed to implement the requirements listed below. Most of these requirements are the same as the requirements we implemented for the CY 2010 payment determination, with some proposed modifications.
1. Administrative Requirements
To participate in the HOP QDRP, several administrative steps must be completed. These steps require the hospital to:
• Identify a QualityNet administrator who follows the registration process located on the QualityNet Web site ( http://www.QualityNet.org) and submits the information to the appropriate CMS-designated contractor. All CMS-designated contractors will be identified on the QualityNet Web site. The same person may be the QualityNet administrator for both the RHQDAPU program and the HOP QDRP. From our experience, we believe that the QualityNet administrator typically fulfills a variety of tasks related to the hospital's ability to participate in the HOP QDRP, such as: creating, approving, editing and/or terminating QualityNet user accounts within the organization; monitoring QualityNet usage to maintain proper security and confidentiality measures; and serving as a point of contact for information regarding QualityNet and the HOP QDRP.
In the past, we have required not only that the hospital designate a QualityNet administrator for purposes of registering the hospital to participate in the HOP QDRP, but also that the hospital continually maintain a QualityNet administrator for as long as the hospital participates in the program. We have become aware that the required maintenance of the QualityNet administrator is creating an undue technical burden for some hospitals and that, in some cases, is preventing the hospital from meeting all of the HOP QDRP requirements. Therefore, we proposed to no longer require that a hospital maintain current designation of a QualityNet administrator. We invited public comment on this proposed change. Nevertheless, we strongly urged hospitals to maintain current designation of a QualityNet administrator, regardless of whether the hospital submits data directly to the CMS-designated contractor or uses a vendor for transmission of data.
Comment: Many commenters agreed with CMS' proposal to remove the requirement to maintain current designation of a QualityNet administrator. Some of these commenters expressed their belief that it is in a hospital's best interest to Start Printed Page 60643 maintain a QualityNet administrator if possible. One commenter greatly appreciated the proposal to no longer require hospitals to maintain a QualityNet administrator to oversee the collection of HOP QDRP data because of the undue technical burden, particularly for hospitals in rural areas. This commenter believed that giving hospitals the option will lead to better quality data collection by lessening this burden on certain rural hospitals.
Response: We thank these commenters for their support; we agree that removing this program requirement will relieve a technical burden especially for some small or rural hospitals. However, due to information systems security requirements, we have now determined that we are prohibited from removing the requirement for a QualityNet Security Administrator at this time. We remind hospitals that are submitting their own data without the use of a vendor that the hospital must have at least one active QualityNet account with the appropriate role assigned in order to submit data. We note that those hospitals with QualityNet accounts (Security Administrator and non-Security Administrator) that are in danger of lapsing receive multiple e-mail notifications that contain reminders that they must sign in or the account will be deactivated.
After consideration of the public comments we received, due to systems requirements, we are not adopting our proposal to no longer require that a hospital maintain current designation of a QualityNet Administrator. Instead, hospitals must continue to maintain a QualityNet Security Administrator as part of the HOP QDRP requirements.
- Register with QualityNet, regardless of the method used for data submission.
- Complete and submit an online participation form if this form (or a paper Notice of Participation form) has not been previously completed, if a hospital has previously withdrawn, or if the hospital acquires a new CCN. For HOP QDRP decisions affecting the CY 2011 payment determination, hospitals that share the same CCN must complete a single online participation form. In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68772), we implemented an online registration form and eliminated the paper form. At this time, the participation form for the HOP QDRP is separate from the RHQDAPU program and completing a form for each program is required. Agreeing to participate includes acknowledging that the data submitted to the CMS-designated contractor will be submitted to CMS and may also be shared with one or more other CMS contractors that support the implementation of the HOP QDRP and be publicly reported.
Under our current requirements, the deadline for submitting the participation form is 30 days following receipt of a CCN form from CMS (73 FR 68772). In the CY 2010 OPPS/ASC proposed rule (74 FR 35400), we proposed to change this requirement as follows:
Hospitals with Medicare acceptance dates on or after January 1, 2010: For the CY 2011 payment update, we proposed that any hospital that has a Medicare acceptance date on or after January 1, 2010 (including a new hospital and hospitals that have merged) must submit a completed participation form no later than 180 days from the date identified as its Medicare acceptance date on the CMS Online System Certification and Reporting (OSCAR) system. Hospitals typically receive a package notifying them of their new CCN after they receive their Medicare acceptance date. The Medicare acceptance date is the earliest date that a hospital can receive Medicare payment for the services that it furnishes. Completing the participation form includes supplying the name and address of each hospital campus that shares the same CCN.
The use of the Medicare acceptance date as beginning the timeline for HOP QDRP participation will allow CMS to monitor more effectively hospital compliance with the requirement to complete a participation form because a hospital's Medicare acceptance date is readily available to CMS through its data systems. In addition, providing an extended time period to register for the program will allow newly functioning hospitals sufficient time to get their operations up and running before having to collect and submit quality data. We invited public comment on these proposed changes.
Hospitals with Medicare acceptance dates before January 1, 2010, that want to participate or withdraw: For the CY 2011 payment update, we proposed that any hospital that has a Medicare acceptance date on or before December 31, 2009 that wants to withdraw from participation in the CY 2011 HOP QDRP or that is not currently participating in the HOP QDRP and wishes to participate in the CY 2011 HOP QDRP must submit a participation form by March 31, 2010. We proposed a deadline of March 31, 2010, because we believe it will give hospitals sufficient time to decide whether they wish to participate in the HOP QDRP, as well as put into place the necessary staff and resources to timely report data for first quarter CY 2010 services. This requirement applies to all hospitals whether or not the hospital has billed for payment under the OPPS. We invited public comment on these proposed changes.
Comment: Several commenters agreed with CMS' proposal to provide additional time for hospitals to submit an HOP QDRP participation form. One commenter believed that, for hospitals with Medicare acceptance dates prior to January 2010, a 3-month window ending March 31, 2010, is reasonable in which to make a decision regarding participation.
Response: We thank the commenters for their support.
Comment: One commenter stated that, based on its experience with hospital mergers, the surviving facility does not typically receive a new CCN and asked that the requirement that merged facilities submit a new participation form be confirmed.
Response: Annual payment update decisions are made for a hospital's CCN. If a hospital's CCN does not change in a merger situation and the hospital is currently participating in the HOP QDRP, the hospital with that CCN would continue to be subject to HOP QDRP requirements, so a new participation form would not be required. However, the participation form requests that hospitals submit National Provider Identifier (NPI) information for any facilities connected to the hospital that bill under the hospital's CCN. The hospital may want to update its participation form to include any facilities added due to a merger, but there is no HOP QDRP requirement to do so at this time. If the hospital's CCN did not change in a merger situation and it was not participating in the HOP QDRP and now wishes to do so, or was participating and now wishes to withdraw, it must comply with the March 31, 2010 timeframe for completing a participation form.
After consideration of the public comments we received, we are adopting as final our proposed administrative requirements with one exception. We are not adopting our proposal to no longer require that a hospital maintain current designation of a QualityNet Administrator. Instead, hospitals must continue to maintain a QualityNet Security Administrator as part of HOP QDRP requirements. Start Printed Page 60644
2. Data Collection and Submission Requirements
a. General Data Collection and Submission Requirements
In the CY 2010 OPPS/ASC proposed rule (74 FR 35401), we proposed that, to be eligible for the full CY 2011 OPPS payment update, hospitals must:
- Submit data: Hospitals that are participating in the HOP QDRP must submit data for each applicable quarter by the deadline posted on the QualityNet Web site; there must be no lapse in data submission. For the CY 2011 annual payment update, the applicable quarters will be as follows: 3rd quarter CY 2009, 4th quarter CY 2009, 1st quarter CY 2010, and 2nd quarter CY 2010. Hospitals that did not participate in the CY 2010 HOP QDRP, but would like to participate in the CY 2011 HOP QDRP, and that have a Medicare acceptance date on the OSCAR system before January 1, 2010, must begin data submission for 1st quarter CY 2010 services using the CY 2011 measure set that we are finalizing in this final rule with comment period. For those hospitals with Medicare acceptance dates on or after January 1, 2010, data submission must begin with the first full quarter following the submission of a completed online participation form. For the four claims-based measures, we will calculate the measures using the hospital's Medicare claims data. For the CY 2011 payment update, we will utilize paid Medicare fee-for-service (FFS) claims submitted prior to January 1, 2010, to calculate these four measures.
Sampling and Case Thresholds: It will not be necessary for a hospital to submit data for all eligible cases for some measures if sufficient eligible case thresholds are met. Instead, for those measures where a hospital has a sufficiently large number of cases, it can sample cases and submit data for these sampled cases rather than submitting data from all eligible cases. This sampling scheme which includes the minimum number of cases based upon case volume will be set out in the HOPD Specifications Manual at least 4 months in advance of the required data collection. Hospitals must meet the sampling requirements for required quality measures each reporting quarter.
In addition, in order to reduce the burden on hospitals that treat a low number of patients but otherwise meet the submission requirements for a particular quality measure, hospitals that have five or fewer claims (both Medicare and non-Medicare) for any measure included in a measure topic in a quarter will not be required to submit patient level data for the entire measure topic for that quarter. Even if hospitals are not required to submit patient level data because they have five or fewer claims (both Medicare and non-Medicare) for any measure included in a measure topic in a quarter, they may voluntarily do so.
Hospitals must submit all required data according to the data submission schedule that will be available on the QualityNet Web site ( https://www.QualityNet.org). This Web site meets or exceeds all current Health Insurance Portability and Accountability Act requirements. Submission deadlines will, in general, be four months after the last day of each calendar quarter. Thus, for example, the submission deadline for data for services furnished during the first quarter of CY 2010 (January–March 2010) will be on or around August 1, 2010. The actual submission deadlines will be posted on the http://www.QualityNet.org Web site.
Hospitals must submit data to the OPPS Clinical Warehouse using either the CMS Abstraction and Reporting Tool for Outpatient Department (CART–OPD) measures or the tool of a third-party vendor that meets the measure specification requirements for data transmission to QualityNet.
Hospitals must submit quality data through My QualityNet, the secure portion of the QualityNet Web site, to the OPPS Clinical Warehouse. The OPPS Clinical Warehouse, which is maintained by a CMS-designated contractor, will submit the OPPS Clinical Warehouse data to CMS. OPPS Clinical Warehouse data are not currently considered to be Quality Improvement Organization (QIO) data; rather, we consider such data to be CMS data. However, it is possible that the information in the OPPS Clinical Warehouse may at some point become QIO information. If this occurs, these data would also become protected under the stringent QIO confidentiality regulations in 42 CFR part 480.
Hospitals must collect HOP QDRP data from outpatient episodes of care to which the required measures apply. For the purposes of the HOP QDRP, an outpatient “episode of care” is defined as care provided to a patient who has not been admitted as an inpatient, but who is registered on the hospital's medical records as an outpatient and receives services (rather than supplies alone) directly from the hospital. Every effort will be made to ensure that data elements common to both inpatient and outpatient settings are defined consistently for purposes of quality reporting (such as “time of arrival”).
Hospitals are to submit required quality data using the CCN under which the care was furnished.
To be accepted into the OPPS Clinical Warehouse, data submissions, at a minimum, must be timely, complete, and accurate. Data submissions are considered to be “timely” when data are successfully accepted into the OPPS Clinical Warehouse on or before the reporting deadline. A “complete” submission is determined based on whether the data satisfy the sampling criteria that are published and maintained in the HOPD Specifications Manual, and must correspond to both the aggregate number of cases submitted by a hospital and the number of Medicare claims the hospital submits for payment. We are aware of “data lags” that occur due to when hospitals submit claims, then cancel and correct those claims; efforts will be made to take such events into account that can change the aggregate Medicare case counts. To be considered “accurate,” submissions must pass validation, if applicable.
CMS strongly recommends that hospitals review OPPS Clinical Warehouse feedback reports and the HOP QDRP Provider Participation Reports that are accessible through their QualityNet accounts. These reports enable hospitals to verify whether the data they or their vendor submitted was accepted into the OPPS Clinical Warehouse and the date/time that such acceptance occurred. We also note that irrespective of whether a hospital submits data to the OPPS Clinical Warehouse itself or uses a vendor to complete the submissions, the hospital is responsible for ensuring that HOP QDRP requirements are met.
Finally, although not required, hospitals may submit, on a voluntary basis, the aggregate numbers of outpatient episodes of care which are eligible for submission under the HOP QDRP and sample size counts. These aggregated numbers of outpatient episodes represent the number of outpatient episodes of care in the universe of all possible cases eligible for data reporting under the HOP QDRP. We do not wish to require this submission at this time because we continue to see evidence that some hospitals would not be able to meet this requirement. However, as it is vital for quality data reporting for hospitals to be able to determine their population sizes, we believe it is highly beneficial for hospitals to develop systems that can determine whether or not they have furnished services or billed for five or fewer cases for a particular measure topic on a quarterly basis. CMS strongly Start Printed Page 60645 recommends that all hospitals work to develop systems that can accurately determine their population and sample sizes for purposes of quality reporting.
In the future, we plan to use the aggregate population and sample size data to assess data submission completeness and adherence to sampling requirements for Medicare and non-Medicare patients.
For the reporting of aggregate numbers of outpatient episodes of care and sample size counts, we proposed that the deadlines for this reporting will be the same as they are for the reporting of quality measures, and these deadlines will be posted on the data submission schedule that will be available on the QualityNet Web site.
We invited public comment on these proposed changes.
Comment: One commenter appreciated CMS' clear and concise definition of an outpatient episode of care. Another commenter asked for a clear definition of what constitutes an outpatient setting.
Response: We thank the first commenter for its support for our definition of an outpatient episode of care. This definition is drawn from the CMS Claims Processing Manual. Chapter 1, Section 50.3.1 of the CMS Claims Processing Manual (issued 06–23–09; Effective Date: 07–01–09) states “Outpatient' means a person who has not been admitted as an inpatient but who is registered on the hospital or critical access hospital (CAH) records as an outpatient and receives services (rather than supplies alone) directly from the hospital or CAH.” Thus, based upon the definition of outpatient, an outpatient setting would be a health care setting where a person is registered on the hospital or CAH records as an outpatient and receives services from the hospital or CAH. Under the HOP QDRP, hospitals are defined as including all the facilities connected to a hospital that are operating and billing for OPPS services under the same CCN. We note that the above definition does not restrict the outpatient setting to the hospital or CAH itself.
Comment: Several commenters stated that many of the program's processes were specified in detail for the first time and that this specificity was appreciated, as it is helpful for hospitals to have clear direction on both the requirements and the process of the program.
Response: We thank the commenters for their feedback.
Comment: One commenter supported CMS' proposal that all hospitals sharing the same CCN be required to combine data across multiple campuses for all clinical data submissions. The commenter believed reporting by CCN is appropriate to align clinical and financial reporting. This commenter also supported this proposal because this approach is similar to the approach being taken by the RHQDAPU program and noted that consistency between administrative aspects of the two programs is appreciated. One commenter stated that while in situations where a new facility is opened or remains under the same ownership, the statement that hospitals are to submit required quality data using the CCN under which the care was furnished is true. However, the commenter believed that, for mergers, there will be a tremendous resource burden placed on the hospital and measure vendor if abstracted data must be separated according to the CCN that applied to the hospital at the time the care was furnished. This commenter stated that because data are submitted and published for public view on a quarterly basis, there is tremendous concern that the public could select a hospital for patient care based on data that does not represent a full quarter. The commenter recommended that if a merger does not occur at the very beginning/end of a quarter, the data be combined for both facilities under the parent/surviving facility CCN because the child or absorbed facility, under its old CCN, no longer exists once the merger occurs.
Response: We thank the commenters that supported our proposal to have quality measure data reported by the CCN that applies to the hospital at the time the care is furnished. We understand that there could be issues regarding burden and completeness of data reporting in the case of mergers. We point out that hospitals operating under separate CCNs participating in the HOP QDRP would be collecting quality measure data separately prior to a merger, and that the data could be kept separate after the merger. Regarding the issue of incomplete data, we acknowledge that the surviving hospital would have less data for the quarter in which the merger took place than if the absorbed hospital's data were included, but the surviving hospital will have all the data for care furnished under its CCN. Because the CCN is the financial and certification identifier for hospitals and is the identifier used by the HOP QDRP to monitor completeness of data reporting, we believe it is important that all quality measure data be reported under the CCN that was in place when the care was delivered. We urge hospitals and vendors to have data reporting systems sufficiently robust to be able to efficiently handle data by the CCN under which the care was delivered.
Comment: One commenter strongly agreed that hospitals with five or fewer claims (Medicare and non-Medicare) for a specific measure should not be required to submit patient-level data for the entire measure topic while being allowed to report data voluntarily, but believed that the allowable time period to not report data was a year. Another commenter urged CMS to modify this provision so that it would apply to hospitals with five or fewer Medicare claims, not five or fewer claims across all payers.
Response: We thank the first commenter for supporting our policy not to require hospitals with five or fewer claims for a specific measure to submit data while allowing these hospitals to report data voluntarily. However, we are clarifying that hospitals that have five or fewer claims (both Medicare and non-Medicare) for any measure included in a measure topic in a quarter will not be required to submit patient-level data for the entire measure topic only for that quarter.
With respect to the second commenter's suggestion that we modify our policy to apply to five or fewer Medicare claims (rather than five or fewer Medicare and non Medicare claims), we selected more than 5 cases per quarter (more than 20 cases per year) as the minimum threshold to ensure that the vast majority of hospitals with sufficient caseload would be required to submit data, while easing the burden on hospitals whose patient counts were too small to reliably predict hospital performance. Because we collect quality measure data on both Medicare and non-Medicare patients, we believe it is appropriate to set our case thresholds using the population for which we are collecting data, which includes both Medicare and non-Medicare patients.
Comment: One commenter questioned the proposal under which hospitals that have five or fewer claims (both Medicare and non-Medicare) for any measure included in a measure topic in a quarter will not be required to submit patient-level data for the entire measure topic for that quarter. The commenter stated that one of the goals of the CMS quality improvement programs is to improve the care given to Medicare beneficiaries and that, by allowing hospitals with five or fewer cases in a quarter to not report, the very hospitals that need improvement the most may be missed. The commenter also believed Start Printed Page 60646 that the burden of reporting is minor for hospitals that would abstract five or fewer charts as this would take less than 30 minutes in most cases, which would not be much of a burden over a 3-month period.
Response: We agree with the commenter that abstracting five charts over a quarter would not be overly burdensome. In implementing the reporting of quality measure data where there are five or fewer cases in a quarter, we are also addressing the reported excessive burden associated with determining the individual cases and submitting the data for those hospitals with small case numbers for a measure. We continue to strive to collect quality of care data while limiting burden. We acknowledge that it is possible that quality of care concerns may exist with hospitals with small case numbers for a particular quality measure. We note that quality of care is also monitored through other mechanisms, including, but not limited to, the Medicare beneficiary complaint process through QIOs and the survey and certification process.
Comment: One commenter agreed that data abstraction processes for outpatient services are sufficiently different from inpatient services as to require the hospital to spend time creating processes to ensure that they capture the accurate population and abstracted data. One commenter agreed with keeping the submission deadline for population and sampling counts the same as the deadline for case-level data, and stated that last minute updates to the population occur because of coding changes. The commenter also stated that synchronizing the deadlines aided hospitals in capturing the most accurate information possible. One commenter agreed with CMS' proposal to keep the submission of population and sampling counts voluntary, and noted that this can be very time consuming for hospitals.
Response: We thank these commenters for confirming our views on the ability of some hospitals to meet a requirement to submit population and sampling data at this time. We reiterate that it is vital, for quality data reporting, that hospitals are able to determine their population sizes and that all hospitals work to develop systems that can accurately determine their population and sample sizes for purposes of quality reporting.
Comment: One commenter urged immediate adoption of an effective mechanism that allows hospitals and their vendors to resubmit quality measure data if an error is discovered and emphasized that the point of public reporting is to put accurate and useful information into the hands of the public and this is facilitated by allowing known reporting mistakes to be corrected.
Response: We agree with the commenter that publicly reporting accurate and useful information is important and that a mechanism or process for correcting errors should be implemented. While a proposal addressing this concern was not included in this current rulemaking, we will consider ways to address this concern in a future rulemaking.
Comment: One commenter disagreed with CMS' approach that sampling requirements should apply based on both Medicare and non-Medicare cases and believed that CMS should focus only on the population of patients for which the agency is responsible.
Response: We believe the commenter is arguing that we should not apply sampling criteria to non-Medicare claims and should focus only on Medicare claims. As we collect quality measure data on both Medicare and non-Medicare patients, we believe it is appropriate to set our sampling criteria so that they apply to the same population, which includes both Medicare and non-Medicare patients.
Comment: One commenter supported the concept of sampling and the development of eligible thresholds and recommended that CMS distribute sampling criteria when new measures are implemented (that is, any measures proposed in a calendar year should include the sampling criteria as part of the OPPS/ASC proposed rule).
Response: We thank the commenter for this suggestion and will consider it in future planning. We note that sampling criteria are included in each release of the HOPD Specifications Manual.
After consideration of the public comments we received, we are adopting as final our proposals regarding HOP QDRP data collection and submission requirements for the CY 2011 payment determination.
b. Extraordinary Circumstance Extension or Waiver for Reporting Quality Data
In our experience, there have been times when hospitals have been unable to submit required quality data due to extraordinary circumstances that are not within their control. It is our goal to not penalize hospitals for such circumstances and we do not want to unduly increase their burden during these times. Therefore, in the CY 2010 OPPS/ASC proposed rule (74 FR 35402), we proposed a process for hospitals to request and for CMS to grant extensions or waivers with respect to the reporting of required quality data when there are extraordinary circumstances beyond the control of the hospital.
Under the proposed process, in the event of extraordinary circumstances not within the control of the hospital, for the hospital to receive consideration for an extension or waiver of the requirement to submit quality data for one or more quarters, a hospital must—
(1) Submit to CMS a request form that will be made available on the QualityNet Web site. The following information should be noted on the form:
- Hospital CCN;
- Hospital Name;
- CEO and any other designated personnel contact information, including name, e-mail address, telephone number, and mailing address (must include a physical address, a post office box address is not acceptable);
- Identified reason for requesting an extension or waiver;
- Hospital's reason for requesting an extension or waiver;
- Evidence of the impact of the extraordinary circumstances, including but not limited to photographs, newspaper and other media articles; and
- A date when the hospital will again be able to submit HOP QDRP data, and a justification for the proposed date.
The request form must be signed by the hospital's CEO. A request form must be submitted within 30 days of the date that the extraordinary circumstance occurred.
Following receipt of such a request, CMS will—
(1) Provide a written acknowledgement using the contact information provided in the request, to the CEO and any additional designated hospital personnel, notifying them that the hospital's request has been received; and
(2) Provide a formal response to the CEO and any additional designated hospital personnel using the contact information provided in the request notifying them of our decision.
We invited public comment on these proposed procedures for requesting an extraordinary circumstance extension or waiver of the requirement to submit quality data for one or more quarters.
Comment: Several commenters expressed their appreciation for CMS' recognition that hospitals facing certain extraordinary circumstances should be granted an extension or waiver. The commenters believed that, while decisions on granting an extension or waiver would best be made on a case-by-case basis depending on each hospital's unique situation, they Start Printed Page 60647 suggested that CMS adopt some general criteria to apply when it determines whether such extensions or waivers would be granted. The commenters also expressed concern that it might not be feasible for a hospital to file a request form for an extraordinary circumstances waiver within 30 days of such an event and urged a creative and flexible approach to working with hospitals in these situations to ensure that an undue burden is not placed on hospitals during a time of hardship.
Response: We will consider these comments as we further develop program procedures for extraordinary circumstance extensions or waivers. We are mindful that many hospitals operating in these adverse situations cannot access the Internet or mail service. We note that we currently use a variety of means to communicate with hospitals in these circumstances, including using our HOP QDRP support contractor and both national and State hospital associations, and will continue to do so. Regarding the ability to file a request form for an extraordinary circumstances waiver within 30 days of such an event, we believe that 30 days is sufficient in the vast majority of circumstances. However, we agree that additional time may be warranted in some extreme circumstances.
After consideration of the public comments we received, we are adopting our proposals regarding extraordinary circumstance extensions or waivers for the reporting of quality data under the HOP QDRP, with a modification that the request form must be submitted within 45 calendar days of the date that the extraordinary circumstance occurred, rather than the 30 days we proposed.
3. HOP QDRP Validation Requirements
In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68776), we announced a voluntary test validation program, the results of which would not affect the CY 2010 payment update for any hospital. Due to resource constraints, we were not able to implement this test validation plan.
a. Data Validation Requirements for CY 2011
Validation, as discussed in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66871), is intended to provide assurance of the accuracy of the hospital abstracted data. For the CY 2011 payment determination, in the CY 2010 OPPS/ASC proposed rule (74 FR 35402), we proposed to implement a validation program that will require hospitals to supply requested medical documentation to a CMS contractor for purposes of being validated. However, the results of the validation will not affect the CY 2011 payment update for any hospital. We believe that it is important for hospitals to have some experience and knowledge of the HOP QDRP validation process before payment determinations are made based upon validation results. We proposed to implement a validation program that will both limit burden upon hospitals, especially small hospitals, as well as provide feedback to all hospitals on validation performance.
Specifically, we proposed to select a random sample of 7,300 cases from all cases successfully submitted to the OPPS Clinical Warehouse by all participating hospitals for the relevant time period described below and validate those data. Based upon the quality data submitted for the CY 2009 payment update and our methodology for drawing the sample, we estimate that the sample will include up to 20 cases per participating hospital; the same number of cases sampled on an annual basis for validation under the RHQDAPU program. A sample size of 7,300 was chosen because it will enable us to detect a relative difference of 10 percent in the measured overall accuracy rate with a 95 percent (two-tailed) confidence interval and should provide sufficient data to conduct post-hoc stratified analyses that provide meaningful feedback. These figures are based upon a power analysis assuming a population measure mismatch rate of five percent with the outcomes being either a match or a mismatch between what the hospital submitted versus what was determined by validation. We intend to supply feedback on the validation results to all hospitals.
We proposed to request medical documentation from hospitals for April 1, 2009 through March 31, 2010 episodes of care, which will allow us to gather one full year of submitted data for validation purposes.
Once we have completed the random selection, a designated CMS contractor will use certified mail to request that each selected hospital send to the CMS contractor supporting medical record documentation that corresponds to each selected episode of care. Each hospital must submit this documentation to the designated CMS contractor within 45 calendar days of the date of the request (as documented on the request letter). If the hospital fails to comply within 30 days of the initial medical documentation request, the designated CMS contractor will send a second certified letter to the hospital reminding the hospital that the requested documentation must be received within 45 calendar days following the date of the initial request. If the hospital still fails to comply, a “zero” score will be assigned to each data element for each selected case and the case will fail for all measures in the same topic (for example, OP–6 and OP–7 measures for a surgical care case).
Once the CMS contractor receives the requested medical documentation, the CMS contractor will independently reabstract the same quality measure data elements that the hospital previously abstracted and submitted and compare the two sets of data to determine whether they match. Specifically, the CMS contractor will conduct a measures level validation by calculating each measure within a submitted record using the independently reabstracted data and then comparing this to the measure reported by the hospital; a percent agreement will then be calculated.
As we stated above, the results of the validation will not affect a hospital's CY 2011 annual payment update because we want to give hospitals time to gain experience with the medical documentation requests and the validation process before these results are used in payment determinations. However, hospitals must supply the medical documentation for each requested case; failure to provide this documentation may result in a 2.0 percentage point reduction in a hospital's CY 2011 annual payment update.
Comment: Many commenters expressed their support for a validation approach where they could receive feedback on their validation results without having their payment affected for the CY 2011 payment update. Several commenters applauded the approach, stating that hospitals will benefit from the program as they continue to gain experience with outpatient quality reporting. One of these commenters specifically agreed with requiring hospitals to submit charts for validation for the CY 2011 payment determination. One commenter expressed support for a 12-month validation period in CY 2011. One commenter expressed support for a different validation process than the one in use for FY 2010 under the inpatient RHQDAPU program.
Response: We thank these commenters and appreciate their support. We agree that it is important that hospitals gain experience with validation for HOP QDRP data collection prior to using validation results to make payment determinations. We also believe that hospitals will benefit from the results of our proposed validation plan, both by Start Printed Page 60648 reviewing results on selected individual cases as well as the aggregate results.
Comment: One commenter stated that hospitals must incur the additional burden of paying to copy and ship medical records to CMS because this funding was not incorporated into the outpatient project.
Response: The issue of costs for copying and shipping medical records to CMS was not discussed in the CY 2010 OPPS/ASC proposed rule, and we are currently considering our policy approach regarding this issue. We are aware that these costs are not insignificant.
Comment: One commenter believed that it is unrealistic to conduct validation and calculate reliable measures from collected HOP QDRP quality measure data because there have been so many changes in the abstraction instructions over the past year. The commenter argued that validation should not be part of the determination for payment decisions until the entire measure set is tested and proven to be reliable and valid. Another commenter stated that the two statements “the results of the validation will not affect a hospital's CY 2011 annual payment update” and “failure to provide this documentation may result in a 2.0 percentage point reduction in the hospital's CY 2011 payment update” were inconsistent.
Response: We agree that there have been issues with the HOP QDRP data, including changes in abstraction instructions over the past year. However, we believe that the validation approach that we have proposed addresses these concerns. For CY 2011 payment determinations, we have proposed to conduct validation on April 1, 2009 to March 31, 2010 episodes of care reported to the OPPS Clinical Warehouse; this is one year after the initial data reported, which was for April 1, 2008 to June 30, 2008 episodes of care. With one exception, most of the significant changes in abstraction instructions during the program's life were incorporated by April 1, 2009. The exception, the exclusion of cancelled surgery cases from the Surgical measures, went into effect with July 1, 2009 episodes of care. We intend to determine whether a selected Surgical Care measure case was cancelled based upon the submitted medical documentation rather than drop April 1, 2009 to June 30, 2009 cases from selection in order to maintain a 12-month sampling frame for validation. We intend to either drop any selected April to June 2009 cancelled surgery cases or otherwise account for this factor.
While it would be optimal to use the preliminary results of the validation effort for CY 2011 in the final design of the validation process for the CY 2012 payment determinations, due to resource constraints, we will not obtain the results of the CY 2011 validation before we must put forth our proposal for CY 2012. We do intend to conduct further analyses of collected HOP QDRP data and will utilize these results in developing our CY 2012 proposals.
Regarding our statement that the results of this validation will not affect a hospital's annual payment update, we wish to clarify that when we referred to “results,” we meant the results of the validation process where what is independently abstracted from a hospital's submitted documentation is compared to what the hospital self-reported. Therefore, while the validation “results” will not affect a hospital's CY 2011 payment update factor, to ensure that we receive an adequate supply of records for our test validation, a hospital must submit required medical record documentation for the selected cases and if it fails to do so, that failure would affect its CY 2011 payment update factor.
Comment: Several commenters supported CMS' proposal to document the validation medical record request process. Specifically, the commenters supported CMS' plans to send two certified letter requests for medical records for data validation in case the hospital does not respond to the first request. The commenters suggested that phone calls be placed to hospitals that do not respond to the first letter to ensure that every effort is made to communicate the request to the appropriate staff; and suggested that this phone call should be placed to the QualityNet Administrator for those hospitals that have a person serving in this role. One commenter suggested that a telephone call to the hospital chief executive officer be made before assigning a “zero” score for validation, as there may be circumstances in which the CEO is unaware of an insufficient response to a request for records.
Response: We thank the commenters and agree that certified letters provide hospitals with multiple written documented notification and reminder attempts; we believe that this alone is sufficient notification. However, we note that the planned contractor for this work as standard practice attempts to call hospitals at least three times about 30 calendar days after it sends the initial medical record request. As a practice, we intend to continue attempting to call hospitals at least three times around the 30th calendar day following the initial request, in addition to sending written certified letters. We believe that these attempted calls at different time periods around the 30th calendar day following the initial request demonstrate our commitment to notify hospitals of medical record requests using multiple communication modes.
Comment: One commenter expressed concern that a random sampling of 7,300 cases is not sufficient to provide hospitals meaningful feedback on the accuracy of their data collection and recommended that CMS select a larger number of cases over a longer period of time. The commenter also suggested that individual feedback on validation results be provided to those hospitals that submit records for this initial validation process.
Response: The major purpose of our proposed sampling scheme for CY 2011 payment determinations is to provide aggregate level feedback to hospitals on data elements abstracted and to validate the quality measure data collected while limiting hospital burden. However, we do intend to provide individual feedback on validation results to those hospitals that submit records for this initial validation effort. Regarding the suggestion to select cases over a longer time period, we have selected the April 1, 2009 to March 31, 2010 timeframe because this time period provides the most recent 12-month period possible. We believe that it is important to use the most recent data possible while maintaining at least a full year's worth of data. We have not included any data from the April 1, 2008 to March 31, 2009 time period because we believe that this will minimize the use for validation purposes of HOP QDRP data that may be unreliable because of changes in data abstraction guidelines that occurred, or because it was collected in the early stages of the program when hospitals were still developing HOP QDRP data collection systems. We believe that hospitals will be able to gain meaningful information from aggregate results produced under our validation sampling scheme, although we agree that it would be more useful to select an increased number of cases. Selecting an increased number of cases is not possible with present resource constraints and we note that this approach would increase hospital burden.
Comment: Several commenters disagreed with randomly selecting only a subset of hospitals for validation because they believe that all hospitals should be held to the same threshold/expectation. One commenter believed that “random validation” would not produce accurate results, and another Start Printed Page 60649 commenter expressed concern that the proposed validation approach is not robust enough given the ever increasing scope of measures included in the measure set.
Response: We appreciate the concern that these commenters express for treating all hospitals similarly, both in costs and benefits of the validation process and for maintaining hospital performance in regard to data validation.
Under the HOP QDRP, all hospitals are responsible for meeting reliability levels for submitted abstracted data. Because hospitals will not know in advance whether they will be selected for validation and because selection will be random, we believe that hospitals will have sufficient incentive to maintain data quality.
Comment: Several commenters suggested that CMS build safeguards into the sampling process to ensure that no more than 20 patient cases are selected from each hospital.
Response: We appreciate the commenters' concerns about the number of cases selected under the proposed sampling design. However, it is highly unlikely, given the number of hospitals and the cases submitted, that any hospital will have more than 20 cases selected. In addition, building in a threshold for the number of cases selected would take away the “random” element of the sampling process.
Comment: One commenter suggested that rather than randomly selecting hospitals each year, CMS adopt a validation process that results in those hospitals not selected in one year having a greater likelihood of being selected in the next/future years.
Response: While we did not propose this approach in our validation plan for CY 2011, in the CY 2010 OPPS/ASC proposed rule (74 FR 35403 through 35404), we discussed additional data validation conditions under consideration for CY 2012 and subsequent years, including the use of targeting criteria. Examples of possible targeting criteria include considering whether a hospital had not been previously selected for validation for 2 or more consecutive years.
Comment: One commenter urged CMS to set strict timelines so that the public has access to data that have undergone the test validation process as soon as possible by publicly reporting validated 2nd and 3rd quarter 2009 data no later than June 2010.
Response: We agree that it is important to make validated data publicly available as soon as possible and will make every effort to do so. However, with present resource constraints, it is not possible to meet the commenter's suggested timeline.
Comment: One commenter urged that State QIOs be supportive not only during the validation appeals process, but proactively during data collection and reporting.
Response: The HOP QDRP was implemented separately from the QIO program and State QIOs have not been involved with the HOP QDRP to date. State QIOs, however, are involved in the RHQDAPU program. We note that QIOs are available for quality of care concerns for individual Medicare cases and that their purview includes the outpatient setting.
Comment: One commenter preferred a validation approach that would select five cases per quarter stratified by measure/topic for all hospitals. The commenter argued that such an approach provides hospitals an opportunity to focus on mismatches by measure set.
Response: We interpret the commenter's statement to mean that the commenter prefers to have five cases that are stratified by measure/topic be selected each quarter for all hospitals so that information on each hospital's individual abstraction accuracy can be assessed by measure/topic. We acknowledge the commenter's belief that such an approach would continually improve data abstraction, but we believe that the improved reliability under the proposed validation process coupled with the reduction of overall hospital burden associated with validation participation will outweigh the potential benefits of validating a smaller number of records for all hospitals. Regardless of whether a hospital was included in any validation selection, we intend to provide aggregate validation information to all hospitals that submit quality measure data.
Comment: Several commenters preferred that CMS give continued attention to individual data element level analysis for validation for a variety of reasons, including: Increasing the denominator and minimizing the impact of a small number of errors; and looking at the individual data elements with a threshold based on sample size. Some of these commenters doubted CMS' statement that higher accuracy rates are possible using the proposed measure level match approach versus a data element level approach and believed that the proposed approach appeared to place hospitals at high risk for not receiving the full CY 2012 payment update. The commenters recommended a period or process where any changes in the validation process be tested without penalty against any payment update prior to broad implementation.
Response: We agree that there should be continued attention to the data element level as the individual data elements are used to calculate quality measures. We proposed a measure level match approach to replace the data element match approach because of what we have observed in the RHQDAPU program for inpatient quality measure data reporting. The intent of the measure match approach is to minimize the impact of errors for noncritical data elements. As we explain in more detail below, we are not finalizing our validation proposal for CY 2012. Instead, we will be conducting further analyses on collected HOP QDRP data and considering all public comments we received on validation before we propose a validation process for the CY 2012 payment determination.
We agree that hospitals should be allowed to gain some experience with validation under the HOP QDRP before we consider such results toward payment determinations, and we are doing so through our validation approach for the CY 2011 payment determination.
Comment: Many commenters agreed with the adoption of a measure score match for validation instead of a data element matching approach. Several commenters believed that it is appropriate to focus on the hospital's measure rate, as opposed to individual data elements, because the measure rate captures the information that is truly important to patient care. The commenters observed that, for data validation in the RHQDAPU (inpatient reporting) program, there have been several instances in which a mismatch between single data elements unrelated to the quality of care provided by a hospital, such as the patient's birth date, have caused hospitals to fail validation and that validating the hospital's measure rate should eliminate these unfortunate incidents.
Response: We thank these commenters for their support. The proposed validation process focuses on validating whether hospital abstracted data results in accurate measure rates and measure denominator counts. We will continue to use the data elements to calculate the measure values and, subsequently, the validation scores.
Comment: One commenter urged CMS to extend the turnaround time for chart selection to 60 days and believed that hospitals should have the option to submit validation cases electronically rather than printed copies because this would avoid shipping delays and allow faster turnaround time. Start Printed Page 60650
Response: We understand the commenter's concern about the deadline for hospitals to submit requested medical records. However, extending this time period increases the amount of time between when services are furnished, initial hospital data are submitted, data are validated, and, ultimately, when the results can be compiled for program purposes. We will consider accepting electronic submission of validation cases using compact disc and electronic health record submission in future years. We must consider both the cost to accept and review these submissions and the added benefit to the hospitals using electronic methods to store medical record information.
After consideration of the public comments we received, we are adopting as final, without modification, our proposals for validation for the CY 2011 payment determination.
b. Data Validation Approach for CY 2012 and Subsequent Years
Similar to our proposal for the FY 2012 RHQDAPU program (74 FR 24178), in the CY 2010 OPPS/ASC proposed rule (74 FR 35403), we proposed to validate data from 800 randomly selected hospitals (approximately 20 percent of all participating HOP QDRP hospitals) each year, beginning with the CY 2012 payment determination. We note that because the 800 hospitals will be selected randomly, every HOP QDRP-participating hospital will be eligible each year for validation selection. For each selected hospital, we proposed to randomly validate per year up to 48 patient episodes of care (12 per quarter) from the total number of cases that the hospital successfully submitted to the OPPS Clinical Warehouse. However, if a selected hospital has submitted less than 12 cases in one or more quarters, only those cases available will be validated. For each selected episode of care, a designated CMS contractor will request that the hospital submit the supporting medical record documentation that corresponds to the episode. We will not be selecting cases stratified by measure or topic; our interest is whether the data submitted by hospitals accurately reflect the care delivered and documented in the medical record, not what the accuracy is by measure or whether there are differences by measure or topic. We proposed to sample data for April 1, 2010 to March 31, 2011 services because this will provide a full year of the most recent data possible to use for purposes of completing the validation in time to make the CY 2012 payment determination.
For the CY 2012 and subsequent years' payment determinations, we proposed to use the validation methodology proposed for the CY 2011 payment update with validation being done for each selected hospital. Specifically, we would conduct a measures level validation by calculating each measure within a submitted record using the independently reabstracted data and then comparing this to the measure reported by the hospital; a percent agreement will then be calculated.
To receive the full OPPS payment update, we proposed that hospitals must attain at least a 90 percent reliability score, based upon our validation process, for the designated time period. We will use the lower bound of a two-tailed 95 percent confidence interval to estimate the validation score. If the calculated upper limit is above the required 90 percent reliability threshold, we will consider a hospital's data to be “validated” for payment purposes. We believe that hospitals will be able to attain higher accuracy rates based on the proposed measure level match approach versus a data element level approach; therefore, we proposed to implement a higher threshold for accuracy than we currently use (and are using) for validation purposes under the RHQDAPU program. We believe that a hospital will be able to achieve a higher accuracy rate under this validation process because we are not calculating whether each data element matches. Instead, we are determining whether or not the reabstracted measure result (for example, was aspirin given at arrival as part of an episode of care that was properly included in the reported data) matches the measure result that was submitted by the hospital. In other words, we are more interested in whether the measure as a whole has been accurately reported than we are in whether each data element that makes up the measure has been accurately reported. Thus, we are focusing on whether the quality measure as a whole that a hospital reports matches what is in the medical record as determined by our re-abstraction. The reason we proposed to implement a measure level match for the HOP QDRP, rather than a data element match, is that in our experience with the RHQDAPU program, hospitals sometimes receive low validation scores due to data element mismatching and not because the care furnished did not match what was documented in the medical record.
We believe that validating a larger number of cases per hospital, but only for 800 randomly selected hospitals, and validating these cases at the measure level (rather than at the data element level) has several benefits. We believe that this approach is suitable for the HOP QDRP because it will: produce a more reliable estimate of whether a hospital's submitted data have been abstracted accurately; provide more statistically reliable estimates of the quality of care delivered in each selected hospital as well as at a national level; and reduce overall hospital burden because most hospitals will not be selected to undergo validation each year.
We solicited public comments on this proposed validation methodology. The public comments we received and our responses are discussed below.
Comment: Several commenters stated that CMS' proposed process for validating hospitals' quality data beginning with CY 2012 holds promise as a reasonable approach to ensure the accuracy of the quality data and improves upon the deficiencies in the current inpatient program validation process.
Response: We agree with the commenters that the proposed new validation process beginning with CY 2012 is an improved approach, and we thank these commenters for their support. However, we have decided that we want to further evaluate and refine the approach and have decided to not finalize a validation approach for CY 2012 in this final rule with comment period. We intend to put forth a proposal for a CY 2012 HOP QDRP validation process in the CY 2011 OPPS/ASC proposed rule.
Comment: One commenter supported the proposed validation process for CY 2012 and subsequent years; however, this commenter believed that the data from the CY 2011 test year should be reviewed to ensure the process is functioning as it was intended and that CMS should make modifications to the process if necessary.
Response: We thank the commenter for supporting our proposed validation process. We agree that it would be helpful to review the data from the CY 2011 test year to evaluate the extent to which the process is functioning as it was intended and to use the results to assist us in determining whether to propose modifications to the validation process for CY 2012 and subsequent years. However, due to resource constraints, we will not receive the results of the CY 2011 validation before we must put forth a proposed validation plan for CY 2012. Instead, we will be conducting further analyses on collected HOP QDRP data and intend to utilize these results as well as all comments we Start Printed Page 60651 received in developing our CY 2012 proposals.
Comment: Several commenters recommended that if validation is limited to randomly selected hospitals each year, a hospital that is selected in one year should be excluded from the selection process for some period, which could be an indeterminate number of subsequent years, the following year, or the next 2 years, or, alternatively, CMS could limit the number of times during a 5-year period a hospital could be randomly selected. Many of these commenters suggested that such an exemption could apply to all hospitals that pass validation, those that pass with high reliability scores, or all hospitals regardless if they pass. Some of these commenters based their suggestions on limiting burden to hospitals and/or rewarding hospitals for participation or for achieving a high reliability or accuracy score. Some commenters believed that exempting a hospital from subsequent validation for some time period for high reliability scores would encourage hospitals to ensure that their data are as accurate as possible and would increase data quality.
Response: We understand the commenters' desire to limit hospital burden on a guaranteed basis and/or to reward hospitals for performance. However, we do not agree that exempting hospitals from validation because they were selected in a previous year or achieved a high reliability score will encourage increased data quality or that it should be a “reward” for meeting a program requirement. It is our belief that any guaranteed exclusion from participating in the validation process also has the potential to undermine a hospital's incentive to maintain data quality.
Comment: One commenter asserted that random selection of hospitals for validation will reduce the hospitals' focus on accuracy because hospitals will have the chance of not being chosen in a given year.
Response: We understand and appreciate the commenter's concern for data accuracy. We believe that each hospital having a chance at selection for validation each year will provide incentive to hospitals to maintain data quality.
Comment: Several commenters made suggestions regarding the sampling scheme for validation. One commenter suggested a random selection of fewer hospitals while increasing the number of records selected and that a random selection of hospitals be done quarterly to reduce the demands on any one hospital while increasing CMS' ability to monitor performance throughout the industry. One commenter supported a random sample of 200 hospitals per quarter with a minimum number of 20 charts reviewed with hospitals not to be selected for validation any more frequently than one time per year. Another commenter agreed with having a separate random selection process for small and rural hospitals that have five or less cases per condition each quarter.
Response: We chose 800 as the number of hospitals we would select for validation each year because this comprises about 25 percent of the total number of HOP QDRP participating hospitals and will provide us with enough data to be statistically assured that we have obtained a representative sample of all hospital data. Regarding randomly sampling hospitals quarterly, we have increased the sample size to gain increased statistical reliability for individual hospital data; lowering the number of cases per hospital or sampling different hospitals each quarter would not enable us to achieve the same result. We agree with the commenters that stratifying sampling by quarter is a possible approach and will consider this as we develop our proposal for a validation process for the CY 2012 payment determination.
Regarding having a separate random selection process for small and rural hospitals that have five or less cases per condition each quarter, we did not propose to stratify by hospital size or by a threshold number of cases per condition. However, we will strongly consider this approach when we develop our CY 2012 validation proposal.
Comment: One commenter supported the random sampling of 800 hospitals (which would require selected hospitals to submit supporting medical records for 48 randomly selected patient episodes of care (12 per quarter)) if the sampling methodology to select the 800 hospitals considers the proportion of rural to urban hospitals. This commenter also believed that this sample must take into account the large number of hospitals that have sample sizes that are too small to make justified decisions based on gathered data. One commenter argued that random validation on a larger number of cases per hospital is excessive for small PPS hospitals.
Response: We agree that hospital size and urban/rural status are important considerations regarding burden and representation of hospital type in any sampling scheme utilized for validation and view these as possible characteristics to stratify sampling of hospitals for CY 2012 and subsequent year's validation. We intend to consider these factors as we further evaluate any proposed validation methodology for CY 2012. Regarding the commenter's belief that this sample must take into account the large number of hospitals that have sample sizes that are too small to make justified decisions based on gathered data, we interpret this statement to mean that the commenter believes these hospitals would not have a sufficient number of cases for us to reliably determine that the hospitals have submitted valid data. We will further assess the numbers of cases submitted by hospitals and, as discussed here in this final rule with comment period, we will be considering whether we should refine our proposed validation methodology to take into account hospital size or a threshold number of case counts in any proposed sampling scheme.
Comment: Several commenters argued that the 90 percent reliability proposal is too stringent for the first year of data validation. Many of these commenters suggested that CMS establish a lower reliability threshold (for example, 70 percent, 75 percent, or 80 percent). Commenters suggesting a 75 percent reliability threshold for the HOP QDRP noted that a 75 percent threshold will be used in the RHQDAPU program for FY 2012 when that program adopts a similar validation approach. One commenter recommended that CMS use data and experience from the CY 2011 test year to determine what an appropriate threshold should be, and until that is determined, the threshold should be the same as the 75 percent threshold that will be required in the inpatient setting for FY 2012. One commenter believed that more analysis of validation results is necessary before establishing the threshold at 90 percent.
Response: After consideration of the public comments regarding the 90-percent threshold, we agree with these commenters that this level is too stringent for the first year of validation where the results may affect a hospital's annual payment update. We appreciate the suggestion that the experience from the CY 2011 test year should be utilized to determine an appropriate rate. However, due to resource constraints, we will not be able to determine any results of the CY 2011 validation prior to proposing and finalizing validation requirements for the CY 2012 payment update factor. However, we will be analyzing collected HOP QDRP data and will take any analyses we complete, as well as the public comments we have received on this proposal, into account as we develop a new proposed Start Printed Page 60652 validation process for CY 2012 and subsequent years.
Comment: One commenter suggested that no hospital be penalized in terms of its payment update if it fails the validation requirement for a single quarter.
Response: We interpret this comment to apply to the proposed validation methodology for CY 2012 and subsequent years because the results of the proposed CY 2011 validation methodology will not affect the CY 2011 payment determination. We did not address whether a hospital would be penalized in terms of its payment update if it fails the validation requirement for a single quarter in the CY 2010 OPPS/ASC proposed rule with comment period. We will take the commenter's suggestion regarding this aspect of validation into consideration as we develop our new proposal for validation for CY 2012 and subsequent years.
We appreciate all the public comments we received regarding the validation process we proposed for CY 2012 and subsequent years and will take them into account as we develop our validation proposals for these years.
c. Additional Data Validation Conditions Under Consideration for CY 2012 and Subsequent Years
In the CY 2010 OPPS/ASC proposed rule (74 FR 35403), we stated that we are considering building upon what we proposed as a validation approach for CY 2012 and subsequent years. We are considering, in addition to selecting a random sample of hospitals for validation purposes, selecting targeted hospitals based on criteria designed to measure whether the data they have reported raises a concern regarding data accuracy. Because little data have been collected under the HOP QDRP at this point, we are considering this approach for possible use beginning with the CY 2012 payment determination. Examples of targeting criteria could include:
- Abnormal data patterns identified such as consistently high HOP QDRP measure denominator exclusion rates resulting in unexpectedly low denominator counts;
- Whether a hospital had previously failed validation; and/or
- Whether a hospital had not been previously selected for validation for 2 or more consecutive years.
Another example of a possible targeting criterion would involve some combination of the some or all of the criteria discussed above.
We solicited public comments on whether these criteria, or another approach, should be applied in future years. We especially solicited suggestions for additional criteria that could be used to target hospitals for validation.
Comment: One commenter opposed the targeting criteria that might supplement random validation as the commenter opposed random validation and instead preferred that all hospitals have cases selected for validation.
Response: We appreciate the commenter's desire for us to validate data from all hospitals. However, we believe that the increased reliability that will be achieved by increasing the number of cases that we validate per selected hospital, coupled with the overall reduction of burden on hospitals that our proposed validation methodology will achieve, outweighs any potential benefit from requiring all HOP QDRP participating hospitals to undergo validation each year of a small number of cases. We chose the sample size of 800 hospitals because this comprises about 25 percent of the total number of HOP QDRP participating hospitals and will provide us with sufficient data to be statistically assured that we have obtained a representative sample of all hospital data.
Comment: One commenter recommended that CMS use similar statistical processes to those used by the Joint Commission to identify outliers in scoring, as well as low denominators compared to population sizes, as these are processes that many hospitals already know.
Response: We thank the commenter for its suggestions, and we will take them into account for our validation proposals for CY 2012.
We greatly appreciate all the public comments we received regarding the validation process proposed for CY 2012. However, as we stated above, we are not finalizing a validation process for CY 2012 at this time. We will take all of the comments we received into account when we develop our validation proposals for CY 2012.
F. 2010 Publication of HOP QDRP Data
In the CY 2009 OPPS/ASC final rule with comment period, we stated our intention to make the information collected under the HOP QDRP available to the public in 2010 (74 FR 68778). In the CY 2008 OPPS/ASC final rule with comment period, we stated that “[i]nformation from non-validated data, including the initial reporting period (April–June 2008) will not be posted” (72 FR 66874). However, section 1833(t)(17)(E) of the Act requires that the Secretary establish procedures to make data collected under the HOP QDRP available to the public, and does not require that such data be validated before it is made public. Moreover, under existing procedures for the RHQDAPU program, data submitted by hospitals are publicly reported regardless of whether those data are successfully validated for payment determination purposes. For these reasons, in the CY 2010 OPPS/ASC proposed rule (74 FR 35404), we proposed to make data collected for quarters beginning with the third quarter of CY 2008 (July–September 2008) under the HOP QDRP publicly available, regardless of whether those data have been validated for payment determination purposes. We invited public comment on this proposal.
Comment: Many commenters opposed the public reporting of unvalidated HOP QDRP data. The commenters stated that these data would not be accurate and may mislead the public. The commenters also argued that because data for the inpatient reporting program were validated before they were publicly posted, the outpatient data should be as well. The commenters stated that making these data available on Hospital Compare and in the downloadable public use file accompanying a Hospital Compare release may lead to inappropriate use of the data in research and policy deliberations, and may result in inaccurate portrayals of the data on various other Web sites that currently utilize Hospital Compare data. The commenters argued that public reporting of unvalidated data may cause confusion among consumers utilizing these data on different Web sites. The commenters were concerned that it is not known now how the non-validated data compare to the validated data. The commenters argued that because data for cases from quarters earlier than the second quarter of 2009 will not have been validated, HOP QDRP data should not be publicly reported prior to this time period.
Response: The validation process for both the RHQDAPU program and the HOP QDRP pertains only to chart-abstracted measures. Validation under these programs for the purposes of payment determination seeks to validate the accurate application of the abstraction rules described in the HOPD Specifications Manual when data are abstracted from medical records and submitted to CMS. Neither the HOP QDRP nor the RHQDAPU program has any validation process for claims data. Thus, the context of our discussion of the public reporting of unvalidated HOP QDRP data in previous rulemakings, our proposal in the CY 2010 OPPS/ASC proposed rule, and our discussion of Start Printed Page 60653 public comments we received regarding whether to post unvalidated data and which quarters of data would be appropriate to post on Hospital Compare pertain only to chart-abstracted measures.
We note that the Secretary is required under section 1833(t)(17)(E) of the Act to establish procedures to make the data submitted under the HOP QDRP available to the public, and we intend to use generally the same procedures that we currently use for the RHQDAPU program. In the RHQDAPU program, we currently publicly report the data as they have been submitted by the hospitals, and we report these data regardless of whether the hospital passed validation. Also, no changes are made to quality data that have been submitted by hospitals that fail validation in the inpatient RHQDAPU program. However, for the RHQDAPU program, we have suppressed data from display on Hospital Compare in circumstances where we have become aware of inaccuracies in the calculation of the measure rates due to systematic issues with the data submitted. We believe that the finalized methodological improvements in the validation process for the CY 2011 HOP QDRP will allow CMS to better assess the overall accuracy of the chart-abstracted data that are submitted by hospitals to CMS. We also believe that our approach will encourage hospitals to optimize their chart abstraction processes and improve the accuracy of their data because it is data that hospitals are responsible for that are ultimately posted on Hospital Compare.
Although we appreciate the concerns raised about the public reporting of unvalidated data, prior to public reporting hospitals are given an opportunity to preview the results to be reported. Additionally, should our consumer testing suggest a different approach to public reporting, or should our validation process for CY2011 reveal a low reliability of self-reported data, we will reconsider our approach for future public reporting.
Comment: Many commenters were opposed to the publication of data beginning with 3rd quarter 2008 services because, during part of that period: (1) Some antibiotics needed to be updated in the specifications but these updates were not present at that time; (2) some procedures needed to be removed from the specifications but these exclusions were not present at that time; and (3) canceled procedures were not able to be excluded from the calculation of certain measures reported at that time. Because these changes were later put into effect, many commenters suggested suppressing earlier quarters of data, and beginning public reporting with the 1st quarter of 2009 data when these changes had been made, and the data specifications were stabilized.
Response: The issues raised by the commenters apply only to the chart-abstracted HOP QDRP surgical care measures OP–6 and OP–7. We have considered the issues raised by the commenters, and because there was an issue with surgeries that were cancelled prior to incision that was not resolved in the specifications until July 1, 2009, we have decided not to report either of the surgery-related process measures (OP–6 & OP–7) for any quarter before the 3rd quarter of 2009.
Comment: Some commenters expressed concern that the 1 year to 2 year time lag in the public reporting of administrative claims-based measures would not be useful to the public.
Response: In the CY 2009 OPPS/ASC final rule with comment period we adopted the 4 claims based imaging efficiency measures for the CY 2010 payment determination and indicated that we would calculate the claims-based imaging efficiency measures for that payment determination using CY 2008 claims (73 FR 68761–68763). We recognize that the time lag for the claims-based measures is a concern, but because of claims submission and claims processing timeframes, this time lag ensures that the most complete and accurate paid claims are used in the measure calculations. Part of the time lag is also due to the time needed for data extraction, data processing, calculation of the measures for the payment determination and subsequent public reporting, quality assurance processes, and the Hospital Compare preview and update schedule. We intend to publicly report the claim-based measures as soon as possible, and the earliest we anticipate being able to make these claims-based measures available to the public is June 2010.
Comment: Some commenters commended CMS for its efforts to publicly report hospital outpatient measures on Hospital Compare in 2010, and encouraged CMS to continue to engage multi‐stakeholder groups in testing and preparing the measures for display on Hospital Compare, as is done with inpatient measures.
Response: We appreciate the supportive comments regarding public reporting of hospital outpatient measures on Hospital Compare in 2010. We began stakeholder and consumer focus group testing of the HOPD measures in the Fall of 2009. We will continue to engage in consumer testing and obtain stakeholder input regarding public reporting of HOP QDRP measures.
Comment: Commenters made numerous suggestions for enhancing the public reporting of HOP QDRP data including:
- Reporting comparative hospital outpatient (OP) and ambulatory surgical center (ASC) quality information on surgical services.
- Providing a mechanism for providers to raise concerns with information to be posted prior to its publication.
- Including a provider narrative section allowing the providers to advise consumers of any concerns regarding reliability or accuracy of information presented.
- Including information on facility accreditation status, state licensure and Medicare certification.
- Creating clear and explicit names and explanations for the measures geared toward consumers.
- Grouping like measures by condition and distinguishing them by care setting.
- Communicating efficiency measures in a manner that clearly interprets the differences among providers, and explains how consumers should integrate this information into decision making.
- Conducting consumer testing and multi-stakeholder vetting of changes in the Hospital Compare architecture, navigation, display and language.
- Considering how best to display hospital outpatient data in the context of current and anticipated future public reporting efforts for ASCs.
- Adding an identifier to the CCN to enable the reporting and display of data for individual hospitals rather than combining results from two or more hospitals sharing the same CCN.
- Displaying measures in a way that allows greater range and detail in categorizations.
Response: We thank the commenters for these suggestions and will consider them as we further develop our procedures for the public reporting of HOP QDRP quality data.
After consideration of the public comments we received, we have decided to finalize our proposal to publicly report HOP QDRP data on Hospital Compare in 2010 with some modifications in the periods of time to be reported. For measures OP–1 through OP–5, we will publicly report data periods beginning with the 3rd quarter of 2008. For measures OP–6 and OP–7, we will publicly report data periods beginning with the 3rd quarter of 2009. For measures OP–8 through OP–11, we will report CY 2010 payment Start Printed Page 60654 determination calculations using CY 2008 claims.
As we noted in section XVI.A.5.c. of this final rule with comment period, in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68778), we established that, for CY 2010, hospitals sharing the same CCN must combine data collection and submission across their multiple campuses for the clinical measures for public reporting purposes and that we will publish quality data by CCN under the HOP QDRP. This approach is consistent with the approach taken under the RHQDAPU program. In that final rule with comment period, we also stated that we intend to indicate instances where data from two or more hospitals are combined to form the publicly reported measures on the Web site.
Comment: One commenter suggested that combining the results from two or more hospitals that share the same CCN is misleading and will not allow consumers and health care payers to assess and use the information, reducing the effectiveness of Hospital Compare. This commenter stated that hospitals should be required to report at the unit of the hospital rather than the CCN. Another commenter suggested that CMS add an identifier to the CCN in order to enable the reporting and display of data of quality measure data for individual hospitals rather than combining results from two or more hospitals that share the same CCN.
Response: We believe that we should publicly report combined data from hospitals with the same CCN because it is important to align clinical reporting with financial reporting. We proposed to report data by CCN for several reasons. First, the unit affected by the OPPS annual update and that must meet all HOP QDRP requirements is the CCN, not an individual hospital or facility that falls under that CCN. Second, hospitals are obligated to comply with applicable survey and certification requirements by CCN, and not by any other individual facility identifier. Third, the additional Medicare identifier for facilities, the National Provider Identifier (NPI), is not a uniform identifier. Fourth, reporting by CCN aligns the reporting of quality of care data with the reporting of financial data. For these reasons, we consider the CCN to be representative of the entire hospital entity for purposes of public reporting under the HOP QDRP. However, as resources permit, we will evaluate whether the benefits the commenter believes would flow from its approach outweigh the reasons outlined above for using the current CCN and whether this suggestion is feasible.
After consideration of the public comments, we have decided to finalize our proposal to publicly report HOP QDRP data on Hospital Compare starting as early as 2010 with some modifications in the periods of time to be reported. Should our consumer testing suggest a different approach to public reporting, or should our validation process for CY 2011 reveal a low reliability of self-reported data, we will reconsider our approach for future public reporting. For measures OP–1 through OP–5, we will publicly report data periods beginning with the 3rd quarter of 2008. For measures OP–6 and OP–7, we will publicly report data periods beginning with the 3rd quarter of 2009. For measures OP–8 through OP–11, we will report 2010 payment determination calculations using calendar year 2008 claims.
G. HOP QDRP Reconsideration and Appeals Procedures
When the RHQDAPU program was initially implemented, it did not include a reconsideration process for hospitals. Subsequently, we received many requests for reconsideration of those payment decisions and, as a result, established a process by which participating hospitals would submit requests for reconsideration. We anticipated similar concerns with the HOP QDRP and, therefore, in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66875), we stated our intent to implement for the HOP QDRP a reconsideration process modeled after the reconsideration process we implemented for the RHQDAPU program. In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68779), we adopted a mandatory reconsideration process that will apply to the CY 2010 payment decisions. In the CY 2010 OPPS/ASC proposed rule (74 FR 35404), we proposed to continue this process for the CY 2011 payment update. Under this proposed process, the hospitals must—
(1) Submit to CMS, via QualityNet, a Reconsideration Request form that will be made available on the QualityNet Web site; this form must be submitted by February 3, 2011, and must contain the following information:
- Hospital CCN.
- Hospital Name.
- CMS-identified reason for failure (as provided in any CMS notification of failure to the hospital).
- Hospital basis for requesting reconsideration. This must identify the hospital's specific reason(s) for believing it met the HOP QDRP requirements and should receive a full annual payment update.
- CEO and any additional designated hospital personnel contact information, including name, e-mail address, telephone number, and mailing address (must include physical address, not just a post office box).
- A copy of all materials that the hospital submitted in order to receive the full payment update for CY 2011. Such material would include, but may not be limited to, the applicable Notice of Participation form or completed online registration form, and quality measure data that the hospital submitted via QualityNet.
The request must be signed by the hospital's CEO.
(2) Following receipt of a request for reconsideration, CMS will—
- Provide an e-mail acknowledgement, using the contact information provided in the reconsideration request, to the CEO and any additional designated hospital personnel notifying them that the hospital's request has been received.
- Provide a formal response to the hospital CEO and any additional designated hospital personnel, using the contact information provided in the reconsideration request, notifying the hospital of the outcome of the reconsideration process.
If a hospital is dissatisfied with the result of a HOP QDRP reconsideration decision, the hospital may file an appeal under 42 CFR part 405, subpart R (PRRB appeal).
Comment: Several commenters supported the proposed hospital reconsideration and appeals process. Some commenters suggested establishing a timeline for CMS to respond to reconsiderations and appeals. One of these commenters suggested a timeline for CMS to respond so that hospitals can better plan in the event the payment rate update is reduced by 2 percentage points. One commenter urged that the full payment rate update not be reduced for hospitals until the reconsideration and appeals process is complete. One commenter believed that this mandatory reconsideration and appeals process should be a permanent component to the quality reporting program and, therefore, not proposed or renewed each calendar year.
Response: We thank these commenters for their support of a hospital reconsideration and appeals process. We plan to complete any CY 2010 reconsideration reviews and communicate the results of these determinations within 60 to 90 days following the date we receive the Start Printed Page 60655 request for reconsideration. While we recognize the commenter's concern about possibly withholding the full payment rate update before the reconsideration and appeals process is complete, we need to consider that if we waited to reduce the payment, the agency could encounter issues with recouping funds that were improperly paid to a hospital that did not meet the HOP QDRP requirements.
Regarding making the reconsideration and appeals process a permanent component to the quality reporting program and, therefore, not proposing or renewing it each calendar year, we have customarily proposed most of the HOP QDRP requirements and procedures, even those we propose to continue with only minor modifications, through the annual rulemaking process in order to afford the public additional opportunities to comment on them. In the case of the reconsideration and appeals procedures, each year we also propose the date by which hospitals must requests for reconsiderations (for the CY 2011 payment update, these requests must be submitted by February 3, 2011).
After consideration of the public comments we received, we are adopting as final the proposed HOP QDRP reconsideration and appeals procedures for CY 2010.
H. Reporting of ASC Quality Data
As discussed above, section 109(b) of the MIEA–TRHCA amended section 1833(i) of the Act by redesignating clause (iv) as clause (v) and adding new clause (iv) to paragraph (2)(D) and new paragraph (7) to the Act. These amendments authorize the Secretary to require ASCs to submit data on quality measures and to reduce the annual payment update in a year by 2.0 percentage points for ASCs that fail to do so. These provisions permit, but do not require, the Secretary to require ASCs to submit such data and to reduce any annual increase for noncompliant ASCs.
In the CY 2008 OPPS/ASC final rule with comment period (72 FR 66875) and in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68780), we indicated that we intended to implement the provisions of section 109(b) of the MIEA–TRHCA in a future rulemaking. While promoting high quality care in the ASC setting through quality reporting is highly desirable and fully in line with our efforts under other payment systems, the transition to the revised payment system in CY 2008 posed significant challenges to ASCs, and we determined that it would be most appropriate to allow time for ASCs to gain some experience with the revised payment system before introducing other new requirements. Further, by implementing quality reporting under the OPPS prior to establishing quality reporting for ASCs, CMS would gain experience with quality measurement in the ambulatory setting in order to identify the most appropriate measures for quality reporting in ASCs prior to the introduction of the requirement in ASCs. Finally, we are sensitive to the potential burden on ASCs associated with chart abstraction and believe that adopting such measures at this time is in contrast with our desire to minimize collection burden, particularly when measures may be reported via EHRs in the future.
We continue to believe that promoting high quality care in the ASC setting through quality reporting is highly desirable and fully in line with our efforts under other payment systems. However, we continue to have the concerns outlined above for CY 2010 and, therefore, in the CY 2010 OPPS/ASC proposed rule (74 FR 35405), we stated that we intend to implement the provisions of section 109(b) of the MIEA–TRHCA in a future rulemaking. We invited public comment on this deferral of quality data reporting for ASCs and invited suggestions for quality measures geared toward the services provided by ASCs. We again sought public comment on potential reporting mechanisms for ASC quality data, including electronic submission of these data.
Comment: Several commenters agreed with CMS' proposal and reasons for deferring quality data reporting for ASCs. Some commenters supported the CMS rationale for the proposed approach, that is, enabling ASCs to gain experience with the recently launched payment system and permitting CMS to gain experience in the HOPD setting before implementing quality data reporting requirements for ASCs. Several commenters supported CMS' decision to move with caution in expanding quality data reporting to the ASC setting and appreciated CMS' sensitivity to administrative burdens faced by ASCs. Commenters stated that it would be beneficial to allow extra time in order to assess implementation challenges and identify appropriate measures. These commenters also indicated that issues such as preventability, risk adjustment, unintended consequences, coding accuracy, burden, and effect on overall health care costs need to be carefully examined before starting a reporting program for a new setting. Other commenters indicated it would be better to wait until ICD–10 implementation to begin measurement in a new setting in order to allow for more accurate coding and measurement and POA coding. One commenter agreed with the continued delay in implementing a quality data reporting program for ASCs based upon CMS' rationale set forth in the proposed rule and suggested that CMS discuss implementation of the requirements, including when ASC reporting will occur and the potential effects on ASC staff. One commenter argued that requiring ASCs to conduct quality data reporting is unnecessary because quality improvement is a key requirement for ASCs to obtain and maintain accreditation and such reporting would result in additional costs to ASC operations.
Response: We thank these commenters for acknowledging the many operational issues that must be addressed prior to implementing a quality data reporting program for ASCs and supporting our decision to defer ASC quality reporting to a future time. However, with regard to the commenter's suggestion that an ASC quality data reporting program is unnecessary in light of ASC quality improvement accreditation requirements, we believe that quality measure data reporting for ASCs would provide consumers with quality of care information for this type of health care provider as well as support our quality improvement efforts. Therefore, notwithstanding the current issues that have led to our determination to implement an ASC quality reporting program in a future rulemaking, we believe it is important and necessary to require ASCs to submit quality data.
Comment: Numerous commenters advocated that CMS move forward with an ASC quality data reporting program as soon as possible. Many commenters indicated that the collection and reporting of quality data is a common practice for ASC facilities, and that the industry is eager to make quality data available to consumers in a manner that allows direct comparisons between equivalent surgical care delivered in HOPDs and ASCs, particularly as the percentage of outpatient surgical services being provided in ASC settings has grown. Some commenters urged CMS to implement a quality data reporting system for ASCs for CY 2010. One commenter was concerned about the continued delay in quality measurement for the rapidly growing ASC setting and indicated that by now it should be technically feasible for ASCs to report on at least the set of five quality measures that were developed Start Printed Page 60656 by the industry-sponsored ASC Quality Collaboration. Several commenters argued that any quality data reporting system implemented would not create significant administrative burden on ASCs. Some of these commenters recommended the use of administrative claims as a means for quality reporting as both chart abstraction and Internet-based reporting would impose major disadvantages for ASCs, most of which are classified as small businesses. Some commenters suggested beginning with a set of six ASC quality measures that have already been developed. Commenters also suggested that CMS consider claims-based reporting for ASCs, which would eliminate the chart abstraction burden, would capitalize on existing data collection infrastructure, and would be most feasible for the industry at this time. Another commenter indicated that specialty-specific measures for ASCs should be implemented in such a reporting program in order to ensure broad opportunity for participation, including those ASCs that specialize in a few services or procedures. One commenter indicated that at least 35 States collect data on ASCs.
Response: We agree that it would be beneficial for consumers to be able to compare the quality of surgical care across HOPDs and ASCs. Currently, in addition to the reasons we outlined in the proposed rule, we do not have the resources needed to implement a quality data reporting program for ASCs. We are aware of the set of five quality measures that were developed by the ASC Quality Collaboration as well as six NQF-endorsed quality measures. While some of the measures may be feasible to collect using claims data, others (such as the patient safety-related measures) may not be meaningful to report unless all patients treated were captured, and hence all-payer claims were collected to generate the measures. We will evaluate the suggested measures for ASC quality data reporting, as well as the feasibility of claims-based measure reporting for ASCs and the need for specialty-specific measures for ASCs in the future.
Comment: One commenter encouraged CMS to focus on electronic submission of data for quality reporting once it has been determined to move forward with the ASC quality program. One commenter recommended that any ASC reporting program build on the public reporting programs in place for the inpatient and outpatient settings and that the measures reported in the ASC setting be consistent and, where possible, identical to the outpatient department program as the consistency will minimize confusion, simplify data collection, and assure greater comparability across sectors.
Response: We thank the commenters for these suggestions. We will consider them in the planning process for ASC quality measure data reporting.
After consideration of the public comments we received, we are finalizing our proposal to implement quality measure reporting in the ASC setting in a future rulemaking. We continue to believe that promoting high quality care in the ASC setting through quality data reporting is highly desirable and fully in line with our efforts under other payment systems.
I. Electronic Health Records
As stated above, CMS is actively seeking alternatives to manual chart abstraction for the collection of quality measures for its quality data reporting programs. Among these alternatives are claims-based measure calculation, collection of data from systematic registries widely used by hospitals, and electronic submission of quality measures using EHRs. In the CY 2009 OPPS/ASC final rule with comment period, we discussed public commenters' suggestions that we adopt measures that can be collected via EHRs (73 FR 68769). We agree with the commenters about the importance of actively working to move to a system of data collection based on submission from EHRs. We have been engaged with health IT standard-setting organizations to promote the adoption of the necessary standards regarding data capture to facilitate data collection via EHRs, and have been collaborating with such organizations on standards for a number of quality measures. We encourage hospitals to take steps toward the adoption of EHRs that will allow for reporting of clinical quality data from the EHR directly to a CMS data repository. We also encourage hospitals that are implementing, upgrading, or developing EHR systems to ensure that such systems conform to standards adopted by HHS. In the CY 2010 OPPS/ASC proposed rule (74 FR 35405), we invited public comment on the future direction of EHR-based quality measure submission with respect to the HOP QDRP.
Comment: One commenter strongly encouraged CMS to consider aligning the HOP QDRP with the ONC measures for “meaningful use,” and to provide clarity on those measures that appear to be similar to those identified as measures for meaningful use, such as the OPPS CY 2011 “OP–4: Aspirin at Arrival” and the meaningful use matrix measure for CY 2011, “Improve quality, safety, efficiency, and reduce health disparities: Percentage patients at high-risk for cardiac events on aspirin prophylaxis [OP].”
Response: The measure matrix referenced by the commenter is a list of criteria developed by the Health IT Policy Council, an advisory committee to ONC, for consideration by the Department as it develops the initial criteria for determining whether an eligible hospital or eligible professional is a meaningful user of certified EHR technology. Eligible hospitals and eligible professionals who demonstrate that they meaningfully use certified EHR technology will be eligible for payment incentives under Medicare, as authorized under the HITECH Act. To be considered a meaningful user of the certified EHR technology, section 1886(n)(3)(A)(iii) of the Act, as added by section 4102(a) of the HITECH Act, requires that eligible hospitals submit to CMS, using certified EHR technology, the clinical quality measures and such other measures selected by the Secretary. Section 1886(n)(3)(B)(iii) of the Act provides that in selecting these reporting measures, the Secretary shall seek to avoid redundant or duplicative reporting with the reporting otherwise required, including reporting under RHQDAPU. CMS will establish the initial meaningful use criteria in future rulemaking, including the selection of quality measures for hospital reporting purposes under this separate incentive program. Some of the clinical quality measures included on the Health IT Policy Council's matrix are similar to measures adopted into the HOP QDRP. As we stated in the “considerations for measurement” section of this final rule with comment period, because we seek to harmonize applicable measures across settings, and many of the measures for the HOP QDRP that apply to HOPDs have been adapted from the RHQDAPU program, some of the measures that appear on the Health IT Policy Council's matrix are similar to measures adopted into the RHQDAPU program. We thank the commenters and will take these comments into consideration as we consider the future direction of EHR-based quality measure submission with respect to the HOP QDRP.
XVII. Healthcare-Associated Conditions
A. Background
1. Preventable Medical Errors and Hospital-Acquired Conditions (HACs) Under the IPPS
As noted in its landmark 1999 report “To Err is Human: Building a Safer Health System,” the Institute of Medicine found that medical errors are Start Printed Page 60657 a leading cause of morbidity and mortality in the United States. Total national costs of these errors due to lost productivity, disability, and health care costs were estimated at $17 billion to $29 billion.[2] As one approach to combating healthcare-associated conditions, in 2005, Congress authorized CMS to adjust Medicare IPPS hospital payments to encourage the prevention of these conditions. Section 1886(d)(4)(D) of the Act (as added by section 5001(c) of the Deficit Reduction Act (DRA) of 2005, Pub. L. 109–171) required the Secretary to select by October 1, 2007, at least two conditions that are: (1) High cost, high volume, or both; (2) assigned to a higher paying diagnosis-related group (DRG) when present as a secondary diagnosis; and (3) could reasonably have been prevented through the application of evidence-based guidelines. CMS has titled this initiative “Hospital-Acquired Conditions (HAC) and Present on Admission (POA) Indicator Reporting.” Since October 1, 2008, Medicare no longer assigns a hospital inpatient discharge to a higher paying Medicare Severity Diagnosis-Related Group (MS–DRG) if a selected HAC is not present on admission. That is, if there is an HAC, the case is paid as though the secondary diagnosis was not present. However, if any nonselected complications or comorbidities appear on the claim, the claim will be paid at the higher MS–DRG rate; to cause a lower MS–DRG payment, all complications or comorbidities on the claim must be selected conditions for the HAC payment provision. Since October 1, 2007, CMS has required hospitals to submit information on Medicare hospital inpatient claims specifying whether diagnoses were POA.
2. Expanding the Principles of the IPPS HACs Payment Provision to the OPPS
In the CY 2009 OPPS/ASC proposed rule and final rule with comment period (73 FR 41547 and 68781, respectively), we discussed whether the principle of Medicare not paying more for preventable HACs during inpatient stays paid under the IPPS could be applied more broadly to other Medicare payment systems in other settings for conditions that occur or result from health care delivered in those settings. We also acknowledged that implementation of this concept would be different for each setting, as each Medicare payment system is unique. As we have used in past rulemaking and general notices, in the following discussion in this final rule with comment period, we refer to conditions that occur in the hospital inpatient setting as “hospital-acquired conditions (HACs),” to conditions that occur in HOPDs as “hospital outpatient healthcare-associated conditions (HOP–HACs),” and to conditions that result from care in settings other than the hospital inpatient and HOPD settings as “healthcare-associated conditions.”
In both the CY 2009 OPPS/ASC proposed rule and final rule with comment period, we specifically presented our rationale for considering the HOPD as a possible appropriate setting for Medicare to extend to the OPPS the concept of not paying more for preventable healthcare-associated conditions that occur as a result of care provided during a hospital encounter. For example, hospitals provide a broad array of services in their HOPDs that may overlap or precede the inpatient activities of the hospital, including many surgical procedures and diagnostic tests that are commonly performed on both hospital inpatients and outpatients. Similarly, individuals who are eventually admitted as hospital inpatients often initiate their hospital encounter in the HOPD, where they receive care during clinic or emergency department visits or observation care that precede their inpatient hospital admission. In addition, like the IPPS, the OPPS is also subject to the “pay-for-reporting” provision that affects the hospital outpatient annual payment update by the authority of section 1833(t)(17) of the Act (as amended by section 109(a) of Pub. L. 109–432 (MIEA–TRHCA)). (We refer readers to section XVI. of this final rule with comment period for a discussion of the HOP QDRP provisions for hospitals that fail to meet the reporting requirements established for the hospital outpatient payment update.)
The risks of preventable medical errors leading to the occurrence of healthcare-associated conditions are likely to be high in outpatient settings, given the large number of encounters and exposures that occur in these settings. Approximately 530,000 preventable drug-related injuries are estimated to occur each year among Medicare beneficiaries in outpatient clinics.[3] These statistics clearly point to the significant magnitude of the problem of healthcare-associated conditions in outpatient settings. Recent trends have shown a shift in services from the inpatient setting to the HOPD, and we expect the occurrence of healthcare-associated conditions stemming from outpatient care to grow directly as a result of this shift in sites of service.
For the CY 2009 OPPS, we did not adopt any new Medicare policy in our discussion of healthcare-associated conditions as they relate to the OPPS. Instead, in the CY 2009 OPPS/ASC proposed rule, we solicited public comments on options and considerations, including the statutory authority related to expanding the IPPS HAC provision to the OPPS. Our discussion addressed the following areas:
- Criteria for possible candidate OPPS conditions;
- Collaboration process;
- Potential OPPS HOP–HACs, including object left in during surgery; air embolism; blood incompatibility; and falls and trauma, including fractures, dislocations, intracranial injuries, crushing injuries, and burns; and
- OPPS infrastructure and payment for encounters resulting in healthcare-associated conditions, including the necessity of POA reporting for hospital outpatient services, methods for risk stratification, and potential methods for adjusting hospital payment.
3. Discussion in the CY 2009 OPPS/ASC Final Rule With Comment Period
In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68784 through 68787), we responded to the public comments we received on healthcare-associated conditions in the context of the OPPS. Several commenters fully supported expanding the IPPS HAC policy to other settings such as HOPDs and ASCs, but many commenters stated that CMS should not implement a related policy in other settings without gaining implementation experience with the IPPS HACs. A number of commenters addressed concerns regarding some of the potential specific HOP–HACs discussed in the CY 2009 OPPS/ASC proposed rule (73 FR 41549), and some commenters suggested other conditions that should be considered or identified those that should not be considered. Many commenters stated that the attribution of HOP–HACs in the HOPD setting is difficult and stated that there was a need to develop risk adjustment techniques to account for differences in patient severity of illness or other patient characteristics. Many commenters asserted that the POA Start Printed Page 60658 indicators may need to be modified for use in the HOPD or ASC setting. Some commenters suggested that a “present on encounter” indicator or another form of incorporation of preexisting conditions into an episode-of-care might be more useful than a POA indicator. Several commenters believed that, without changes to the existing OPPS payment structure, there would be no straightforward methodology for adjusting hospital payment.
While we acknowledged these challenges in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68787), we noted that we view addressing the ongoing problem of preventable healthcare-associated conditions in outpatient settings, including the HOPD, as a key value-based purchasing strategy to sharpen the focus on such improvements beyond hospital inpatient care to those settings where the majority of Medicare beneficiaries receive most of their health care services. We also noted that we looked forward to continuing to work with stakeholders to improve the quality, safety, and value of health care provided to Medicare beneficiaries, beginning with a joint IPPS/OPPS listening session.
B. Public Comments and Recommendations on Issues Regarding Healthcare-Associated Conditions From the Joint IPPS/OPPS Listening Session
Subsequent to the issuance of the CY 2009 OPPS/ASC final rule with comment period, we held a joint Hospital-Acquired Conditions and Hospital Outpatient Healthcare-Associated Conditions Listening Session on December 18, 2008. (The listening session was announced in a notice published in the Federal Register on October 30, 2008 (73 FR 64618).) During the listening session, we provided an overview of the HAC program under the IPPS and our previous discussions of extending the underlying concepts to the HOPD, including OPPS infrastructure concerns such as the lack of a POA indicator and the need to address current ICD–9–CM POA reporting guidelines, attribution of conditions in the HOPD, and payment adjustment considerations. In addition to the initial candidate HOP–HACs that we had previously identified based on their adoption under the IPPS, we discussed other potential HOP–HACs, such as medication errors, conditions related to complications of hospital outpatient surgery or other procedures, and infections related to HOPD care. A transcript of the listening session is available on the CMS Web site at: http://www.cms.hhs.gov/HospitalAcqCond/07_EducationalResources.asp#TopOfPage.
Of the many public comments presented orally at the listening session or submitted in writing, approximately one-half commented on expansion of the IPPS HAC payment provision to other settings. Some commenters were in favor of an expansion to the HOPD and other settings. Many commenters requested that CMS delay any expansion, citing the short duration of experience with HACs and POA indicator reporting for inpatient hospitalizations and the need to evaluate the current program prior to its expansion to other settings.
We appreciate these commenters' perspectives and note that now that we have early data on the HAC program, in the near future we plan to evaluate the impact of the HAC payment provision through a joint program evaluation with CDC, AHRQ, and the Office of Public Health and Science.
Many commenters pointed to the need to define the boundaries of an episode-of-care for healthcare-associated conditions in the HOPD and other settings in order to define when, how, and to whom an expanded policy would apply. These commenters also noted that hospital outpatients have frequently received care from numerous practitioners and providers over an extended period of time and the hospitals' or clinics' role would be supportive, rather than prescriptive, with respect to that patient care. They requested that CMS develop a comprehensive and accurate definition of an episode-of-care in order to appropriately attribute responsibility and the additional costs associated with HOP–HACs.
We have previously acknowledged that short-term consideration of HOP–HACs would necessarily be limited to conditions that occur during and result from care provided in a single hospital outpatient encounter because a broader definition of an episode-of-care has not yet been developed.
Many commenters believed that detailed information should be gathered and analyzed from the IPPS POA indicator reporting experience before an expansion of the HAC payment provision and POA indicator reporting to the HOPD. Other commenters pointed out that the initial four conditions under consideration for HOPDs based on their adoption under the IPPS would likely require emergency admission for treatment of the event. Though secondary to an initial encounter in the HOPD, they indicated that these conditions would be coded as POA for the IPPS according to current reporting guidelines and would not be captured as HOP–HACs. Several commenters stated that, in the HOPD, it would be particularly important to make an assessment over an entire episode-of-care; thus, POA might be better defined in terms of “present on encounter” for this purpose. Other commenters pointed to the need for the development of new codes and determinations of when the codes should apply in order to capture POA conditions under the OPPS, an activity that would potentially significantly increase hospitals' administrative burden. Some commenters suggested waiting to expand the HAC payment provision to other settings until implementation of the ICD–10 classification system, which would provide more precise coding to identify preexisting conditions.
We have acknowledged a number of these challenges already, and we will continue to consider these reporting issues as we refine our views regarding potential HOP–HACs.
Many commenters highlighted that patients receiving hospital outpatient care may receive care in multiple departments of the hospital, both during a single outpatient encounter and longitudinally over many outpatient encounters of relatively short duration. These commenters stated that, because of these common patterns of care, the timely identification of HOP–HACs and their provider attribution would be particularly challenging. In addition, the commenters pointed out that patient factors may play a role in the development of potential HOP–HACs, such as adverse drug events. Several of these commenters suggested targeting the HOP–HAC policy to specific APCs, specific HCPCS codes, or specific HOPD settings, such as the emergency department. In the CY 2009 OPPS/ASC proposed rule and final rule with comment period (73 FR 41549 through 41550 and 68785 through 68787, respectively), we discussed the challenge of provider attribution under the OPPS, particularly for conditions that may develop over time and involve multiple encounters and other care settings. We understand the importance of this issue and will continue to be cognizant of it in future policy development.
Several commenters asserted that CMS should consider risk adjustment models that incorporate population risk adjustments to avoid creating barriers to access for more complex patients or to avoid unduly placing providers treating more complex patients at higher risk for payment consequences due to HOP–HACs. A number of commenters endorsed the use of rate-based measures Start Printed Page 60659 of conditions on a provider-specific level so that the level of preventability of specific clinical conditions could be determined and compared. Several commenters stated that, under the best of circumstances, falls may not be “reasonably preventable,” particularly in the HOPD. Many commenters also believed that adverse drug events would require further definition in order to appropriately address medication errors that were not directly under the control of the hospital providing the treatment of the medication-related problem and were, therefore, not “reasonably preventable.” Similarly, some commenters stated that it would be difficult to appropriately attribute metabolic derangements in the HOPD to the hospital treating the resulting clinical problem.
We appreciate these public comments and will use our collaborative process with CDC, AHRQ, and the Office of Public Health and Science to help define potential HOP–HACs that are clinically meaningful for patient safety, as well as attributable to care furnished by providers.
Numerous commenters urged CMS to generally proceed with care, to promote the use of evidence-based guidelines and care coordination, and to ensure that any HOP–HAC program is aligned with other CMS quality programs. Many commenters believed that the challenges involved might be better addressed operationally within a full-scale value-based purchasing program. We appreciate these suggestions and will consider them as we advance policies that will ensure paying for the highest quality, safest, and most effective health care for Medicare beneficiaries.
C. CY 2010 Approach to Healthcare-Associated Conditions Under the OPPS
For CY 2010, we did not propose to expand the principles behind the IPPS HAC payment provision to the OPPS through a HOP–HAC program (74 FR 35407). We stated that we continue to believe that it may be appropriate to expand the principles of the IPPS HAC payment provision to the OPPS in the future. However, we acknowledged that, at this time, there are many operational challenges to such an expansion that will require further consideration and infrastructure development. We appreciate the input and guidance provided by the many public commenters to date on how to approach these challenges. Most stakeholders have strongly encouraged CMS to evaluate the impact of the IPPS HAC payment provision before further considering any expansion to other settings. We explained that we are evaluating the impact of the HAC and POA indicator reporting initiative on Medicare payment and that we plan to consider any relevant findings as part of our future decision making regarding any expansion of the HAC payment provision to other settings. We welcomed additional suggestions and comments from stakeholders on potential HOP–HACs as additional information becomes available and health care delivery continues to evolve.
Comment: Several commenters commended CMS for considering an extension of the current IPPS HAC policy to other care settings and payment systems, including the OPPS. These commenters suggested that it would be reasonable to begin with patient safety-related conditions such as an object left in during surgery, air embolism, blood incompatibility, falls and trauma, including fractures, dislocations, intracranial injuries, crushing injuries, and burns.
However, the majority of commenters supported CMS' position in not proposing to expand the IPPS HAC payment provision to the OPPS at this time. They agreed with the plan to consider any relevant findings from the agency's evaluation of the impact of the HAC and POA indicator reporting initiatives (74 FR 35407) as part of CMS' future decision-making regarding expansion of the IPPS HAC policy to other settings. Several commenters reiterated their concerns related to technical challenges in expanding the IPPS HAC program to the OPPS, with particular emphasis on the need to develop a type of POA coding for hospital outpatient services and the fact that current POA guidelines designate conditions that develop during an outpatient encounter, such as clinic and emergency department visits, observation services, or outpatient surgery, as POA for hospital inpatient reporting. Other commenters encouraged CMS to develop a comprehensive definition of an episode-of-care, with the potential for inclusion of related care settings to appropriately attribute accountability. Some commenters urged CMS to evaluate the role of ICD–10 classification in the HAC program and to consider postponing implementation of HOP–HACs until the adoption of ICD–10. A number of commenters recommended that CMS focus on areas of patient safety in a future HOP–HACs program, including outpatient surgery and outpatient procedures that are correlated with the potential for injury and medication errors. Finally, several commenters encouraged CMS to ensure that any future HOP–HAC program provides an incentive for care coordination, aligns with other CMS quality initiatives, and has no detrimental effect on patient access to care.
Response: We appreciate the thoughtful and detailed suggestions from commenters. We will continue to consider the concerns and suggestions from commenters as we evaluate the impact of the HAC and POA indicator reporting initiative and as part of our future decision making regarding any expansion of the HAC payment provision to other settings.
XVIII. Files Available to the Public Via the Internet
A. Information in Addenda Related to the CY 2010 Hospital OPPS
Addenda A and B to this final rule with comment period provide various data pertaining to the CY 2010 payment for items and services under the OPPS. Addendum A, which includes a list of all APCs payable under the OPPS, and Addendum B, which includes a list of all active HCPCS codes with their CY 2010 OPPS payment status and comment indicators, are available to the public by clicking “Hospital Outpatient Regulations and Notices” on the CMS Web site at: http://www.cms.hhs.gov/HospitalOutpatientPPS/.
For the convenience of the public, we also are including on the CMS Web site a table that displays the HCPCS code data in Addendum B sorted by APC assignment, identified as Addendum C.
Addendum D1 defines the payment status indicators that are used in Addenda A and B. Addendum D2 defines the comment indicators that are used in Addendum B. Addendum E lists the HCPCS codes that are only payable to hospitals as inpatient procedures and are not payable under the OPPS. Addendum L contains the out-migration wage adjustment for CY 2010. Addendum M lists the HCPCS codes that are members of a composite APC and identifies the composite APC to which each is assigned. This addendum also identifies the status indicator for the HCPCS code and a comment indicator if there is a change in the code's status with regard to its membership in the composite APC. Each of the HCPCS codes included in Addendum M has a single procedure payment APC, listed in Addendum B, to which it is assigned when the criteria for assignment to the composite APC are not met. When the criteria for payment of the code through the composite APC are met, one unit of the composite APC payment is paid, thereby providing packaged payment for all services that are assigned to the composite APC Start Printed Page 60660 according to the specific I/OCE logic that applies to the APC. We refer readers to the discussion of composite APCs in section II.A.2.e. of this final rule with comment period for a complete description of the composite APCs.
These addenda and other supporting OPPS data files are available on the CMS Web site at: http://www.cms.hhs.gov/HospitalOutpatientPPS/.
B. Information in Addenda Related to the CY 2010 ASC Payment System
Addenda AA and BB to this final rule with comment period provide various data pertaining to the CY 2010 payment for the covered surgical procedures and covered ancillary services for which ASCs may receive separate payment. Addendum AA lists the CY 2010 ASC covered surgical procedures, their payment indicators, and their payment rates. Addendum BB displays the CY 2010 ASC covered ancillary services, their payment indicators, and their payment rates. All ASC relative payment weights and payment rates for CY 2010 are a result of applying the revised ASC payment system methodology established in the final rule for the revised ASC payment system published in the Federal Register on August 2, 2007 (72 FR 42470 through 42548) to the CY 2010 OPPS and MPFS ratesetting information.
Addendum DD1 defines the payment indicators that are used in Addenda AA and BB. Addendum DD2 defines the comment indicators that are used in Addenda AA and BB.
Addendum EE (available only on the CMS Web site) lists the surgical procedures that are excluded from Medicare payment if furnished in ASCs. The excluded procedures listed in Addendum EE are surgical procedures that are assigned to the OPPS inpatient list, are not covered by Medicare, are reported using a CPT unlisted code, or have been determined to pose a significant safety risk or are expected to require an overnight stay when performed in ASCs.
These addenda and other supporting ASC data files are included on the CMS Web site at: http://www.cms.hhs.gov/ASCPayment/. The MPFS data files are located at: http://www.cms.hhs.gov/PhysicianFeeSched/.
The links to all of the FY 2010 IPPS wage index-related tables (that are used for the CY 2010 OPPS) that were published in the FY 2010 IPPS/LTCH PPS final rule (74 FR 44032 through 44125) are accessible on the CMS Web site at: http://www.cms.hhs.gov/AcuteInpatientPPS/WIFN.
XIX. Collection of Information Requirements
A. Legislative Requirement for Solicitation of Comments
Under the Paperwork Reduction Act of 1995, we are required to provide 30-day notice in the Federal Register and solicit public comment before a collection of information requirement is submitted to the Office of Management and Budget (OMB) for review and approval. In order to fairly evaluate whether an information collection should be approved by OMB, section 3506(c)(2)(A) of the Paperwork Reduction Act of 1995 requires that we solicit comment on the following issues:
- The need for the information collection and its usefulness in carrying out the proper functions of our agency.
- The accuracy of our estimate of the information collection burden.
- The quality, utility, and clarity of the information to be collected.
- Recommendations to minimize the information collection burden on the affected public, including automated collection techniques.
The CY 2010 OPPS/ASC proposed rule and this final rule with comment period do not specify any information collection requirements through regulatory text. However, in the proposed rule and in this final rule with comment period, we make reference to associated information collection requirements that are not discussed in the regulation text contained in this document. The following is a discussion of those requirements, for which we solicited public comment in the CY 2010 OPPS/ASC proposed rule (74 FR 35232).
B. Associated Information Collections Not Specified in Regulatory Text
1. Hospital Outpatient Quality Data Reporting Program (HOP QDRP)
As previously stated in section XVI. of this final rule with comment period, the quality data reporting program for hospital outpatient care, known as the Hospital Outpatient Quality Data Reporting Program (HOP QDRP), has been generally modeled after the quality data reporting program for hospital inpatient services, the Reporting Hospital Quality Data for Annual Payment Update (RHQDAPU) program. Section 109(a) of the MIEA–TRHCA (Pub. L. 109–432) amended section 1833(t) of the Act by adding a new subsection (17) that affects the payment rate update applicable to OPPS payments for services furnished by hospitals in outpatient settings on or after January 1, 2009. Section 1833(t)(17)(A) of the Act, which applies to hospitals as defined under section 1886(d)(1)(B) of the Act, states that subsection (d) hospitals that fail to report data required for the quality measures selected by the Secretary in the form and manner required by the Secretary under section 1833(t)(17)(B) of the Act will receive a 2.0 percentage point reduction to their annual payment update factor. Section 1833(t)(17)(B) of the Act requires that hospitals submit quality data in a form and manner, and at a time, that the Secretary specifies. Section 1833(t)(17)(C)(i) of the Act requires the Secretary to develop measures appropriate for the measurement of the quality of care (including medication errors) furnished by hospitals in outpatient settings, that these measures reflect consensus among affected parties and, to the extent feasible and practicable, that these measures include measures set forth by one or more national consensus building entities.
2. HOP QDRP Quality Measures for the CY 2010 and CY 2011 Payment Determinations
In the CY 2009 final rule with comment period (73 FR 68766), we adopted 4 claims-based imaging measures for use in CY 2010, bringing the total number to 11 measures. For the CY 2010 payment update, we are requiring hospitals to submit data related to the seven data abstracted measures; we will calculate the claims-based measures using administrative paid claims data and do not require additional hospital data submissions. Similarly, as we proposed, we are using the same 11 measures and the same data submission requirements related to the seven data abstracted measures for CY 2011 payment determinations.
| HOP QDRP measurement set to be used for CY 2010 and CY 2011 payment determination |
|---|
| OP–1: Median Time to Fibrinolysis |
| OP–2: Fibrinolytic Therapy Received Within 30 Minutes |
| OP–3: Median Time to Transfer to Another Facility for Acute Coronary Intervention |
| OP–4: Aspirin at Arrival |
| Start Printed Page 60661 |
| OP–5: Median Time to ECG |
| OP–6: Timing of Antibiotic Prophylaxis |
| OP–7: Prophylactic Antibiotic Selection for Surgical Patients |
| OP–8: MRI Lumbar Spine for Low Back Pain |
| OP–9: Mammography Follow-up Rates |
| OP–10: Abdomen CT—Use of Contrast Material |
| OP–11: Thorax CT—Use of Contrast Material |
As part of the data submission process pertaining to the 11 measures listed above, hospitals must also complete and submit a notice of participation in the HOP QDRP. By submitting this document, hospitals agree that they will allow CMS to publicly report the quality measures as required by the HOP QDRP.
The burden associated with this section is the time and effort associated with completing the notice of participation as well as collecting and submitting the data on the seven data abstracted measures. We estimate that there will be approximately 3,500 respondents per year. For hospitals to collect and submit the information on the required measures, we estimate it will take 30 minutes per sampled case. We estimate there will be a total of 1,800,000 cases per year, approximately 514 cases per respondent. The estimated annual burden associated with the aforementioned submission requirements is 900,000 hours ((1,800,000 cases/year) × (0.5 hours/case)).
We did not receive any public comments on the burden associated with these information collection requirements.
3. HOP QDRP Validation Requirements
In addition to requirements for submitting of quality data, hospitals must also comply with the requirements for data validation in CY 2011. As specified in detail in section XVI.E. of this final rule with comment period, for the CY 2011 payment determination, as we proposed, we are implementing a validation program that will require hospitals to supply requested medical documentation to a CMS contractor for purposes of validating those data. However, the results of the validation will not affect the CY 2011 payment update for any hospital, although the payment update may be affected if a hospital fails to submit the requested data. We believe that it is important for hospitals to have some experience and knowledge of the HOP QDRP validation process before payment determinations are made based upon validation results. As we proposed, we are implementing a validation program that will both limit burden upon hospitals, especially small hospitals, as well as provide feedback to all hospitals on validation performance. We are requesting medical documentation from hospitals for April 1, 2009 through March 31, 2010 episodes of care, which, with a modification for two of the quality measures, will allow us to gather one full year of submitted data for validation purposes.
The burden associated with the CY 2011 requirement is the time and effort necessary to submit validation data to a CMS contractor. We estimate that it will take each hospital approximately 38 minutes to comply with these data submission requirements. To comply with the requirements, we estimate each hospital must submit between 2 to 3 cases on average for review. We estimate that 3,200 hospitals will need to comply with these requirements in order for us to collect a total of 7,300 charts across all sampled hospitals. The estimated annual burden associated with the data validation process for CY 2011 is 2,026 hours.
Similar to our policy for the FY 2012 RHQDAPU program (74 FR 43884 through 43889), we proposed (74 FR 35403) to validate data from 800 randomly selected hospitals each year under the HOP QDRP, beginning with the CY 2012 payment determination. We note that, because the 800 hospitals will be selected randomly, every HOP QDRP-participating hospital will be eligible each year for validation selection. For each selected hospital, we will randomly validate per year up to 48 patient episodes of care (12 per quarter) from the total number of cases that the hospital successfully submitted to the OPPS Clinical Warehouse during the applicable time period. However, if a selected hospital has submitted less than 12 cases in one or more quarters, only those cases available will be validated. However, we did not adopt that proposal in this final rule with comment period. Instead, we indicated that we would take into account results of further analyses of collected data, as well as public comments we received on our proposal and propose a validation process for the CY 2012 payment rate update in next year's rulemaking process.
The burden associated with the proposed CY 2012 requirement, if we adopt it next year is the time and effort necessary to submit validation data to a CMS contractor. We estimate that it will take each of the 800 sampled hospitals approximately 12 hours to comply with these data submission requirements. To comply with the requirements, we estimate each hospital must submit 48 cases for the affected year for review. We estimate that 800 hospitals must comply with these requirements to submit a total of 38,400 charts across all sampled hospitals. The estimated annual burden associated with the data validation process for CY 2012 and subsequent years is 9,600 hours.
We discuss public comments on this information collection requirement in section XVI.E.3.b. of this final rule with comment period.
4. HOP QDRP Reconsideration and Appeals Procedures
In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68779), we adopted a mandatory reconsideration process that will apply to the CY 2010 payment decisions. As we proposed in the CY 2010 OPPS/ASC proposed rule, we will continue this process for the CY 2011 payment update. Under this process, the hospitals must meet all of the requirements specified in section XVI.G. of this final rule with comment period. We did not assign burden to the aforementioned information collection requirements in the CY 2010 OPPS/ASC proposed rule because we believed the associated information collection requirements were exempt under 5 CFR 1320.4 (that is, information collected subsequent to an administrative action is not subject to the PRA). However, upon further review, in this final rule with comment period, we are assigning burden to the reconsideration and appeals procedures. The burden associated with the reconsideration and appeals procedures is the time and effort necessary to gather the required information and submit it to CMS. The required information, as specified in section XVI.G. of this final rule with comment period, involves the submission of a completed reconsideration request form that is Start Printed Page 60662 signed by the hospital's chief financial officer. We estimate that 25 hospitals will avail themselves of the reconsideration and appeals procedures on an annual basis. We estimate that it will take each hospital approximately 40 minutes to gather the required information, complete the required reconsideration request form, obtain the signiture of the chief financial officer, and forward the documentation to CMS. The total annual estimated burden associated with these requirements is 1,000 minutes.
We did not receive any public comments on these information collection requirements.
5. Additional Topics
While we are seeking OMB approval for the information collection requirements associated with the HOP QDRP and the data validation processes, in the CY 2010 OPPS/ASC proposed rule (74 FR 35232), we also sought public comment on several issues that have the potential to ultimately affect the burden associated with HOP QDRP and the data validation processes. Specifically, that proposed rule listed the possible quality measures under consideration for CY 2012 and subsequent years. We also solicited public comments to explore the use of registries to comply with the HOP QDRP submission requirements, the use of EHRs as a data submission tool, the use of a standardized process for the retirement of HOP QDRP quality measures, the use of an extraordinary circumstance extension or waiver for reporting quality data, and the implementation of additional data validation conditions. We discussed the comments we received on these issues in section XVI. of the preamble of this final rule with comment period.
XX. Response to Comments
Because of the large number of public comments we normally receive on Federal Register documents, we are not able to acknowledge or respond to them individually. We will consider all comments we receive by the date and time specified in the DATES section of this final rule with comment period, and, when we proceed with a subsequent document(s), we will respond to those comments in the preamble to that document(s).
XXI. Regulatory Impact Analysis
A. Overall Impact
We have examined the impacts of this final rule with comment period as required by Executive Order 12866 (September 1993, Regulatory Planning and Review), the Regulatory Flexibility Act (RFA) (September 19, 1980, Pub. L. 96–354), section 1102(b) of the Social Security Act, the Unfunded Mandates Reform Act of 1995 (Pub. L. 104–4), Executive Order 13132 on Federalism, and the Congressional Review Act (5 U.S.C. 804(2)).
1. Executive Order 12866
Executive Order 12866 directs agencies to assess all costs and benefits of available regulatory alternatives and, if regulation is necessary, to select regulatory approaches that maximize net benefits (including potential economic, environmental, public health and safety effects, distributive impacts, and equity). A regulatory impact analysis (RIA) must be prepared for major rules that have economically significant effects ($100 million or more in any 1 year) or adversely affect in a material way the economy, a sector of the economy, productivity, competition, jobs, the environment, public health or safety, or State, local, or tribal government or communities (58 FR 51741).
We estimate that the effects of the OPPS provisions that are being implemented in this final rule with comment period will result in expenditures exceeding $100 million in any 1 year. We estimate the total increase (from changes in this final rule with comment period as well as enrollment, utilization, and case-mix changes) in expenditures under the OPPS for CY 2010 compared to CY 2009 to be approximately $1.9 billion. Because this final rule with comment period for the OPPS is “economically significant” as measured by the $100 million threshold and also a major rule under the Congressional Review Act, we have prepared a regulatory impact analysis that, to the best of our ability, presents the costs and benefits of this rulemaking. Table 73 of this final rule with comment period displays the redistributional impact of the CY 2010 changes on OPPS payment to various groups of hospitals.
We estimate that the effects of the ASC provisions that are being implemented in this final rule with comment period for the ASC payment system will not exceed $100 million in any 1 year and, therefore, are not economically significant. We estimate the total increase (from changes in this final rule with comment period as well as enrollment, utilization, and case-mix changes) in expenditures under the ASC payment system for CY 2010 compared to CY 2009 to be approximately $80 million. However, because this final rule with comment period for the ASC payment system substantially affects ASCs, we have prepared a regulatory impact analysis of changes to the ASC payment system that, to the best of our ability, presents the costs and benefits of this rulemaking. Table 75 and Table 76 of this final rule with comment period display the redistributional impact of the CY 2010 changes on ASC payment, grouped by specialty area and then by procedures with the greatest ASC expenditures, respectively.
2. Regulatory Flexibility Act (RFA)
The RFA requires agencies to analyze options for regulatory relief of small businesses if a rule has a significant impact on a substantial number of small entities. For purposes of the RFA, small entities include small businesses, nonprofit organizations, and small governmental jurisdictions. Many hospitals, other providers, ASCs, and other suppliers are considered to be small entities, either by being nonprofit organizations or by meeting the Small Business Administration (SBA) definition of a small business (hospitals having revenues of $34.5 million or less in any 1 year and ASCs having revenues of $10 million or less in any 1 year). (For details on the latest standards for health care providers, we refer readers the SBA's Web site at: http://sba.gov/idc/groups/public/documents/sba_homepage/serv_sstd_tablepdf.pdf (refer to the 620000 series).)
For purposes of the RFA, we have determined that many hospitals and most ASCs would be considered small entities according to the SBA size standards. Individuals and States are not included in the definition of a small entity. Therefore, the Secretary has determined that this final rule with comment period will have a significant impact on a substantial number of small entities. Because we acknowledge that many of the affected entities are small entities, the analyses presented throughout this final rule with comment period constitute our regulatory flexibility analysis. Therefore, in the CY 2010 OPPS/ASC proposed rule (74 FR 35410), we solicited public comments on our estimates and analyses of the impact of the proposed rule on those small entities.
3. Small Rural Hospitals
In addition, section 1102(b) of the Act requires us to prepare a regulatory impact analysis if a rule may have a significant impact on the operations of a substantial number of small rural hospitals. This analysis must conform to the provisions of section 603 of the RFA. With the exception of hospitals located in certain New England Start Printed Page 60663 counties, for purposes of section 1102(b) of the Act, we now define a small rural hospital as a hospital that is located outside an urban area and has fewer than 100 beds. Section 601(g) of the Social Security Amendments of 1983 (Pub. L. 98–21) designated hospitals in certain New England counties as belonging to the adjacent urban areas. Thus, for OPPS purposes, we continue to classify these hospitals as urban hospitals. We believe that the changes to the OPPS in this final rule with comment period will affect both a substantial number of rural hospitals as well as other classes of hospitals and that the effects on some may be significant. Also, the changes to the ASC payment system in this final rule with comment period will affect rural ASCs. Therefore, the Secretary has determined that this final rule with comment period will have a significant impact on the operations of a substantial number of small rural hospitals.
4. Unfunded Mandates
Section 202 of the Unfunded Mandates Reform Act of 1995 (UMRA) also requires that agencies assess anticipated costs and benefits before issuing any rule whose mandates require spending in any 1 year of $100 million in 1995 dollars, updated annually for inflation. That threshold level is currently approximately $133 million. This final rule with comment period will not mandate any requirements for State, local, or tribal governments, nor will it affect private sector costs.
5. Federalism
Executive Order 13132 establishes certain requirements that an agency must meet when it promulgates a proposed rule (and subsequent final rule) that imposes substantial direct costs on State and local governments, preempts State law, or otherwise has Federalism implications.
We have examined the OPPS and ASC provisions included in this final rule with comment period in accordance with Executive Order 13132, Federalism, and have determined that they will not have a substantial direct effect on State, local or tribal governments, preempt State law, or otherwise have a Federalism implication. As reflected in Table 73 below, we estimate that OPPS payments to governmental hospitals (including State and local governmental hospitals) will increase by 1.8 percent under this final rule with comment period. While we do not know the number of ASCs with government ownership, we anticipate that it is small. We believe that the provisions related to payments to ASCs in CY 2010 will not affect payments to any ASCs owned by government entities.
The following analysis, in conjunction with the remainder of this document, demonstrates that this final rule with comment period is consistent with the regulatory philosophy and principles identified in Executive Order 12866, the RFA, and section 1102(b) of the Act.
This final rule with comment period will affect payments to a substantial number of small rural hospitals and a small number of rural ASCs, as well as other classes of hospitals and ASCs, and some effects may be significant.
B. Effects of OPPS Changes in This Final Rule With Comment Period
We are making several changes to the OPPS that are required by the statute. We are required under section 1833(t)(3)(C)(ii) of the Act to update annually the conversion factor used to determine the APC payment rates. We also are required under section 1833(t)(9)(A) of the Act to revise, not less often than annually, the wage index and other adjustments, including pass-through payments and outlier payments. In addition, we must review the clinical integrity of payment groups and weights at least annually. Accordingly, in this final rule with comment period, we are updating the conversion factor and the wage index adjustment for hospital outpatient services furnished beginning January 1, 2010, as we discuss in sections II.B. and II.C., respectively, of this final rule with comment period. We also are revising the relative APC payment weights using claims data for services furnished from January 1, 2008, through December 31, 2008, and updated cost report information. We are continuing the current payment adjustment for rural SCHs, including EACHs. Finally, we list the 6 drugs and biologicals in Table 30 of this final rule with comment period that we are removing from pass-through payment status for CY 2010.
Under this final rule with comment period, we estimate that the update change to the conversion factor and other adjustments as provided by the statute will increase total OPPS payments by 2.1 percent in CY 2010. The changes to the APC weights, the changes to the wage indices, and the continuation of a payment adjustment for rural SCHs, including EACHs, will not increase OPPS payments because these changes to the OPPS are budget neutral. However, these updates do change the distribution of payments within the budget neutral system as shown in Table 73 below and described in more detail in this section. We also estimate that the total change in payments between CY 2010 and CY 2009, considering all payments, including changes in estimated total outlier payments and expiration of additional money for specified wages indices outside of budget neutrality, will increase total OPPS payments by 1.9 percent.
1. Alternatives Considered
Alternatives to the changes we are making and the reasons that we have chosen the options are discussed throughout this final rule with comment period. Some of the major issues discussed in this final rule with comment period and the options considered are discussed below.
a. Alternatives Considered for Pass-Through Payment for Implantable Biologicals
We are finalizing our proposal to change the way we evaluate transitional pass-through applications for implantable biologicals and the way we pay for implantable biologicals newly eligible for transitional pass-through status beginning in CY 2010. As discussed in detail in section V.A.4. of this final rule with comment period, we are finalizing a policy that the pass-through evaluation process and pass-through payment methodology for implantable biologicals that are surgically inserted or implanted (through a surgical incision or a natural orifice) and that are newly approved for pass-through payment beginning on or after January 1, 2010, be the device pass-through process and payment methodology only. As a result, implantable biologicals that are surgically inserted or implanted (through a surgical incision or a natural orifice) will no longer be eligible to submit biological pass-through applications and to receive biological pass-through payment at ASP+6 percent. Rather, implantable biologicals that are eligible for device pass-through payment will be paid based on the charges-adjusted-to-cost methodology used for all pass-through device categories.
We considered three alternatives for the pass-through evaluation process and payment methodology for implantable biologicals that are surgically inserted or implanted (through a surgical incision or a natural orifice), as indicated in the CY 2010 OPPS/ASC proposed rule (74 FR 35411). The first alternative we considered was to make no change to the current pass-through evaluation process and payment methodology for Start Printed Page 60664 implantable biologicals that are surgically inserted or implanted. We did not select this alternative because this approach would continue the separate pass-through evaluation processes and payment methodologies for implantable biologicals and implantable nonbiological devices that are sometimes used for the same clinical indications, and where the implantable biologicals are often FDA-approved as devices. Moreover, under our current policy, implantable biologicals could potentially have two periods of pass-through payment, one as a biological and one as a device. We believe that it is most appropriate for a product to be eligible for a single period of OPPS pass-through payment, rather than a period of device pass-through payment and a period of drug or biological pass-through payment.
The second alternative we considered was to add a criterion requiring the demonstration of substantial clinical improvement to the biological pass-through evaluation process in order for a biological to be approved for pass-through payment. This alternative would provide pass-through payment only for those biologicals that demonstrate clinical superiority, consistent with the pass-through evaluation process for devices, and ensure that a product could receive only one period of pass-through payment. We did not choose this alternative because this approach would continue the different pass-through payment methods for implantable biological and nonbiological devices. Pass-through payment for biologicals is made at ASP+6 percent as required for drug and biological pass-through payment, while pass-through devices are paid at charges adjusted to cost. Therefore, this second alternative would result in continued inconsistent pass-through payment methodologies for biological and nonbiological devices that may substitute for one another.
The third alternative we considered and the one we are adopting for CY 2010 is to provide that, beginning in CY 2010, the pass-through evaluation process and pass-through payment methodology for implantable biologicals that are surgically inserted or implanted (through a surgical incision or a natural orifice) be the device pass-through process and payment methodology only. As we discuss in section V.A.4. of this final rule with comment period, after consideration of the public comments we received on the proposed rule, we are adopting this alternative because we believe that a consistent pass-through payment policy is to evaluate all such devices, both biological and nonbiological, under the device pass-through process. We believe that implantable biologicals that function as and may be substitutes for implantable devices are most similar to devices because of their required surgical insertion or implantation, and that it would be appropriate to evaluate them as devices because they share significant clinical similarity with implantable nonbiological devices.
b. Alternatives Considered for Payment of the Acquisition and Pharmacy Overhead Costs of Drugs and Biologicals That Do Not Have Pass-Through Status
For CY 2010, we are finalizing a transition payment for separately payable drugs and biologicals at ASP+4 percent, which will continue to represent combined payment for both the acquisition and pharmacy overhead costs of separately payable drugs and biologicals. As discussed in detail in section V.B.3. of this final rule with comment period, we are redistributing $200 million total of packaged drug cost ($150 million of the pharmacy overhead cost currently attributed to coded packaged drugs and biologicals with an ASP and $50 million of the estimated pharmacy overhead cost currently attributed to uncoded packaged drugs and biologicals) to separately payable drugs and biologicals to provide an adjustment for the pharmacy overhead costs of these separately payable products. As a result, we are proportionally reducing the cost of packaged drugs and biologicals that is included in the separate payment for procedural APCs to offset the $200 million adjustment to provide payment for separately payable drugs and biologicals at ASP+4 percent. We received favorable public comments on our proposal to redistribute cost within drugs and biologicals to adjust payment for separately payable drugs and biologicals. The public commenters also agreed that our estimated total cost for all drugs and biologicals in our claims data is accurate. Therefore, we are redistributing a portion of pharmacy overhead cost in the CY 2010 final rule claims data from some packaged drugs and biologicals to separately payable drugs and biologicals. A redistribution within drug cost maintains the estimated total cost of drugs and biologicals under the OPPS.
We considered three alternatives for payment of the acquisition and pharmacy overhead costs of drugs and biologicals that do not have pass-through status for CY 2010. The first alternative we considered was to continue our standard policy of comparing the estimated aggregate cost of separately payable drugs and biologicals in our claims data to the estimated aggregate ASP dollars for separately payable drugs and biologicals, using the ASP as a proxy for average acquisition cost, to calculate the estimated percent of ASP that would serve as the proxy for the combined acquisition and pharmacy overhead costs of separately payable drugs and biologicals (70 FR 68642). Under this standard methodology, using July 2009 ASP information and updated final rule costs derived from CY 2008 OPPS claims data, we estimated the combined acquisition and pharmacy overhead costs of separately payable drugs and biologicals to be ASP–3 percent. We also determined the combined acquisition and pharmacy overhead costs of coded packaged drugs and biologicals with an ASP to be 258 percent of ASP. As discussed in section V.B.3. of this final rule with comment period, we did not choose this alternative because we believe that this analysis indicates that our standard drug payment methodology has the potential to “compress” the calculated costs of separately payable drugs and biologicals and inflate the calculated costs of packaged drugs and biologicals to some degree. Further, we recognize that the attribution of pharmacy overhead costs to packaged or separately payable drugs and biologicals through our standard drug payment methodology of a combined payment for acquisition and pharmacy overhead costs also depends on the determination of separate or packaged payment for all drugs and biologicals each year based on our annual drug packaging threshold. Changes to the packaging threshold and the packaged status of drugs or biologicals may result in changes to the estimated combined acquisition and pharmacy overhead costs of drugs and biologicals that do not reflect actual changes in hospital pharmacy overhead cost for those products.
The second alternative we considered was to adopt the February 2009 APC Panel recommendation to accept the pharmacy stakeholders' recommended methodology for payment of drugs and biologicals that do not have pass-through status. This recommended methodology would establish ASP+6 percent as the cost of packaged drugs and biologicals, including all pharmacy overhead costs; establish ASP+6 percent as the acquisition cost of separately payable drugs and biologicals with some overhead cost included; and reallocate the residual cost of packaged drugs and biologicals currently reflected in the claims data across three categories of Start Printed Page 60665 pharmacy overhead cost that would then be paid separately for each administration of separately payable drugs and biologicals in CY 2010. The pharmacy stakeholders recommended that we pay the pharmacy overhead amount specific to the overhead category to which a drug or biological is assigned, in addition to the ASP+6 percent payment for the separately payable drug or biological, each time a separately payable drug or biological is administered. We refer readers to section V.B.3. of this final rule with comment period for a more detailed discussion of the pharmacy stakeholders' recommended methodology. We did not choose this alternative because we do not believe that ASP+6 percent would pay appropriately for the acquisition and pharmacy overhead costs of packaged drugs. We believe the amount of redistribution of pharmacy overhead costs from packaged to separately payable drugs and biologicals incorporated in the recommendation of the pharmacy stakeholders would be too great. In addition, we do not believe that it would be appropriate to establish separate payment for pharmacy overhead costs, thereby unbundling payment for the acquisition and overhead costs of separately payable drugs and biologicals when hospitals report a single charge for these products that represents both types of costs. For these reasons, we are not accepting the APC Panel recommendation to adopt the pharmacy stakeholders' recommended methodology.
The third alternative we considered and the one we selected for CY 2010 is to make a transition payment for nonpass-through separately payable drugs and biologicals at ASP+4 percent, which will continue to represent a combined payment for both the acquisition costs of separately payable drugs and biologicals and the pharmacy overhead costs applicable to these products. We also are reducing the cost of packaged drugs and biologicals that is included in the payment for procedural APCs to offset the $200 million adjustment to payment for separately payable drugs and biologicals. The $200 million consists of $150 million (one-third of the pharmacy overhead cost) from the cost of coded packaged drugs and biologicals with an ASP and $50 million from the uncoded packaged drug and biological cost. To model this policy for the CY 2010 final rule with comment period, we reduced the cost of coded packaged drugs and biologicals with an ASP by 24 percent (based on final rule data; the reduction was 27 percent based on proposed rule data) and the cost of uncoded packaged drugs and biologicals by 8 percent when we calculated the median costs of the CY 2010 procedural APCs. We chose this transition alternative because we believe that it provides an appropriate redistribution of pharmacy overhead costs associated with drugs and biologicals and is consistent with the principles of a prospective payment system.
c. Alternatives Considered for the Physician Supervision of Hospital Outpatient Services
We are revising or further defining several policies related to the physician supervision of services in the HOPD for CY 2010. We refer readers to section XII.D. of this final rule with comment period for the full discussion of these policies. Specifically, for the CY 2010 OPPS, we are revising our existing policy that requires direct supervision to be provided by a physician to allow, when statutorily permitted under the Social Security Act, specified nonphysician practitioners to supervise the hospital outpatient therapeutic services that they are able to personally perform within their State scope of practice and hospital-granted privileges. We note that section 144(a)(1) of Public Law 100–275 imposes strict requirements for the direct physician supervision of PR, CR, and ICR services and gives us no flexibility to modify the requirement beyond direct physician supervision by a doctor of medicine or a doctor of osteopathy. We also are establishing a policy for hospital outpatient therapeutic services furnished in the main hospital buildings or in on-campus provider-based departments (PBDs) that “direct supervision” means that the supervisory practitioner must be on the same campus and immediately available to furnish assistance and direction throughout the performance of the procedure. In addition, we are establishing in regulations a policy that applies the MPFS physician supervision requirements for diagnostic tests to all hospital outpatient diagnostic tests performed directly by the hospital or under arrangement.
We considered three alternatives for the physician supervision of hospital outpatient services for CY 2010 in the CY 2010 OPPS/ASC proposed rule (74 FR 35412 through 35413). The first alternative we considered was to make no changes to the existing supervision policies for hospital outpatient therapeutic and diagnostic services and to provide no new policy guidance in this area. This approach would require hospitals to ensure that only physicians supervise services that may currently be ordered or performed by nonphysician practitioners within their State scope of practice and hospital-granted privileges. Hospitals would not receive payment for outpatient services for which they were unable to provide supervision by a physician. In addition, there could continue to be confusion regarding what “direct supervision” means for services provided in an area of the hospital that may not be a PBD of the hospital. Lastly, there would be potential for misunderstanding regarding the appropriate level of physician supervision required for hospital outpatient diagnostic services without a clearly stated policy, codified in regulations, that would apply the same level of physician supervision to all hospital outpatient diagnostic services, whether provided directly or under arrangement, as applies to those services currently furnished in physicians' offices and independent diagnostic testing facilities. We did not choose this alternative because we believe that it is important to address the issues outlined above, including areas of potential confusion or limited current policy guidance, to ensure that hospitals are able to comply with the hospital outpatient supervision requirements while providing access to care for Medicare beneficiaries.
The second alternative we considered was to permit specified nonphysician practitioners to supervise the hospital outpatient therapeutic services that they are able to personally perform within their State scope of practice and hospital-granted privileges, but to make no changes that would provide clearer statements of policy regarding other concerns raised by hospitals regarding physician supervision for hospital outpatient therapeutic and diagnostic services. We did not choose this alternative because we believe it is important to clearly specify the policies that apply to the supervision of both therapeutic and diagnostic services in all hospital outpatient settings in order to ensure the safety and effectiveness of hospital outpatient services furnished to Medicare beneficiaries.
The third alternative we considered and the one we selected for CY 2010 was to revise our existing policy to permit specified nonphysician practitioners, when statutorily permitted in the Social Security Act, to supervise the services that they are able to personally perform within their State scope of practice and hospital-granted privileges; to establish a specific definition of “direct supervision” for hospital outpatient therapeutic services Start Printed Page 60666 furnished in the hospital or in on-campus PBDs that was consistent for services furnished by the hospital on-campus; and to apply the MPFS supervision requirements for diagnostic tests to all hospital outpatient diagnostic tests provided directly by the hospital or under arrangement. We selected this alternative because we believe that it is appropriate that, unless the Act imposes strict requirements for the direct supervision of certain services, such as PR, CR, and ICR services, a licensed nonphysician practitioner who may bill and be paid by Medicare for the practitioner's professional services should be able to supervise the therapeutic services that he or she may personally perform within his or her State scope of practice and hospital-granted privileges. Furthermore, we believe that it is necessary and appropriate to establish consistent and operationally feasible policies regarding the supervision requirements for hospital outpatient therapeutic and diagnostic services in order to ensure safe and effective health care services for Medicare beneficiaries. We refer readers to section XII.D. of this final rule with comment period for a complete discussion of the final physician supervision policies.
2. Limitations of Our Analysis
The distributional impacts presented here are the projected effects of the CY 2010 policy changes on various hospital groups. We post on the CMS Web site our hospital-specific estimated payments for CY 2010 with the other supporting documentation for this final rule with comment period. To view the hospital-specific estimates, we refer readers to the CMS Web site at: http://www.cms.hhs.gov/HospitalOutpatientPPS/. Select “regulations and notices” from the left side of the page and then select “CMS–1414–P” from the list of regulations and notices. The hospital-specific file layout and the hospital-specific file are listed with the other supporting documentation for this final rule with comment period. We show hospital-specific data only for hospitals whose claims were used for modeling the impacts shown in Table 73 below. We do not show hospital-specific impacts for hospitals whose claims we were unable to use. We refer readers to section II.A.2. of this final rule with comment period for a discussion of the hospitals whose claims we do not use for ratesetting and impact purposes.
We estimate the effects of the individual policy changes by estimating payments per service, while holding all other payment policies constant. We use the best data available, but do not attempt to predict behavioral responses to our policy changes. In addition, we do not make adjustments for future changes in variables such as service volume, service mix, or number of encounters. As we have done in previous rules, in the CY 2010 OPPS/ASC proposed rule (74 FR 35413), we solicited public comment and information about the anticipated effects of our proposed changes on providers and our methodology for estimating them.
We received many public comments on the proposed changes to payment policies and to proposed payment rates for the CY 2010 OPPS. We have summarized these public comments and provided our responses to them in other sections of this final rule with comment period as part of our discussions of the specific topics to which the comments pertained. We did not receive any public comments on our methodology for estimating the anticipated effects of our proposed changes on providers or other parties.
3. Estimated Effects of This Final Rule With Comment Period on Hospitals
Table 73 below shows the estimated impact of this final rule with comment period on hospitals. Historically, the first line of the impact table, which estimates the change in payments to all hospitals, has always included cancer and children's hospitals, which are held harmless to their pre-BBA payment-to-cost ratio. We also are including CMHCs in the first line that includes all providers because we included CMHCs in our weight scaler estimate.
We present separate impacts for CMHCs in Table 73 because CMHCs are paid under only two APCs for services under the OPPS: APC 0172 (Level 1 Partial Hospitalization (3 services)) and APC 0173 (Level II Partial Hospitalization (4 or more services)). We note that CMHS are also a different provider type. We discuss the impact on CMHCs in section XXI.B.4. of this final rule with comment period.
The estimated increase in the total payments made under the OPPS is limited by the increase to the conversion factor set under the methodology in the statute. The distributional impacts presented do not include assumptions about changes in volume and service mix. The enactment of Public Law 108–173 on December 8, 2003, provided for the additional payment outside of the budget neutrality requirement for wage indices for specific hospitals reclassified under section 508. Public Law 108–173 extended section 508 reclassifications through September 30, 2008. Section 124 of Public Law 110–275 further extended section 508 reclassifications through September 30, 2009. The amounts attributable to these reclassifications are incorporated into the CY 2009 estimates in Table 73.
Table 73 shows the estimated redistribution of hospital and CMHC payments among providers as a result of APC reconfiguration and recalibration; wage indices; the combined impact of the APC recalibration, wage effects, and the market basket update to the conversion factor; and, finally, estimated redistribution considering all payments for CY 2010 relative to all payments for CY 2009, including the impact of changes in the outlier threshold, expiring section 508 wage indices, and changes to the pass-through payment estimate. We did not model an explicit budget neutrality adjustment for the rural adjustment for SCHs because we are not making any changes to the policy for CY 2010. Because the updates to the conversion factor, including the update of the market basket and the subtraction of additional money dedicated to pass-through payment for CY 2010, are applied uniformly across services, observed redistributions of payments in the impact table for hospitals largely depend on the mix of services furnished by a hospital (for example, how the APCs for the hospital's most frequently furnished services will change), and the impact of the wage index changes on the hospital. However, total payments made under this system and the extent to which this final rule with comment period will redistribute money during implementation also will depend on changes in volume, practice patterns, and the mix of services billed between CY 2009 and CY 2010 by various groups of hospitals, which CMS cannot forecast.
Overall, the final OPPS rates for CY 2010 will have a positive effect for providers paid under the OPPS, resulting in a 1.9 percent estimated increase in Medicare payments. Removing cancer and children's hospitals, because their payments are held harmless to the pre-BBA ratio between payment and cost, and CMHCs suggests that these changes also will result in a 1.9 percent estimated increase in Medicare payments to all other hospitals.
To illustrate the impact of the final CY 2010 changes, our analysis begins with a baseline simulation model that uses the final CY 2009 weights, the FY 2009 final post-reclassification IPPS wage indices, and the final CY 2009 conversion factor. Column 2 in Table 73 Start Printed Page 60667 shows the independent effect of the changes resulting from the reclassification of services among APC groups and the recalibration of APC weights, based on 12 months of CY 2008 OPPS hospital claims data and the most recent cost report data. We modeled the effect of the APC recalibration changes for CY 2010 by varying only the weights (the final CY 2009 weights versus the final CY 2010 weights calculated using the service mix and volume in the CY 2008 claims used for this final rule with comment period) and calculating the percent difference in weight. Column 2 also reflects the effect of the changes resulting from the APC reclassification and recalibration changes and any changes in multiple procedure discount patterns or conditional packaging that occur as a result of the changes in the relative magnitude of payment weights.
Column 3 reflects the independent effects of the updated wage indices, including the application of budget neutrality for the rural floor policy on a statewide basis. We did not model a budget neutrality adjustment for the rural adjustment for SCHs because we are making no changes to the policy for CY 2010. We modeled the independent effect of updating the wage indices by varying only the wage indices, holding APC relative weights, service mix, and the rural adjustment constant and using the CY 2010 scaled weights and a CY 2009 conversion factor that included a budget neutrality adjustment for the effect of changing the wage indices between CY 2009 and CY 2010.
Column 4 demonstrates the combined “budget neutral” impact of APC recalibration (that is, Column 2), the wage index update (that is, Column 3), as well as the impact of updating the conversion factor with the market basket update. We modeled the independent effect of the budget neutrality adjustments and the market basket update by using the weights and wage indices for each year, and using a CY 2009 conversion factor that included the market basket update and a budget neutrality adjustment for differences in wage indices.
Finally, Column 5 depicts the full impact of the CY 2010 policies on each hospital group by including the effect of all the changes for CY 2010 (including the APC reconfiguration and recalibration shown in Column 2) and comparing them to all estimated payments in CY 2009 (these CY 2009 estimated payments include the payments resulting from the non-budget neutral increases to wage indices under section 508 of Pub. L. 108–173 as extended by Pub. L. 110–275). Column 5 shows the combined budget neutral effects of Columns 2 through 4, plus the impact of the change to the fixed-dollar outlier threshold from $1,800 to $2,175; the impact of the expiration of section 508 reclassifications; the change in the HOP QDRP payment reduction for the small number of hospitals in our impact model that failed to meet the reporting requirements; and the impact of increasing the estimate of the percentage of total OPPS payments dedicated to transitional pass-through payments. We discuss our CY 2010 change to the outlier threshold in section II.F. of this final rule with comment period. Of the 85 hospitals that failed to meet the HOP QDRP reporting requirements for the full CY 2009 update (and assumed, for modeling purposes, to be the same number for CY 2010), we included 28 in our model because they had both CY 2008 claims data and recent cost report data. We estimate that the cumulative effect of all changes for CY 2010 will increase payments to all providers by 1.9 percent for CY 2010. We modeled the independent effect of all changes in Column 5 using the final weights for CY 2009 and the final weights for CY 2010. We used the final conversion factor for CY 2009 of $66.059 and the final CY 2010 conversion factor of $67.406. Column 5 also contains simulated outlier payments for each year. We used the charge inflation factor used in the FY 2010 IPPS/RY 2010 LTCH PPS final rule of 6.86 percent (1.0686) to increase individual costs on the CY 2008 claims, and we used the most recent overall CCR in the July 2009 Outpatient Provider-Specific File (OPSF) (74 FR 44010). Using the CY 2008 claims and a 6.86 percent charge inflation factor, we currently estimate that outlier payments for CY 2009, using a multiple threshold of 1.75 and a fixed-dollar threshold of $1,800, will be approximately 1.03 percent of total payments. Outlier payments of 1.03 percent are incorporated in the CY 2009 comparison in Column 5. We used the same set of claims and a charge inflation factor of 14.18 percent (1.1418) and the CCRs in the July 2009 OPSF, with an adjustment of 0.9880 to reflect relative changes in cost and charge inflation between CY 2008 and CY 2010, to model the CY 2010 outliers at 1.0 percent of total payments using a multiple threshold of 1.75 and a fixed-dollar threshold of $2,175.
Column 1: Total Number of Hospitals
The first line in Column 1 in Table 73 shows the total number of providers (4,222), including cancer and children's hospitals and CMHCs for which we were able to use CY 2008 hospital outpatient claims to model CY 2009 and CY 2010 payments, by classes of hospitals. We excluded all hospitals for which we could not accurately estimate CY 2009 or CY 2010 payment and entities that are not paid under the OPPS. The latter entities include CAHs, all-inclusive hospitals, and hospitals located in Guam, the U.S. Virgin Islands, Northern Mariana Islands, American Samoa, and the State of Maryland. This process is discussed in greater detail in section II.A. of this final rule with comment period. At this time, we are unable to calculate a disproportionate share (DSH) variable for hospitals not participating in the IPPS. Hospitals for which we do not have a DSH variable are grouped separately and generally include freestanding psychiatric hospitals, rehabilitation hospitals, and long-term care hospitals. We show the total number (3,942) of OPPS hospitals, excluding the hold-harmless cancer and children's hospitals and CMHCs, on the second line of the table. We excluded cancer and children's hospitals because section 1833(t)(7)(D) of the Act permanently holds harmless cancer hospitals and children's hospitals to a proportion of their pre-BBA payment relative to their pre-BBA costs and, therefore, we removed them from our impact analyses. We show the isolated impact on 221 CMHCs in the last row of the impact table and discuss that impact separately below.
Column 2: APC Changes Due to Reassignment and Recalibration
This column shows the combined effects of the reconfiguration, recalibration, and other policies (such as setting payment for separately payable drugs and biologicals at ASP+4 percent with an accompanying reduction in the amount of cost associated with packaged drugs and biologicals, payment for brachytherapy sources based on median unit cost, and changes in payment for PHP services. Overall, we estimate that changes in APC reassignment and recalibration across all services paid under the OPPS will increase payments to urban hospitals by 0.1 percent. We estimate that both large and other urban hospitals will see an increase of 0.1 percent, all attributable to recalibration. We estimate that urban hospitals billing fewer than 11,000 lines for OPPS services will experience decreases of 0.2 to 1.0 percent, while urban hospitals billing 11,000 or more lines for OPPS services will see no change or an increase of 0.1 percent.
Overall, we estimate that rural hospitals will experience a decrease of 0.1 percent as a result of changes to the APC structure. We estimate that rural Start Printed Page 60668 hospitals of all bed sizes will experience no change or decreases of up to 0.2 percent as a result of APC recalibration. We estimate that rural hospitals that report fewer than 5,000 lines for OPPS services will experience a decrease of 0.8 percent, while rural hospitals that report more than 5,000 lines for OPPS services will see decreases of 0.1 percent to 0.4 percent.
Among teaching hospitals, we estimate that the largest observed impact resulting from APC recalibration will include an increase of 0.2 percent for major teaching hospitals and a increase of 0.1 percent for minor teaching hospitals.
Classifying hospitals by type of ownership suggests that proprietary and governmental hospitals will see no change, and voluntary hospitals will see an estimated increase of 0.1 percent.
Finally, we estimate that hospitals for which DSH payments are not available will experience decreases of 2.5 to 2.7 percent that are largely attributable to the reduction in PHP payment for APC 0172. We estimate that most other classes of hospitals will not experience any change from CY 2009 to CY 2010 or will experience a modest increase.
Column 3: New Wage Indices and the Effect of the Rural Adjustment
This column estimates the impact of applying the FY 2010 IPPS wage indices for the CY 2010 OPPS. We are not changing the rural payment adjustment for CY 2010. We estimate that the combination of updated wage data and statewide application of rural floor budget neutrality will redistribute payment among regions. We also updated the list of counties qualifying for the section 505 out-migration adjustment. Overall, we estimate that urban hospitals will not experience any change from CY 2009 to CY 2010, and that rural hospitals will experience a decrease of 0.1 percent as a result of the updated wage indices. However, we estimate that hospitals in rural New England States and rural West South Central States will experience decreases of 0.9 and 0.7 percent, respectively. We estimate that urban and rural Mountain States will experience increases of 0.6 percent.
Column 4: All Budget Neutrality Changes and Market Basket Update
We estimate that the addition of the market basket update of 2.1 percent will mitigate any negative impacts on hospital payments for CY 2010 created by the budget neutrality adjustments made in Columns 2 and 3, with the exception of hospitals not paid under the IPPS, including freestanding psychiatric, rehabilitation, and long-term care hospitals, that we estimate will continue to experience decreases of between -0.6 and -0.7 percent. In general, Column 4 shows that all hospitals will experience an estimated increase of 2.1 percent, attributable to the 2.1 percent market basket increase.
Overall, we estimate that these changes will increase payments to urban hospitals by 2.2 percent. We estimate that large urban hospitals will experience an increase of 2.3 percent, and “other” urban hospitals will experience a 2.1 percent increase.
Overall, we estimate that rural hospitals will experience a 1.9 percent increase as a result of the market basket update and other budget neutrality adjustments. We estimate that rural hospitals that bill less than 5,000 lines of OPPS services will experience an increase of 1.5 percent and that rural hospitals that bill more than 5,000 lines of OPPS services will experience increases of 1.8 to 1.9 percent.
Among teaching hospitals, we estimate that the observed impacts resulting from the market basket update and other budget neutrality adjustments will include an increase of 2.4 and 2.2 percent, respectively, for major and minor teaching hospitals.
Classifying hospitals by type of ownership suggests that voluntary, proprietary, and governmental hospitals will experience estimated increases of 2.2 percent, 2.1 percent, and 2.0 percent, respectively.
Column 5: All Changes for CY 2010
Column 5 compares all estimated changes for CY 2010 to estimated final payment for CY 2009, including the expiration of the reclassifications under section 508, the change in the outlier threshold, payment reductions for hospitals that failed to meet the HOP QDRP reporting requirements, and the difference in pass-through estimates that are not included in the combined percentages shown in Column 4. This column includes estimated payment for a handful of hospitals receiving reduced payment because they did not meet their hospital outpatient quality measure reporting requirements; however, we estimate that the anticipated change in payment between CY 2009 and CY 2010 for these hospitals will be negligible. Overall, we estimate that providers will experience an increase of 1.9 percent under this final rule with comment period in CY 2010 relative to total spending in CY 2009. The projected 1.9 percent increase for all providers in Column 5 of Table 73 reflects the 2.1 percent market basket increase, less 0.03 percent for the change in the pass-through estimate between CY 2009 and CY 2010, less 0.03 percent for the difference in estimated outlier payments between CY 2009 (1.03 percent) and CY 2010 (1.0 percent), and less 0.14 percent due to the expiration of the special, non-budget neutral wage index payments made under section 508. We estimate that when we exclude cancer and children's hospitals (which are held harmless to their pre-OPPS costs) and CMHCs, the increase remains at 1.9 percent.
We estimate that the combined effect of all changes for CY 2010 will increase payments to urban hospitals by 2.0 percent. We estimate that large urban hospitals will experience a 2.1 percent increase, while “other” urban hospitals will experience an increase of 1.9 percent. We estimate that urban hospitals that bill less than 5,000 lines of OPPS services will experience an increase of 1.2 percent, and we estimate that all urban hospitals that bill more than 5,000 lines of OPPS services will experience increases between 1.9 percent and 2.0 percent.
Overall, we estimate that rural hospitals will experience a 1.6 percent increase as a result of the combined effects of all changes for CY 2010. We estimate that rural hospitals that bill less than 5,000 lines of OPPS services will experience an increase of 1.3 percent and rural hospitals that bill greater than 5,000 lines of OPPS services will experience increases ranging from 1.4 percent to 1.8 percent.
Among teaching hospitals, we estimate that the impacts resulting from the combined effects of all changes will include an increase of 2.0 percent for major teaching hospitals and an increase of 1.9 percent for minor teaching hospitals.
Classifying hospitals by type of ownership, we estimate that proprietary hospitals will gain 2.0 percent, governmental hospitals will experience an increase of 1.8 percent, and voluntary hospitals will experience an increase of 1.9 percent.
4. Estimated Effects of This Final Rule With Comment Period on CMHCs
The last row of the impact analysis in Table 73 demonstrates the impact on CMHCs. We modeled this impact assuming that CMHCs will continue to provide the same number of days of PHP care, with each day having either three services or four or more services, as seen in the CY 2008 claims data. We excluded days with one or two services. Using these assumptions, we estimate that there would be a 5.0 percent decrease in payments to CMHCs due to these APC policy changes (shown in Start Printed Page 60669 Column 2). The relative weight for low intensity partial hospitalization APC 0172 (Level 1 Partial Hospitalization (3 services)) declines between CY 2009 and CY 2010 under this final rule with comment period. CMHCs perform a greater proportion of low intensity partial hospitalization days than freestanding psychiatric hospitals. Table 73 demonstrates our estimate that hospitals not paid under the IPPS for which a disproportionate patient percentage is not available (DSH Not Available), consisting largely of freestanding psychiatric hospitals, will experience a more moderate decline in payments of 2.7 percent. Psychiatric hospitals provide a greater proportion of APC 0173 (Level II Partial Hospitalization (4 or more services)) for which the relative weight increases between CY 2009 and CY 2010 under this final rule with comment period.
Column 3 shows that the estimated impact of adopting the CY 2010 wage index values will be no change in payments to CMHCs. We note that all providers paid under the OPPS, including CMHCs, will receive a 2.1 percent market basket increase. Combining this market basket increase, along with changes in APC policy for CY 2010 and the CY 2010 wage index updates, and changes in outlier payments, we estimate that the combined impact on CMHCs for CY 2010 will be a 3.0 percent decrease.
Start Printed Page 60670 Start Printed Page 60671 Start Printed Page 60672 Start Printed Page 606735. Estimated Effect of This Final Rule With Comment Period Rule on Beneficiaries
For services for which the beneficiary pays a copayment of 20 percent of the payment rate, the beneficiary share of payment will increase for services for which the OPPS payments will rise and will decrease for services for which the OPPS payments will fall. For example, for a service assigned to Level IV Needle Biopsy/Aspiration Except Bone Marrow (APC 0037) in the CY 2009 OPPS, the national unadjusted copayment is $228.76, and the minimum unadjusted copayment is $178.60. For CY 2010, the national unadjusted copayment for APC 0037 will be $228.76, the same rate in effect for CY 2009. The minimum unadjusted copayment for APC 0037 will be $208.97 or 20 percent of the CY 2010 national unadjusted payment rate for APC 0037 of $1,044.81. The minimum unadjusted copayment will rise because the payment rate for APC 0037 will rise for CY 2010. In all cases, the statute limits beneficiary liability for copayment for a procedure to the hospital inpatient deductible for the applicable year. The CY 2009 hospital inpatient deductible is $1,068. The CY 2010 hospital inpatient deductible is $1,100.
In order to better understand the impact of changes in copayment on beneficiaries, we modeled the percent change in total copayment liability using CY 2008 claims. We estimate, using the claims of the 4,222 hospitals and CMHCs on which our modeling is based, that total beneficiary liability for copayments will decline as an overall percentage of total payments, from 23.0 percent in CY 2009 based on updated claims data for this final rule with comment period to 22.6 percent in CY 2010.
6. Conclusion
The changes in this final rule with comment period will affect all classes of hospitals and CMHCs. We estimated that some classes of hospitals will experience significant gains and others less significant gains, but all classes of hospitals will experience positive updates in OPPS payments in CY 2010 with one exception. We estimate that hospitals not paid under the IPPS will see an overall decrease in payment of 0.6 to 0.8 percent because they are largely freestanding psychiatric hospitals that bill mostly PHP services. As we discuss in substantial detail in section X. of this final rule with comment period, payment for APC 0172 will decline for CY 2010 and, therefore, we estimate that payments to CMHCs and hospitals that furnish mostly PHP services will also decline. In general, we estimate that CMHCs will experience an overall decline of 3.0 percent in total payment due to the recalibration of the payment rates.
Table 73 demonstrates the estimated distributional impact of the OPPS budget neutrality requirements that will result in a 1.9 percent increase in payments for all services paid under the OPPS in CY 2010, after considering all changes to APC reconfiguration and recalibration, as well as the market basket increase, wage index changes, estimated payment for outliers, and changes to the pass-through payment estimate. The accompanying discussion, in combination with the rest of this final rule with comment period, constitutes a regulatory impact analysis.
7. Accounting Statement
As required by OMB Circular A–4 (available at http://www.whitehouse.gov/omb/circulars/a004/a-4.pdf), in Table 74, we have prepared an accounting statement showing the CY 2010 estimated hospital OPPS incurred benefit impact associated with the CY 2010 hospital outpatient market basket update shown in this final rule with comment period based on the baseline for the 2009 Trustees Report. All estimated impacts are classified as transfers.
| Category | Transfers |
|---|---|
| Annualized Monetized Transfers | $0.5 billion. |
| From Whom to Whom | Federal Government to outpatient hospitals and other providers who received payment under the hospital OPPS. |
| Total | $0.5 billion. |
C. Effects of ASC Payment System Changes in This Final Rule With Comment Period Rule
On August 2, 2007, we published in the Federal Register the final rule for the revised ASC payment system, effective January 1, 2008 (72 FR 42470). In that final rule, we adopted the methodologies to set payment rates for covered ASC services to implement the revised payment system so that it would be designed to result in budget neutrality as required by section 626 of Public Law 108–173; established that the OPPS relative payment weights would be the basis for payment and that we would update the system annually as part of the OPPS rulemaking cycle; and provided that the revised ASC payment rates would be phased-in over 4 years. During the 4-year transition to full implementation of the ASC payment rates, payments for surgical procedures paid in ASCs in CY 2007 are made using a blend of the CY 2007 ASC payment rate and the ASC payment rate calculated according to the ASC standard ratesetting methodology for the applicable transitional year. In CY 2009, we are paying ASCs using a 50/50 blend, in which payment is calculated by adding 50 percent of the CY 2007 ASC rate for a surgical procedure on the CY 2007 ASC list of covered surgical procedures and 50 percent of the CY 2009 ASC rate calculated according to the ASC standard ratesetting methodology for the same procedure. For CY 2010, we are transitioning the blend to a 25/75 blend of the CY 2007 ASC rate and the ASC payment rate calculated according to the ASC standard ratesetting methodology. Beginning in CY 2011, we will pay ASCs for all covered surgical procedures, including those on the CY 2007 ASC list, at the ASC payment rates calculated according to the ASC standard ratesetting methodology. Payment for procedures that were not included on the ASC list of covered surgical procedures in CY 2007 is not subject to the transitional payment methodology.
ASC payment rates are calculated by multiplying the ASC conversion factor by the ASC relative payment weight. As discussed fully in section XV. of this final rule with comment period, we set the CY 2010 ASC relative payment weights by scaling CY 2010 ASC relative payment weights by the ASC scaler of 0.9567. These weights take into consideration the 25/75 blend for the Start Printed Page 60674 third year of transitional payment for certain services. If there were no transition, the scaler for the CY 2010 relative payment weights would be 0.9338. The estimated effects of the updated relative payment weights on payment rates during this transitional period are varied and are reflected in the estimated payments displayed in Tables 75 and 78 below.
The CY 2010 ASC conversion factor was calculated by adjusting the CY 2009 ASC conversion factor to account for changes in the pre-floor and pre-reclassified hospital wage indices between CY 2009 and CY 2010 and by applying the CY 2010 CPI–U of a 1.2 percent increase. The CY 2010 ASC conversion factor is $41.873.
1. Alternatives Considered
Alternatives to the changes we are making and the reasons that we have chosen the options are discussed throughout this final rule with comment period. Some of the major ASC issues discussed in this final rule with comment period and the options considered are discussed below.
a. Alternatives Considered for Office-Based Procedures
According to our final policy for the revised ASC payment system, we designate as office-based those procedures that are added to the ASC list of covered surgical procedures in CY 2008 or later years and that we determine are predominantly performed in physicians' offices based on consideration of the most recent available volume and utilization data for each individual procedure HCPCS code and/or, if appropriate, the clinical characteristics, utilization, and volume of related HCPCS codes. We establish payment for procedures designated as office-based at the lesser of the MPFS nonfacility PE RVU amount or the ASC rate developed according to the standard methodology of the revised ASC payment system.
In developing this final rule with comment period, we reviewed the full CY 2008 utilization data for all surgical procedures added to the ASC list of covered surgical procedures in CY 2008 or later years and for those procedures for which the office-based designation is temporary in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68730 through 68733). Based on that review, and as discussed in section XV.C.1.b. of this final rule with comment period, we are newly designating six existing surgical procedures as office-based and making permanent the office-based designations of four existing surgical procedures that have temporary office-based designations in CY 2009. We also are providing temporary office-based designations for 16 CY 2010 procedures reported with new or substantially revised CPT codes and continuing the temporary office-based designations for 6 procedures that were temporarily office-based in CY 2009. We considered two alternatives in developing this policy.
The first alternative we considered was to make no change to the procedure payment designations. This would mean that we would pay for the 6 procedures we are designating as permanently office-based and the 16 procedures we are newly designating as temporarily office-based at an ASC payment rate calculated according to the standard ratesetting methodology of the revised ASC payment system and for the 10 procedures with temporary office-based designations for 2009 according to the office-based methodology. We did not select this alternative because our analysis of the data and our clinical review indicated that all 10 procedures we are designating permanently office-based as well as the 22 procedures that we are designating temporarily office-based could be considered to be predominantly performed in physicians' offices. Consistent with our final policy adopted in the August 2, 2007 final rule (72 FR 42509), we were concerned that making payments at the standard ASC payment rate for the 6 procedures newly designated as office-based and 16 new procedures designated as temporarily office-based could create financial incentives for the procedures to shift from physicians' offices to ASCs for reasons unrelated to clinical decisions regarding the most appropriate setting for surgical care. Further, consistent with our policy, we believe that when adequate data become available to make permanent determinations about procedures with temporary office-based designations, maintaining the temporary designation is no longer appropriate.
The second alternative we considered and the one we selected for CY 2010 is to designate six additional procedures as office-based for CY 2010 and to make permanent the office-based designations of four of the procedures with temporary office-based designations in CY 2009. We also are designating 16 new procedures described by new or substantially revised CPT codes for CY 2010 as temporarily office-based and continuing to designate 6 procedures as temporarily office-based in CY 2010. We chose this alternative because our claims data and clinical review indicate that these procedures could be considered to be predominantly performed in physicians' offices. We believe that designating these procedures as office-based, which results in the CY 2010 ASC payment rate for these procedures potentially being capped at the CY 2010 physicians' office rate (that is, the MPFS nonfacility PE RVU amount), if applicable, is an appropriate step to ensure that Medicare payment policy does not create financial incentives for such procedures to shift unnecessarily from physicians' offices to ASCs, consistent with our final policy adopted in the August 2, 2007 final rule.
b. Alternatives Considered for Covered Surgical Procedures
According to our final policy for the revised ASC payment system, we designate as covered all surgical procedures that we determine would not be expected to pose a significant risk to beneficiary safety or would not be expected to require an overnight stay when performed on Medicare beneficiaries in an ASC.
In developing this final rule with comment period, we reviewed the clinical characteristics and full CY 2008 utilization data, if applicable, for all procedures reported by Category III CPT codes implemented July 1, 2009, and surgical procedures that were excluded from ASC payment for CY 2009. In response to public comments received on the CY 2009 OPPS/ASC proposed rule, we stated in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68724) that, as we developed the CY 2010 OPPS/ASC proposed rule and final rule with comment period, we would perform a comprehensive review of the APCs in order to identify potentially inconsistent ASC treatment of procedures assigned to a single APC under the OPPS. Thus, for this final rule with comment period, we examined surgical procedures that were excluded from the CY 2009 ASC list of covered surgical procedures and the APCs to which they were assigned under the OPPS. Based on this review, we identified 26 surgical procedures that meet the criteria for inclusion on the ASC list of covered surgical procedures, and we are adding those procedures to the list for CY 2010 payment, in addition to the 2 new surgical procedures described by Category III CPT codes that were new for July 2009, and that we determined were appropriate for addition to the ASC list. We considered two alternatives in developing this policy.
The first alternative we considered was to make no change to the ASC list of covered surgical procedures for CY 2010. We did not choose this alternative because our analysis of data and clinical Start Printed Page 60675 review indicated that the 28 procedures we are designating as covered surgical procedures for CY 2010 would not be expected to pose a significant risk to beneficiary safety in ASCs and would not be expected to require an overnight stay. Consistent with our final policy, we were concerned that by continuing to exclude them from the list of ASC covered surgical procedures, we may unnecessarily limit beneficiaries' access to the services in the most clinically appropriate settings.
The second alternative we considered and the one we selected for CY 2010 was to designate 28 additional procedures as ASC covered surgical procedures for CY 2010. We chose this alternative because our claims data and clinical review indicate that these procedures would not be expected to pose a significant risk to beneficiary safety and would not be expected to require an overnight stay, and thus they meet the criteria for inclusion on the list of ASC covered surgical procedures. We believe that adding these procedures to the list of covered surgical procedures is an appropriate step to ensure that beneficiary access to services is not limited unnecessarily.
2. Limitations of Our Analysis
Presented here are the projected effects of the changes for CY 2010 on Medicare payment to ASCs. A key limitation of our analysis is our inability to predict changes in ASC service mix between CY 2008 and CY 2010 with precision. We believe that the net effect on Medicare expenditures resulting from the CY 2010 changes will be small in the aggregate for all ASCs. However, such changes may have differential effects across surgical specialty groups as ASCs continue to adjust to the payment rates based on the policies of the revised ASC payment system. We are unable to accurately project such changes at a disaggregated level. Clearly, individual ASCs will experience changes in payment that differ from the aggregated estimated impacts presented below.
3. Estimated Effects of This Final Rule With Comment Period on Payments to ASCs
Some ASCs are multispecialty facilities that perform the gamut of surgical procedures, from excision of lesions to hernia repair to cataract extraction; others focus on a single specialty and perform only a limited range of surgical procedures, such as eye, digestive system, or orthopedic procedures. The combined effect on an individual ASC of the update to the CY 2010 payments will depend on a number of factors, including, but not limited to, the mix of services the ASC provides, the volume of specific services provided by the ASC, the percentage of its patients who are Medicare beneficiaries, and the extent to which an ASC provides different services in the coming year. The following discussion presents tables that display estimates of the impact of the CY 2010 update to the revised ASC payment system on Medicare payments to ASCs, assuming the same mix of services as reflected in our CY 2008 claims data. Table 75 depicts the estimated aggregate percent change in payment by surgical specialty or ancillary items and services group by comparing estimated CY 2009 payments to estimated CY 2010 payments, and Table 76 shows a comparison of estimated CY 2009 payments to estimated CY 2010 payments for procedures that we estimate will receive the most Medicare payment in CY 2010.
Table 75 shows the estimated effects on aggregate Medicare payments under the revised ASC payment system by surgical specialty or ancillary items and services group. We have aggregated the surgical HCPCS codes by specialty group, grouped all HCPCS codes for covered ancillary items and services into a single group, and then estimated the effect on aggregated payment for surgical specialty and ancillary items and services groups, considering separately the CY 2010 transitional rates and the ASC payment rates calculated according to the ASC standard ratesetting methodology that would apply in CY 2010 if there were no transition. The groups are sorted for display in descending order by estimated Medicare program payment to ASCs. The following is an explanation of the information presented in Table 75.
• Column 1— Surgical Specialty or Ancillary Items and Services Group indicates the surgical specialty into which ASC procedures are grouped or the ancillary items and services group which includes all HCPCS codes for covered ancillary items and services. To group surgical procedures by surgical specialty, we used the CPT code range definitions and Level II HCPCS codes and Category III CPT codes, as appropriate, to account for all surgical procedures to which the Medicare program payments are attributed.
• Column 2— Estimated ASC Payments were calculated using CY 2008 ASC utilization (the most recent full year of ASC utilization) and CY 2009 ASC payment rates. The surgical specialty and ancillary items and services groups are displayed in descending order based on estimated CY 2009 ASC payments.
• Column 3— Estimated CY 2010 Percent Change With Transition (25/75 Blend) is the aggregate percentage increase or decrease, compared to CY 2009, in Medicare program payment to ASCs for each surgical specialty or ancillary items and services group that is attributable to updates to the ASC payment rates for CY 2010 under the scaled, 25/75 blend of the CY 2007 ASC payment rates and the CY 2010 ASC payment rates calculated according to the ASC standard ratesetting methodology.
• Column 4— Estimated CY 2010 Percent Change Without Transition (Fully Implemented) is the aggregate percentage increase or decrease in Medicare program payment to ASCs for each surgical specialty or ancillary items and services group that would be attributable to updates to ASC payment rates for CY 2010 compared to CY 2009 if there were no transition period to the fully implemented payment rates. The percentages appearing in Column 4 are presented only as illustrative comparisons to the percentage changes under the transition policy in Column 3. We are not eliminating or modifying the policy for a 4-year transition that was finalized in the August 2, 2007 final rule (72 FR 42519).
As seen in Table 75, we estimate that the update to ASC rates for CY 2010 will result in no change in aggregate payment amounts for eye and ocular adnexa procedures and in aggregate decreases of 4 percent in payment amounts for both digestive system and nervous system procedures. As shown in Column 4 in the table, we estimate that if there were no transitional payment for these three surgical specialty groups in CY 2010, aggregate payments would decrease by 1 percent for eye and ocular adnexa procedures and by 10 and 6 percent for digestive and nervous system procedures, respectively.
Generally, for the surgical specialty groups that account for less ASC utilization and spending, we estimate that the payment effects of the CY 2010 update are positive. We estimate that ASC payments for procedures in those surgical specialties will increase in CY 2010 with the 25/75 transitional payment rates and, in the absence of the transition, will increase even more. For instance, we estimate that, in the aggregate, payment for integumentary system procedures will increase by 13 percent under the CY 2010 rates and by 20 percent if there were no transition. We estimate similar effects for genitourinary, cardiovascular, Start Printed Page 60676 musculoskeletal, respiratory, hematologic and lymphatic systems, and auditory system procedures as well.
An estimated increase in aggregate payment for the specialty group does not mean that all procedures in the group will experience increased payment rates. For example, the substantial estimated increase for CY 2010 for integumentary procedures is likely due to the significant median cost increase for APC 0137 (Level V Skin Repair) under the OPPS. The highest volume procedure in the integumentary surgical specialty group, described by CPT code 15823 (Blepharoplasty, upper eyelid; with excessive skin weighting down lid), is assigned to that APC under the OPPS. In contrast, the estimated increased payments at the surgical specialty group level may be due to decreased payments for some of the most frequently provided procedures in the group and the moderating effect of the sometimes substantial payment increases for the less frequently performed procedures within the surgical specialty group.
Also displayed in Table 75 is a separate estimate of Medicare ASC payments for the group of separately payable covered ancillary items and services. We estimate that aggregate payments for these items and services will remain the same for CY 2010. The payment estimates for the covered surgical procedures include the costs of packaged ancillary items and services. In prior years' rules, we did not have ASC payment data for covered ancillary items and services because prior to CY 2008, they were paid under other fee schedules or packaged into payment for the covered surgical procedures. Beginning with the CY 2010 proposed rule and this final rule with comment period, we have utilization data for those services as well as for all of the covered surgical procedures provided in ASCs under the revised payment system.
| Surgical specialty group | Estimated CY 2009 ASC payments (in millions) | Estimated CY 2010 percent change with transition (25/75 blend) | Estimated CY 2010 percent change without transition (fully implemented) |
|---|---|---|---|
| (1) | (2) | (3) | (4) |
| Total | 3,077 | 1 | 1 |
| Eye and ocular adnexa | 1,405 | 0 | −1 |
| Digestive system | 731 | −4 | −10 |
| Nervous system | 365 | −4 | −5 |
| Musculoskeletal system | 285 | 15 | 29 |
| Genitourinary system | 112 | 10 | 17 |
| Integumentary system | 106 | 13 | 20 |
| Respiratory system | 27 | 24 | 37 |
| Cardiovascular system | 20 | 17 | 27 |
| Ancillary items and services | 15 | 0 | −1 |
| Auditory system | 8 | 9 | 17 |
| Hematologic & lymphatic systems | 3 | 22 | 40 |
Table 76 below shows the estimated impact of the updates to the revised ASC payment system on aggregate ASC payments for selected surgical procedures during CY 2010 with and without the transitional blended rate. The table displays 30 of the procedures receiving the greatest estimated CY 2009 aggregate Medicare payments to ASCs. The HCPCS codes are sorted in descending order by estimated CY 2009 program payment.
• Column 1— HCPCS code.
• Column 2— Short Descriptor of the HCPCS code.
• Column 3—Estimated CY 2009 Allowed Charges were calculated using CY 2008 ASC utilization (the most recent full year of ASC utilization) and the CY 2009 ASC payment rates. The estimated CY 2009 allowed charges are expressed in millions of dollars.
• Column 4— Estimated CY 2010 Percent Change with Transition (25/75 Blend) reflects the percent differences between the estimated ASC payment for CY 2009 and the estimated payment for CY 2010 based on the update, incorporating a 25/75 blend of the CY 2007 ASC payment rate and the CY 2010 ASC payment rate calculated according to the ASC standard ratesetting methodology.
• Column 5— Estimated CY 2010 Percent Change without Transition (Fully Implemented) reflects the percent differences between the estimated ASC payment for CY 2009 and the estimated payment for CY 2010 based on the update if there were no transition period to the fully implemented payment rates. The percentages appearing in Column 5 are presented as a comparison to the percentage changes under the transition policy in Column 4 for informational purposes only. We are not eliminating or modifying the policy for the 4-year transition that was finalized in the August 2, 2007 final rule (72 FR 42519).
As displayed in Table 76, 24 of the 30 procedures with the greatest estimated aggregate CY 2009 Medicare payment are included in the 3 surgical specialty groups that are estimated to account for the most Medicare payment to ASCs in CY 2009, specifically eye and ocular adnexa, digestive system, and nervous system surgical groups. Consistent with the estimated payment effects on the surgical specialty groups displayed in Table 75, the estimated effects of the CY 2010 update on ASC payment for individual procedures in year 3 of the transition shown in Table 76 are varied. Aggregate ASC payments for many of the most frequently furnished ASC procedures will decrease as the transitional rates more closely align the individual procedure relative ASC payment weights with the relativity of payments under the OPPS.
The ASC procedure for which the most Medicare payment is estimated to be made in CY 2009 is the cataract removal procedure reported with CPT code 66984 (Extracapsular cataract removal with insertion of intraocular lens prosthesis (one stage procedure), Start Printed Page 60677 manual or mechanical technique (e.g., irrigation and aspiration or phacoemulsification)). We estimate that the update to the ASC rates will result in a negligible payment decrease for this procedure in CY 2010. The estimated payment effects on the three other eye and ocular adnexa procedures included in Table 76 will be slightly positive or negative, but for CPT code 66821 (Discission of secondary membranous cataract (opacified posterior lens capsule and/or anterior hyaloid); laser surgery (e.g., YAG laser) (one or more stages)), the estimated CY 2010 payment decrease will be 10 percent, significantly greater than the decreases estimated for any of the other eye and ocular adnexa procedures shown.
We estimate that the transitional payment rates for all but 1 of the 9 digestive system procedures included in Table 76 will decrease by 5 to 8 percent in CY 2010. Those estimated decreases are consistent with decreases in the previous 2 years under the revised ASC payment system and are expected because, under the previous ASC payment system, the payment rates for many high volume endoscopy procedures were almost the same as the payments for the procedures under the OPPS.
The estimated effects of the CY 2010 update on the 9 nervous system procedures for which the most Medicare ASC payment is estimated to be made in CY 2009 will be variable. Our estimates indicate that the CY 2010 update will result in payment decreases of 4 percent or less for 4 of the 9 procedures and in more substantial decreases for 2 others. We estimate that the greatest decreases will be for the add-on procedure described by CPT code 64484 (Injection, anesthetic agent and/or steroid, transforaminal epidural; lumbar or sacral, each additional level) and for the procedure described by CPT code 63685 (Insertion or replacement of spinal neurostimulator pulse generator or receiver, direct or inductive coupling), which we estimate to have 19 and 9 percent payment decreases, respectively, in CY 2010. In contrast, the three nervous system procedures for which we estimate positive effects on CY 2010 payments, CPT code 63650 (Percutaneous implantation of neurostimulator electrode array, epidural); CPT code 64721 (Neuroplasty and/or transposition; median nerve at carpal tunnel); and CPT code 64622 (Destruction by neurolytic agent, paravertebral facet joint nerve; lumbar or sacral, single level), are estimated to have substantial payment increases of 10, 13, and 6 percent, respectively.
The estimated payment effects for most of the remaining procedures listed in Table 76 will be positive. For example, the CY 2010 transitional payment rates for musculoskeletal CPT codes 29880 (Arthroscopy, knee, surgical; with meniscectomy (medial AND lateral, including any meniscal shaving)) and 29881 (Arthroscopy, knee, surgical; with meniscectomy (medial OR lateral, including any meniscal shaving)) are estimated to increase 17 percent over the CY 2009 transitional payment rates. We estimate that musculoskeletal procedures will account for a greater percentage of CY 2010 Medicare ASC spending as we estimate that payment for procedures in that surgical specialty group will increase under the revised payment system in CY 2010.
| HCPCS code * | Short descriptor | Estimated CY 2009 allowed charges (in mil) | Estimated CY 2010 percent change with transition (25/75 blend) | Estimated CY 2010 percent change without transition (fully implemented) |
|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) |
| 66984 | Cataract surg w/iol, 1 stage | 1,064 | 0 | −2 |
| 43239 | Upper gi endoscopy, biopsy | 164 | −6 | −13 |
| 45380 | Colonoscopy and biopsy | 133 | −5 | −11 |
| 45378 | Diagnostic colonoscopy | 124 | −5 | −11 |
| 45385 | Lesion removal colonoscopy | 96 | −5 | −11 |
| 66821 | After cataract laser surgery | 71 | −10 | −20 |
| 62311 | Inject spine l/s (cd) | 69 | −4 | −8 |
| 66982 | Cataract surgery, complex | 62 | 0 | −2 |
| 64483 | Inj foramen epidural l/s | 57 | −4 | −8 |
| 15823 | Revision of upper eyelid | 35 | 15 | 21 |
| 45384 | Lesion remove colonoscopy | 33 | −5 | −11 |
| G0105 | Colorectal scrn; hi risk ind | 33 | −8 | −17 |
| G0121 | Colon ca scrn not hi rsk ind | 32 | −8 | −16 |
| 29881 | Knee arthroscopy/surgery | 25 | 16 | 30 |
| 63650 | Implant neuroelectrodes | 25 | 10 | 14 |
| 43235 | Uppr gi endoscopy, diagnosis | 24 | 2 | 1 |
| 64721 | Carpal tunnel surgery | 23 | 13 | 24 |
| 52000 | Cystoscopy | 22 | −5 | −9 |
| 29880 | Knee arthroscopy/surgery | 20 | 17 | 30 |
| 63685 | Insrt/redo spine n generator | 18 | −9 | −8 |
| 29826 | Shoulder arthroscopy/surgery | 17 | 28 | 54 |
| 62310 | Inject spine c/t | 15 | −4 | −8 |
| 67904 | Repair eyelid defect | 15 | 0 | 2 |
| 28285 | Repair hammertoe | 15 | 14 | 25 |
| 29827 | Arthroscop rotator cuff repr | 14 | 22 | 42 |
| 64622 | Destr paravertebrl nerve l/s | 14 | 6 | 14 |
| 64484 | Inj foramen epidural add-on | 13 | −19 | −38 |
| 43248 | Uppr gi endoscopy/guide wire | 12 | −6 | −13 |
| 64623 | Destr paravertebral n add-on | 12 | −4 | −8 |
| Start Printed Page 60678 | ||||
| 26055 | Incise finger tendon sheath | 12 | 12 | 21 |
| * Note that HCPCS codes deleted for CY 2010 are not displayed in this table. | ||||
The previous ASC payment system served as an incentive to ASCs to focus on providing procedures for which they determined Medicare payments would support their continued operation. We note that, historically, the ASC payment rates for many of the most frequently performed procedures in ASCs were similar to the OPPS payment rates for the same procedures. Conversely, procedures with ASC payment rates that were substantially lower than the OPPS rates have historically been performed least often in ASCs. We believed that the revised ASC payment system will encourage greater efficiency in ASCs and will promote significant increases in the breadth of surgical procedures performed in ASCs because it distributes payments across the entire spectrum of covered surgical procedures based on a coherent system of relative weights that are related to the clinical and facility resource requirements of those procedures.
The CY 2008 claims data that we used to develop the CY 2010 ASC payment system relative weights and rates reflect the first year of utilization under the revised payment system. Although the changes in the claims data are not large, the data reflect increased Medicare ASC spending for procedures that were newly added to the ASC list in CY 2008. Our estimates based on CY 2008 data indicate that for CY 2010 there would be especially noticeable increases in spending for genitourinary and cardiovascular procedures, compared to the previous ASC payment system.
4. Estimated Effects of This Final Rule With Comment Period on Beneficiaries
We estimate that the CY 2010 update to the ASC payment system will be generally positive for beneficiaries with respect to the new procedures that we are adding to the ASC list of covered surgical procedures and for those that we are designating as office-based for CY 2010. First, except for screening colonoscopy and flexible sigmoidoscopy procedures, the ASC coinsurance rate for all procedures is 20 percent. This contrasts with procedures performed in HOPDs, where the beneficiary is responsible for copayments that range from 20 percent to 40 percent of the procedure payment. Second, ASC payment rates under the revised payment system are lower than payment rates for the same procedures under the OPPS; therefore, the beneficiary coinsurance amount under the ASC payment system almost always will be less than the OPPS copayment amount for the same services. (The only exceptions will be if the ASC coinsurance amount exceeds the inpatient deductible. The statute requires that copayment amounts under the OPPS not exceed the inpatient deductible.) For new procedures that we are adding to the ASC list of covered surgical procedures in CY 2010, as well as for procedures already included on the list, and that are furnished in an ASC rather than the HOPD setting, the beneficiary coinsurance amount will be less than the OPPS copayment amount. Furthermore, the additions to the ASC list of covered surgical procedures will provide beneficiaries access to more surgical procedures in ASCs. Beneficiary coinsurance for services migrating from physicians' offices to ASCs may decrease or increase under the revised ASC payment system, depending on the particular service and the relative payment amounts for that service in the physician's office compared to the ASC. However, for those additional procedures that we are designating as office-based in CY 2010, the beneficiary coinsurance amount will be no greater than the beneficiary coinsurance in the physician's office.
In addition, as finalized in the August 2, 2007 final rule (72 FR 42520), in CY 2010, the third year of the 4-year transition to the ASC payment rates calculated according to the ASC standard ratesetting methodology of the revised ASC payment system, ASC payment rates for a number of commonly furnished ASC procedures will continue to be reduced, resulting in lower beneficiary coinsurance amounts for these ASC services in CY 2010.
5. Conclusion
The updates to the ASC payment system for CY 2010 will affect each of the approximately 5,000 ASCs currently approved for participation in the Medicare program. The effect on an individual ASC will depend on its mix of patients, the proportion of the ASC's patients that are Medicare beneficiaries, the degree to which the payments for the procedures offered by the ASC are changed under the revised payment system, and the extent to which the ASC provides a different set of procedures in the coming year.
The CY 2010 update to the revised ASC payment system includes a payment update of 1.2 percent that we estimate will result in a greater amount of Medicare expenditures in CY 2010 than was estimated to be made in CY 2009. We estimate that the update to the revised ASC payment system, including the addition of surgical procedures to the list of covered surgical procedures, will have a modest effect on Medicare expenditures compared to the estimated level of Medicare expenditures in CY 2009.
6. Accounting Statement
As required by OMB Circular A–4 (available at http://www.whitehousegov/omb/circulars/a004/a-4.pdf), in Table 77 below, we have prepared an accounting statement showing the classification of the expenditures associated with the statutorily authorized 1.2 percent update to the CY 2010 revised ASC payment system, based on the provisions of this final rule with comment period and the baseline spending estimates for ASCs in the 2009 Medicare Trustees Report. This table provides our best estimate of Medicare payments to suppliers as a result of the final update to the CY 2010 ASC payment system, as presented in this Start Printed Page 60679 final rule with comment period. All expenditures are classified as transfers.
| Category | Transfers |
|---|---|
| Annualized Monetized Transfers | $33 Million. |
| From Whom to Whom | Federal Government to Medicare Providers and Suppliers. |
| Total | $33 Million. |
D. Effects of Requirements for Hospital Reporting of Quality Data for Annual Hospital Payment Update
In section XVI. of the CY 2009 OPPS/ASC final rule with comment period (73 FR 68758), we discussed our requirements for subsection (d) hospitals to report quality data under the HOP QDRP in order to receive the full payment update for CY 2010. In section XVI. of this final rule with comment period, we established additional policies affecting the CY 2010 and CY 2011 HOP QDRP. We estimate that about 83 hospitals may not receive the full payment update in CY 2010. Most of these hospitals are either small rural or small urban hospitals. However, at this time, information is not available to determine the precise number of hospitals that do not meet the requirements for the full hospital market basket increase for CY 2010. We also estimate that 83 hospitals may not receive the full payment update in CY 2011.
In section XVI.E.3.a. of this final rule with comment period, for the CY 2011 payment update, as part of the validation process, we are requiring hospitals to submit paper copies of requested medical records to a designated contractor within the required timeframe. Failure to submit requested documentation can result in a 2 percentage point reduction in a hospital's update, but the failure to pass the validation itself would not. Of the 83 hospitals that we estimate will not receive the full payment update for CY 2011, we estimate that no more than 20 hospitals would fail the validation documentation submission requirement for the CY 2011 payment update.
For the CY 2011 payment update, our validation sample size is estimated to be about 7,300 medical records. We estimate that this requirement will cost hospitals approximately 12 cents per page for copying and approximately $4.00 per chart for postage. We have found, based on experience, that an average sized outpatient medical chart is approximately 30 pages. We estimate that the total cost to the impacted hospitals will be approximately $55,480, with a maximum expected cost of $152 for an individual hospital based upon an expected maximum of 20 selected records; the expected minimum will be $0.00 if no records were selected from a hospital. We believe that this cost is minimal, compared with the 2.0 percentage point HOP QDRP component of the annual payment update at risk. CMS does not plan to reimburse hospitals for copying and mailing costs. This validation requirement is necessary so that CMS has all the information it needs to validate the accuracy of hospital submitted data abstracted from paper medical records.
In section XVI.E.3.b. of this final rule with comment period, we did not, at this time, adopt our proposal in the CY 2010 OPPS/ASC proposed rule (74 FR 35403) to expand the CY 2011 validation requirement for the CY 2012 payment update. Instead, we will consider the public comments we received on that proposal, as well as any analyses we conduct of the CY 2011 validation process, and propose a CY 2012 validation process as a part of the CY 2011 OPPS/ASC rulemaking. We believe that this approach will give HOP QDRP hospitals additional experience with the validation process and allow these hospitals sufficient time to prepare for the CY 2012 validation.
However, we noted in the CY 2010 OPPS/ASC proposed rule (74 FR 35424) that we expected our proposal to validate data submitted by 800 hospitals for purposes of the CY 2012 HOP QDRP payment determination would not have changed the number of hospitals that fail the validation requirement from CY 2011. For CY 2011, and under our proposal for CY 2012 in the CY 2010 OPPS/ASC proposed rule, we stated that we would calculate the validation matches for CY 2011 (we note, however, that the validation results will not affect the CY 2011 payment update) and CY 2012 by assessing whether the overall measure data submitted by the hospital matches the independently reabstracted measure data. We believe that this methodology will make it easier for hospitals to satisfy the validation requirement than if we calculate the percent agreement between what the hospital submitted and what the CMS-designated contractor independently reabstracted for each individual data element that was submitted. In addition, for the CY 2012 payment update, in the CY 2010 OPPS/ASC proposed rule, we proposed to validate data for only 800 hospitals out of the approximately 3,400 HOP QDRP participating hospitals. As a result, we believe that the effect of our proposed validation process for CY 2012 would have been minimal in terms of the number of hospitals that would not meet all program requirements. In the CY 2010 OPPS/ASC proposed rule (74 FR 35424), we stated that of the 83 hospitals that we estimated would not have received the full payment update for CY 2012, we estimated that approximately 20 hospitals would have failed to meet our proposed CY 2012 validation requirements.
E. Executive Order 12866
In accordance with the provisions of Executive Order 12866, this final rule with comment period rule was reviewed by the OMB.
Start List of SubjectsList of Subjects
42 CFR Part 410
- Health facilities
- Health professions
- Laboratories
- Medicare
- Rural areas
- X-rays
42 CFR Part 416
- Health facilities
- Health professions
- Medicare
- Reporting and recordkeeping requirements
42 CFR Part 419
- Hospitals
- Medicare
- Reporting and recordkeeping requirements
For reasons stated in the preamble of this document, the Centers for Medicare & Medicaid Services is amending 42 CFR chapter IV as set forth below:
End Amendment Part Start PartPART 410—SUPPLEMENTARY MEDICAL INSURANCE (SMI) BENEFITS
End Part Start Amendment Part1. The authority citation for part 410 continues to read as follows:
End Amendment Part Start AuthorityAuthority: Secs. 1102 and 1871 of the Social Security Act (42 U.S.C. 1302 and 1395hh).
End Authority Start Amendment Part2. Section 410.27 is amended by—
End Amendment Part Start Amendment Parta. Revising the section heading.
End Amendment Part Start Amendment Partb. Revising the introductory text of paragraph (a) and paragraph (a)(1).
End Amendment Part Start Amendment Partc. Revising paragraph (e).
End Amendment Part Start Amendment Partd. Revising paragraph (f).
End Amendment Part Start Amendment Parte. Adding new paragraph (g).
End Amendment Part Start Amendment PartThe revisions and additions read as follows:
End Amendment Part(a) Medicare Part B pays for hospital or CAH services and supplies furnished Start Printed Page 60680 incident to a physician or nonphysician practitioner service to outpatients, including drugs and biologicals that cannot be self-administered, if—
(1) They are furnished—
(i) By or under arrangements made by the participating hospital or CAH, except in the case of a SNF resident as provided in § 411.15(p) of this chapter;
(ii) As an integral though incidental part of a physician's or nonphysician practitioner's services;
(iii) In the hospital or CAH or in a department of the hospital or CAH, as defined in § 413.65 of this subchapter; and
(iv) Under the direct supervision of a physician or a nonphysician practitioner as specified in paragraph (f) of this section. Nonphysician practitioners may directly supervise services that they may personally furnish in accordance with State law and all additional requirements, including those specified in §§ 410.71, 410.73, 410.74, 410.75, 410.76, and 410.77.
(A) For services furnished in the hospital or CAH or in an on-campus outpatient department of the hospital or CAH, as defined in § 413.65 of this subchapter, “direct supervision” means that the physician or nonphysician practitioner must be present on the same campus and immediately available to furnish assistance and direction throughout the performance of the procedure. It does not mean that the physician or nonphysician practitioner must be present in the room when the procedure is performed. For pulmonary rehabilitation, cardiac rehabilitation, and intensive cardiac rehabilitation services, direct supervision must be furnished by a doctor of medicine or osteopathy, as specified in §§ 410.47 and 410.49, respectively.
(B) For services furnished in an off-campus outpatient department of the hospital or CAH, as defined in § 413.65 of this subchapter, “direct supervision” means the physician or nonphysician practitioner must be present in the off-campus provider-based department of the hospital or CAH and immediately available to furnish assistance and direction throughout the performance of the procedure. It does not mean that the physician or nonphysician practitioner must be present in the room when the procedure is performed. For pulmonary rehabilitation, cardiac rehabilitation, and intensive cardiac rehabilitation services, direct supervision must be furnished by a doctor of medicine or osteopathy, as specified in §§ 410.47 and 410.49, respectively.
(e) Services furnished by an entity other than the hospital or CAH are subject to the limitations specified in § 410.42(a).
(f) For purposes of this section, “nonphysician practitioner” means a clinical psychologist, licensed clinical social worker, physician assistant, nurse practitioner, clinical nurse specialist, or certified nurse-midwife.
(g) For purposes of this section, “in the hospital or CAH” means areas in the main building(s) of the hospital or CAH that are under the ownership, financial, and administrative control of the hospital or CAH; that are operated as part of the hospital or CAH; and for which the hospital or CAH bills the services furnished under the hospital's or CAH's CMS Certification Number.
3. Section 410.28 is amended by revising paragraph (e) to read as follows:
End Amendment Part(e) Medicare Part B makes payment under section 1833(t) of the Act for diagnostic services furnished by or under arrangements made by the participating hospital, only when the diagnostic services are furnished under the appropriate level of physician supervision specified by CMS in accordance with the definitions in § 410.32(b)(3)(i), (b)(3)(ii), and (b)(3)(iii). Under general supervision, the training of the nonphysician personnel who actually perform the diagnostic procedure and the maintenance of the necessary equipment and supplies are the continuing responsibility of the facility. In addition—
(1) For services furnished directly or under arrangement in the hospital or in an on-campus outpatient department of the hospital, as defined in § 413.65 of this subchapter, “direct supervision” means that the physician must be present on the same campus and immediately available to furnish assistance and direction throughout the performance of the procedure. It does not mean that the physician must be present in the room when the procedure is performed. For this purpose, the definition of “in the hospital” is as specified in § 410.27(g).
(2) For services furnished directly or under arrangement in an off-campus outpatient department of the hospital, as defined in § 413.65 of this subchapter, “direct supervision” means the physician must be present in the off-campus provider-based department of the hospital and immediately available to furnish assistance and direction throughout the performance of the procedure. It does not mean that the physician must be present in the room when the procedure is performed.
(3) For services furnished under arrangement in nonhospital locations, “direct supervision” means the definition specified in § 410.32(b)(3)(ii).
PART 416—AMBULATORY SURGICAL SERVICES
End Part Start Amendment Part4. The authority citation for Part 416 continues to read as follows:
End Amendment Part Start AuthorityAuthority: Secs. 1102 and 1871 of the Social Security Act (42 U.S.C. 1302 and 1395hh).
End Authority Start Amendment Part5. Section 416.30 is amended by revising paragraph (f)(2) to read as follows:
End Amendment Part(f) * * *
(2) The ASC participates and is paid only as an ASC.
PART 419—PROSPECTIVE PAYMENT SYSTEM FOR HOSPITAL OUTPATIENT DEPARTMENT SERVICES
End Part Start Amendment Part6. The authority citation for part 419 continues to read as follows:
End Amendment Part Start AuthorityAuthority: Secs. 1102, 1833(t), and 1871 of the Social Security Act (42 U.S.C. 1302, 1395(t), and 1395(hh).
End Authority Start Amendment Part7. Section 419.64 is amended by adding new paragraphs (a)(4)(iii) and (a)(4)(iv), to read as follows:
End Amendment Part(a) * * *
(4) * * *
(iii) A biological that is not surgically implanted or inserted into the body.
(iv) A biological that is surgically implanted or inserted into the body, for which pass-through payment as a biological is made on or before December 31, 2009.
8. Section 419.66 is amended by revising paragraph (b)(4)(iii) to read as follows:
End Amendment Part(b) * * *
(4) * * *
(iii) A material that may be used to replace human skin (for example, a biological skin replacement material or synthetic skin replacement material).
9. Section 419.70 is amended by revising the heading of paragraph (d)(5) to read as follows:
End Amendment Part(d) * * *
(5) Temporary treatment for small sole community hospitals on or after January 1, 2009 and through December 31, 2009. * * *
(Catalog of Federal Domestic Assistance Program No. 93.773, Medicare—Hospital Insurance; and Program No. 93.774, Medicare—Supplementary Medical Insurance Program)
Dated: October 26, 2009.
Charlene Frizzera,
Acting Administrator, Centers for Medicare & Medicaid Services.
Dated: October 29, 2009.
Kathleen Sebelius,
Secretary.
Start Printed Page 60686
Start Printed Page 60687
Start Printed Page 60688
Start Printed Page 60689
Start Printed Page 60690
Start Printed Page 60691
Start Printed Page 60692
Start Printed Page 60693
Start Printed Page 60694
Start Printed Page 60695
Start Printed Page 60696
Start Printed Page 60697
Start Printed Page 60698
Start Printed Page 60699
Start Printed Page 60700
Start Printed Page 60701
Start Printed Page 60702
Start Printed Page 60703
Start Printed Page 60704
Start Printed Page 60705
Start Printed Page 60706
Start Printed Page 60707
Start Printed Page 60708
Start Printed Page 60709
Start Printed Page 60710
Start Printed Page 60711
Start Printed Page 60712
Start Printed Page 60713
Start Printed Page 60714
Start Printed Page 60715
Start Printed Page 60716
Start Printed Page 60717
Start Printed Page 60718
Start Printed Page 60719
Start Printed Page 60720
Start Printed Page 60721
Start Printed Page 60722
Start Printed Page 60723
Start Printed Page 60724
Start Printed Page 60725
Start Printed Page 60726
Start Printed Page 60727
Start Printed Page 60728
Start Printed Page 60729
Start Printed Page 60730
Start Printed Page 60731
Start Printed Page 60732
Start Printed Page 60733
Start Printed Page 60734
Start Printed Page 60735
Start Printed Page 60736
Start Printed Page 60737
Start Printed Page 60738
Start Printed Page 60739
Start Printed Page 60740
Start Printed Page 60741
Start Printed Page 60742
Start Printed Page 60743
Start Printed Page 60744
Start Printed Page 60745
Start Printed Page 60746
Start Printed Page 60747
Start Printed Page 60748
Start Printed Page 60749
Start Printed Page 60750
Start Printed Page 60751
Start Printed Page 60752
Start Printed Page 60753
Start Printed Page 60754
Start Printed Page 60755
Start Printed Page 60756
Start Printed Page 60757
Start Printed Page 60758
Start Printed Page 60759
Start Printed Page 60760
Start Printed Page 60761
Start Printed Page 60762
Start Printed Page 60763
Start Printed Page 60764
Start Printed Page 60765
Start Printed Page 60766
Start Printed Page 60767
Start Printed Page 60768
Start Printed Page 60769
Start Printed Page 60770
Start Printed Page 60771
Start Printed Page 60772
Start Printed Page 60773
Start Printed Page 60774
Start Printed Page 60775
Start Printed Page 60776
Start Printed Page 60777
Start Printed Page 60778
Start Printed Page 60779
Start Printed Page 60780
Start Printed Page 60781
Start Printed Page 60782
Start Printed Page 60783
Start Printed Page 60784
Start Printed Page 60785
Start Printed Page 60786
Start Printed Page 60787
Start Printed Page 60788
Start Printed Page 60789
Start Printed Page 60790
Start Printed Page 60791
Start Printed Page 60792
Start Printed Page 60793
Start Printed Page 60794
Start Printed Page 60795
Start Printed Page 60796
Start Printed Page 60797
Start Printed Page 60798
Start Printed Page 60799
Start Printed Page 60800
Start Printed Page 60801
Start Printed Page 60802
Start Printed Page 60803
Start Printed Page 60804
Start Printed Page 60805
Start Printed Page 60806
Start Printed Page 60807
Start Printed Page 60808
Start Printed Page 60809
Start Printed Page 60810
Start Printed Page 60811
Start Printed Page 60812
Start Printed Page 60813
Start Printed Page 60814
Start Printed Page 60815
Start Printed Page 60816
Start Printed Page 60817
Start Printed Page 60818
Start Printed Page 60819
Start Printed Page 60820
Start Printed Page 60821
Start Printed Page 60822
Start Printed Page 60823
Start Printed Page 60824
Start Printed Page 60825
Start Printed Page 60826
Start Printed Page 60827
Start Printed Page 60828
Start Printed Page 60829
Start Printed Page 60830
Start Printed Page 60831
Start Printed Page 60832
Start Printed Page 60833
Start Printed Page 60834
Start Printed Page 60835
Start Printed Page 60836
Start Printed Page 60837
Start Printed Page 60838
Start Printed Page 60839
Start Printed Page 60840
Start Printed Page 60841
Start Printed Page 60842
Start Printed Page 60843
Start Printed Page 60844
Start Printed Page 60845
Start Printed Page 60846
Start Printed Page 60847
Start Printed Page 60848
Start Printed Page 60849
Start Printed Page 60850
Start Printed Page 60851
Start Printed Page 60852
Start Printed Page 60853
Start Printed Page 60854
Start Printed Page 60855
Start Printed Page 60856
Start Printed Page 60857
Start Printed Page 60858
Start Printed Page 60859
Start Printed Page 60860
Start Printed Page 60861
Start Printed Page 60862
Start Printed Page 60863
Start Printed Page 60864
Start Printed Page 60865
Start Printed Page 60866
Start Printed Page 60867
Start Printed Page 60868
Start Printed Page 60869
Start Printed Page 60870
Start Printed Page 60871
Start Printed Page 60872
Start Printed Page 60873
Start Printed Page 60874
Start Printed Page 60875
Start Printed Page 60876
Start Printed Page 60877
Start Printed Page 60878
Start Printed Page 60879
Start Printed Page 60880
Start Printed Page 60881
Start Printed Page 60882
Start Printed Page 60883
Start Printed Page 60884
Start Printed Page 60885
Start Printed Page 60886
Start Printed Page 60887
Start Printed Page 60888
Start Printed Page 60889
Start Printed Page 60890
Start Printed Page 60891
Start Printed Page 60892
Start Printed Page 60893
Start Printed Page 60894
Start Printed Page 60895
Start Printed Page 60896
Start Printed Page 60897
Start Printed Page 60898
Start Printed Page 60899
Start Printed Page 60900
Start Printed Page 60901
Start Printed Page 60902
Start Printed Page 60903
Start Printed Page 60904
Start Printed Page 60905
Start Printed Page 60906
Start Printed Page 60907
Start Printed Page 60908
Start Printed Page 60909
Start Printed Page 60910
Start Printed Page 60911
Start Printed Page 60912
Start Printed Page 60913
Start Printed Page 60914
Start Printed Page 60915
Start Printed Page 60916
Start Printed Page 60917
Start Printed Page 60918
Start Printed Page 60919
Start Printed Page 60920
Start Printed Page 60921
Start Printed Page 60922
Start Printed Page 60923
Start Printed Page 60924
Start Printed Page 60925
Start Printed Page 60926
Start Printed Page 60927
Start Printed Page 60928
Start Printed Page 60929
Start Printed Page 60930
Start Printed Page 60931
Start Printed Page 60932
Start Printed Page 60933
Start Printed Page 60934
Start Printed Page 60935
Start Printed Page 60936
Start Printed Page 60937
Start Printed Page 60938
Start Printed Page 60939
Start Printed Page 60940
Start Printed Page 60941
Start Printed Page 60942
Start Printed Page 60943
Start Printed Page 60944
Start Printed Page 60945
Start Printed Page 60946
Start Printed Page 60947
Start Printed Page 60948
Start Printed Page 60949
Start Printed Page 60950
Start Printed Page 60951
Start Printed Page 60952
Start Printed Page 60953
Start Printed Page 60954
Start Printed Page 60955
Start Printed Page 60956
Start Printed Page 60957
Start Printed Page 60958
Start Printed Page 60959
Start Printed Page 60960
Start Printed Page 60961
Start Printed Page 60962
Start Printed Page 60963
Start Printed Page 60964
Start Printed Page 60965
Start Printed Page 60966
Start Printed Page 60967
Start Printed Page 60968
Start Printed Page 60969
Start Printed Page 60970 Start Printed Page 60971 Start Printed Page 60972 Start Printed Page 60973 Start Printed Page 60974 Start Printed Page 60975 Start Printed Page 60976 Start Printed Page 60977 Start Printed Page 60978 Start Printed Page 60979 Start Printed Page 60980 Start Printed Page 60981 Start Printed Page 60982 Start Printed Page 60983 End Supplemental Information
Footnotes
1. A registry is a collection of clinical data for purposes of assessing clinical performance, quality of care, and opportunities for quality improvement.
Back to Citation2. Institute of Medicine: To Err Is Human: Building a Safer Health System, November 1999. Available at: http://www.iom.edu/Object.File/Master/4/117/ToErr-8pager.pdf.
Back to Citation3. Asplen, P., Wolcott, J., Bootman, J.L., Cronenwett, L.R. (editors): Preventing Medication Errors: Quality Chasm Series, The National Academy Press, 2007. Available at: http://www.nap.edu/catalog.php?record_id=11623.
Back to Citation4120–01–P
BILLING CODE 4120–01–C
BILLING CODE 4120–01–P
BILLING CODE 4120–01–C
BILLING CODE 4120–01–P
BILLING CODE 4120–01–C
BILLING CODE 4120–01–P
BILLING CODE 4120–01–C
BILLING CODE 4120–01–P
BILLING CODE 4120–01–C
BILLING CODE 4120–01–P
BILLING CODE 4120–01–C
BILLING CODE 4120–01–P
BILLING CODE 4120–01–C
BILLING CODE 4120–01–P
BILLING CODE 4120–01–C
BILLING CODE 4120–01–P
BILLING CODE 4120–01–C
BILLING CODE 4120–01–P
[FR Doc. E9–26499 Filed 10–30–09; 4:15 pm]
BILLING CODE 4120–01–C